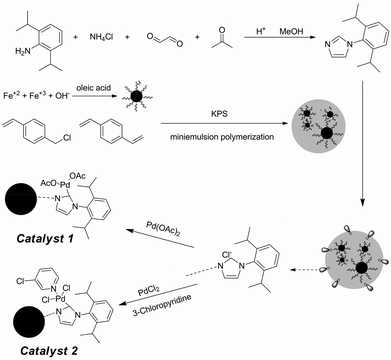
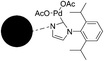
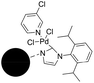
A magnetically separable palladium catalyst containing a bulky N-heterocyclic carbene ligand for the Suzuki–Miyaura reaction†
Z.
Wang
a,
Y.
Yu
a,
Y. X.
Zhang
b,
S. Z.
Li
a,
H.
Qian
*a and
Z. Y.
Lin
*c
aCollege of Material Science and Engineering, Huaqiao University, XiaMen 361021, Fujian, China. E-mail: hquqh@hotmail.com; Fax: (+86) 5926166393
bCollege of Communication Science and Engineering, Huaqiao University, XiaMen 361021, Fujian, China
cEngineering Research Center of Environment-Friendly Functional Materials, Ministry of Education, Huaqiao University, XiaMen 361021, Fujian, China. E-mail: linzy@hqu.eud.cn; Fax: (+86) 5926162220
First published on 8th September 2014
Abstract
This work describes the preparation and characterization of a magnetic palladium catalyst with bulky N-heterocyclic carbene (NHC) ligands for the Suzuki–Miyaura cross-coupling reaction. After the 1-(2,6-diisopropylphenyl)-1H-imidazole (1-arylimidazole) was modified on the surface of magnetic polymer carriers, palladium diacetate was employed to synthesize the Pd-NHC complex, affording a palladium loading of 0.23 mmol g−1. This magnetic catalyst showed high catalytic activity towards the Suzuki–Miyaura reaction of phenylboronic acids with aryl bromides in the ethanol–water solution (TON > 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). After 21 cycling runs, its catalytic activity decreased slightly, and no leaking of palladium was found either in products or in reaction residue. When other sources of palladium (PdCl2 and 3-Cl-pyridinyl) were employed to synthesize the palladium complex, the stability of the magnetic catalyst was greatly improved to perform the catalysis of Suzuki–Miyaura reactions with aryl chlorides at 100 °C.
000). After 21 cycling runs, its catalytic activity decreased slightly, and no leaking of palladium was found either in products or in reaction residue. When other sources of palladium (PdCl2 and 3-Cl-pyridinyl) were employed to synthesize the palladium complex, the stability of the magnetic catalyst was greatly improved to perform the catalysis of Suzuki–Miyaura reactions with aryl chlorides at 100 °C.
Introduction
The palladium-catalyzed Suzuki–Miyaura cross-coupling reaction has been widely used in scientific research and industrial production during the past few decades. Although a large number of homogeneous catalysts showed excellent catalytic activity,1–4 the homogeneous catalysis also suffers from the tedious separation of the expensive catalyst in order to lower costs and avoid pollution.5,6 Moreover, the utilization of recyclable catalysts is an inevitable trend with the development of green chemistry and engineering. Therefore, immobilization of palladium catalysts has attracted much attention and made great achievements.7 However, most of those supported Pd catalysts have a lower activity in comparison with the similar homogeneous systems.A series of carriers have been developed for immobilizing palladium catalysts, such as silica,8–10 alumina,11 microporous polymers,12–16 carbon black,17,18 dendrimers,19,20 and polyoxometates.21 It is known that the size of the carriers is one of the most important factors which influences the catalytic activity of catalysts.22 Large scale carriers benefit the separation and recycling processes, but catalytic efficiency is usually decreased due to its small specific surface area in the heterogeneous reactions. However, nanoparticles, that make the catalyst much closer to the homogeneous system, resulting in high catalytic efficiency, are very difficult to separate by conventional procedures like filtration or precipitation. Thus, some superparamagnetic nanoparticles were developed as the catalyst supports to bridge the gap between heterogeneous and homogeneous catalysis.22 There are many benefits of these nano-magnetic carriers, for example, deposit-free, nanosize distribution, easy magnetic separation, no metal leaking and non-toxicity, which made it possible to integrate high catalytic activity and easy separation.23–26 We have previously reported the preparation of some magnetic nano-spheres and micro-spheres and their applications in bioseparation and water purification.27,28 These magnetic carriers exhibited high efficiency, strong specificity and easy recycling.
Since palladium catalysts were used as the catalysts of the Suzuki–Miyaura reaction, palladium complex and palladium nanoparticles have been widely researched and explored. Compared with the palladium complex, the palladium nanoparticles become less stable and more poorly selective due to their high activity, which merely generated more byproducts in the reaction and easily became inactivated in the process of storage and utilization. Thus, the tetrakis(triphenylphosphine) palladium(0) has been widely applied as the catalyst for the Suzuki–Miyaura reaction in many fields. But this palladium complex is sensitive to air and moisture. Although much work has been carried out to improve the stability of this ligand, the corresponding structure became more complicated and the synthetic processes were tedious.29–31 In contrast, the NHC ligands are more stable than the most electron-rich phosphanes due to the stronger σ electron donation of their carbenes.32 Therefore, NHC ligands have been applied in various catalytic reactions due to their strong σ-donating ability and great steric effect.33
In recent years, Organ, Nolan and other researchers have done a great job on bulky nucleophilic carbene ligands.32,34–38 These studies suggest that some ligands including the structure of imidazol-2-ylidene with bulky substitutions showed much higher stability and catalytic activity when they chelated with a suitable palladium source to form the corresponding palladium complex. However, few reports were found about the immobilization of these bulky NHCs. Thus, we were interested in the preparation of a corresponding magnetic palladium catalyst to look at their catalytic activities.
In this contribution we showed the preparation procedures of the magnetic carriers with miniemulsion polymerization and the synthesis of the bulky NHC ligand of 1-(2,6-diisopropylphenyl)-1H-imidazole. Then, the NHC ligand was grafted on the surface of these magnetic carriers. Two different palladium sources were employed to coordinate with the NHC ligand. Then, the catalytic properties of these magnetic catalysts for the Suzuki–Miyaura reaction were carefully investigated. The resulting magnetic catalysts are of prime interest due to their high catalytic activity, easy separation and good stability.
Experimental
Chemicals and instruments
All the reagents and solvents were purchased from Sinopharm Chemical Reagent Co. Ltd and used without any pretreatment. Reaction yields were analyzed by gas chromatography (GC, Agilent Technologies). The state of palladium was determined using a X-ray diffractometer (XRD, Bruker). The size and morphology of magnetic nanoparticles were observed using transmission electron microscopy (TEM, HITACHI). The magnetic content of nanoparticles was analyzed using a thermogravimetric analyser (TG, SHIMADZU). The magnetization measurements were performed at room temperature using a vibrating sample magnetometer (Mpms XL-7, Quantum Design). The 1H NMR of the compounds were recorded on a 400 MHz Bruker Avance spectrometer. Elemental analysis of dried samples was implemented using vario EL III (elementar). The palladium amount on the carriers was measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES, SHIMADZU). The BET analysis was performed on a surface area analyzer (Quantachrome Instruments).General procedure for the preparation of magnetic palladium catalysts
General procedure for the Suzuki–Miyaura reaction catalyzed by the magnetic catalysts
The catalyst 1 was dispersed in 15 mL ethanol by sonication, then aryl bromides (2 mmol), phenylboronic acid (2.4 mmol) and K2CO3 (6 mmol) were added. The reaction mixture was stirred at 70 °C for 12 h. The catalyst was collected by magnetic separation and the products were extracted with chloroform. After the extract was completely dried, the samples were analyzed by GC and NMR.The catalyst 2 and K2CO3 (3 mmol) were dispersed in DMF (10 mL) with the help of sonication. Then, aryl halide reagent (1 mmol) and arylboronic acid (2 mmol) were added . After the mixture was subjected to sonication for 15 min, it was stirred at 100 °C for 12 h. The catalyst was recovered by magnetic separation. After the solvent was removed, the products were dispersed in 15 mL chloroform and analyzed by GC with the same procedure as above.
Results and discussion
Catalyst preparation and characterization
The miniemulsion polymerization was employed to prepare magnetic carriers, which was necessary for the sensitive magnetic response of the achieved polymer spheres. The size of the synthesized magnetic particles ranged from 42 to 125 nm with an average diameter of 97 nm based on TEM (Fig. 1). The particle size distribution was very wide, and some aggregations of magnetic spheres were observed due to the self-emulsifying behavior of chloromethyl styrene. It was also found that the magnetic response of these samples was so sensitive that one minute was enough to completely separate these magnetic particles by a handheld magnet. From the magnetization curve in Fig. 2, it is seen that the magnetic content of nanoparticles reached 38%. The saturation magnetization of magnetic nanoparticles reached as high as 19.3 emu g−1. After the preparation of magnetic catalyst 1, its magnetic response was almost the same as that of the magnetic carriers. It meant that no leaking of magnetite particles occurred in the process of immobilization of the Pd-NHC complex. Furthermore, the catalyst could be easily and uniformly dispersed in solution again after the magnetic separation due to its superparamagnetism. It may act as a quasi homogeneous phase in the solvent, which greatly benefits the catalytic activity of the palladium complex.41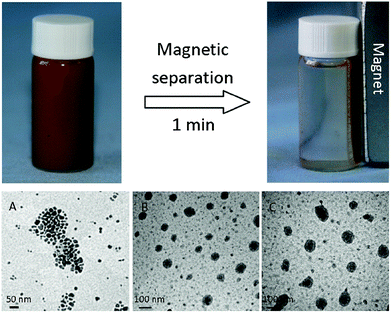 | ||
| Fig. 1 The pictures of the magnetic separation and the TEM photos of magnetite nanoparticles (A), the magnetic polymer carriers (B) and the catalyst 1 (C). | ||
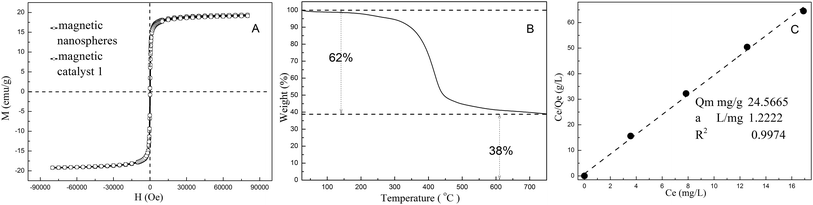 | ||
| Fig. 2 The VSM curves of magnetic particles and catalyst 1 (A); the TG curve of magnetic nanoparticles (B); and the adsorption isotherm of these magnetic carriers with palladium ions (C). | ||
Depending on the functional group of chloromethyl of these magnetic carriers, 1-arylimidazole could be easily modified on their surface. From the nitrogen content of these magnetic particles, which was measured by elemental analysis, about 0.21 mmol NHC ligand was grafted on each gram of magnetic carriers. After the palladium ion was chelated on these NHC ligands, the content of palladium was determined to be 0.23 mmol g−1 using ICP-AES, which was close to the loading levels of 1-arylimidazole on these magnetic carriers. Because the two kinds of catalysts were synthesized with the same batch of modified magnetic nanoparticles, the palladium loading level of those two different catalysts was nearly the same (0.23 mmol g−1, analyzed by ICP-AES). The related absorption isotherm was measured and is shown in Fig. 2. These data were mostly in accord with the Langmuir equation. Thus, it meant that the monolayer of palladium ions was coordinated with these ligands on the surface of these magnetic carriers.
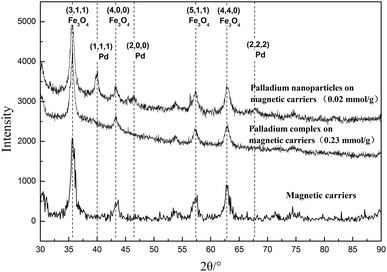
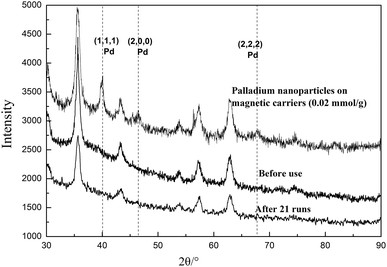
In the process of the coordination between the immobilized NHC ligand and palladium ions, the formation of Pd nanoparticles was completely avoided, as shown in Fig. 3. It was found that the characteristic peaks of palladium nanoparticles were very evident when its palladium content was only 0.02 mmol g−1. But, for the palladium complex, there were no characteristic peaks of palladium nanoparticles on its XRD pattern when its content reached 0.23 mmol g−1. Therefore, the catalytic performances of these magnetic catalysts were mainly attributed to the palladium complex.
Catalytic properties of the magnetic palladium catalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave an excellent yield (99%) at 70 °C. Compared with this solvent, the aprotic solvents always resulted in lower yields. Different bases were investigated, as shown in Table 1.When K2CO3 was used as a base, the catalyst resulted in the highest yield (99%). However, the yields were slightly lower when stronger alkalis (NaOH or KOH) were used. Cesium carbonate and triethylamine were also employed. But, the corresponding yields were obviously low. It indicated that a suitable pH range was available to the catalysis of the Suzuki–Miyaura reaction. Extremely high and very low alkalinity might decrease the catalytic efficiency of our magnetic catalysts. Therefore, based on the above experiments, the solution of ethanol–water (3
1) gave an excellent yield (99%) at 70 °C. Compared with this solvent, the aprotic solvents always resulted in lower yields. Different bases were investigated, as shown in Table 1.When K2CO3 was used as a base, the catalyst resulted in the highest yield (99%). However, the yields were slightly lower when stronger alkalis (NaOH or KOH) were used. Cesium carbonate and triethylamine were also employed. But, the corresponding yields were obviously low. It indicated that a suitable pH range was available to the catalysis of the Suzuki–Miyaura reaction. Extremely high and very low alkalinity might decrease the catalytic efficiency of our magnetic catalysts. Therefore, based on the above experiments, the solution of ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and K2CO3 were usually employed in following experiments.
1) and K2CO3 were usually employed in following experiments.
| Solvent | Yielda | Base | Yieldb |
|---|---|---|---|
| Base: K2CO3 | Solvent: ethanol–H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
||
a Aryl halide (2 mmol), phenylboronic acid (2.4 mmol), catalyst 1 (0.02 mol%) and K2CO3 (3 mmol) were added to 20 mL solvent. The reaction temperature was set to 70 °C, and the reaction time was 12 h.
b Aryl halide (2 mmol), phenylboronic acid (2.4 mmol), catalyst 1 (0.02 mol%) and a base (3 mmol) were added to ethanol–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was also 12 h. The mole fraction of palladium here means the moles of palladium atoms divided by the theoretical conversion moles of the reaction. 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was also 12 h. The mole fraction of palladium here means the moles of palladium atoms divided by the theoretical conversion moles of the reaction.
|
|||
DMF–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50% | K2CO3 | 99% |
DMF–H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
53% | K3PO4 | 93% |
Dioxane–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81% | KOH | 92% |
THF–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76% | NaOH | 91% |
Toluene–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Trace | Na2CO3 | 97% |
Ethanol–H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
95% | Cs2CO3 | 32% |
Ethanol–H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99% | Triethylamine | 56% |
Although numerous research studies have been carried out on the Suzuki–Miyaura reaction with arylboronic acid reagents, a high reaction temperature with a large amount (1−10 mol%) of catalyst was usually required.42–45 Especially for some supported catalysts, the catalytic activity decreased greatly.46–48 In Table 2, it was found that the yield of the reaction reached 96% in the presence of 0.02 mol% of palladium. Even 5 mol ppm (0.0005 mol% of Pd) palladium of catalyst 1 could also promote the catalysis (entry 3), which was unusual among the reported supported catalyst and even exceeded many homogeneous palladium catalysts.49–51 Compared with the commercial heterogeneous palladium catalysts (EnCat TPP30 and NP30), in the presence of 0.15 mol% palladium, the yields were only 80% and 70%, respectively.52 But, in Yamada's work, the corresponding TON values even reached 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570
570![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 when a palladium atom was chelated with two polymeric imidazoles.53,54 This symmetrical structure might have great advantages on the catalytic activity of the palladium complex. Similar work is still being carried out by our group.
000 when a palladium atom was chelated with two polymeric imidazoles.53,54 This symmetrical structure might have great advantages on the catalytic activity of the palladium complex. Similar work is still being carried out by our group.
| Entry | Pd (mol%) | Time (h) | Yield (%) | TON |
|---|---|---|---|---|
a Aryl halide (2 mmol), phenylboronic acid (2.4 mmol), catalyst 1 and K2CO3 (3 mmol) were added to ethanol–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h. 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h.
|
||||
| 1 | 2 × 10−2 | 4 | 96 | 4800 |
| 2 | 1 × 10−3 | 12 | 87 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| 3 | 5 × 10−4 | 12 | 57 | — |
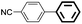
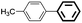
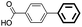
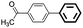
The adaptability of this magnetic catalyst was also investigated, as shown in Table 3. Various aryl bromides with electron-donating or electron-withdrawing groups were employed. It was found that the electron-rich halides gave lower yields compared with the electron-deficient kinds although the oxidative addition is slower for aryl halides with electron-donating groups (entries 3–14).55 The Suzuki–Miyaura coupling of aryl bromides proceeded with high efficiency (yields >90%). However, the steric effect greatly hindered the reaction. When 2,6-dimethylphenylboronic acid was used to react with bromobenzene, the yield decreased to 61% (entry 15).
| Entry | R1 | R2 | Yieldb (%) | TON | Impurity contentc (%) |
|---|---|---|---|---|---|
a Aryl halide (2 mmol), phenylboronic acid (2.4 mmol), catalyst 1 (0.02 mol%) and K2CO3 (3 mmol) were added to ethanol–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h.
b The reaction yield was determined by GC using hexadecane as an internal standard.
c The purity was also determined by GC, which was calculated with the ratio of biphenyl/(biphenyl + product).
d The temperature was 50 °C and the reaction time was 4 h.
e The temperature was 70 °C and the reaction time was 4 h.
f The reaction was performed under an oxygen atmosphere.
g The reaction substrate was 1-naphthaleneboronic acid.
h The reaction substrate was 1-bromonaphthalene. 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h.
b The reaction yield was determined by GC using hexadecane as an internal standard.
c The purity was also determined by GC, which was calculated with the ratio of biphenyl/(biphenyl + product).
d The temperature was 50 °C and the reaction time was 4 h.
e The temperature was 70 °C and the reaction time was 4 h.
f The reaction was performed under an oxygen atmosphere.
g The reaction substrate was 1-naphthaleneboronic acid.
h The reaction substrate was 1-bromonaphthalene.
|
|||||
| 1d | p-COCH3 | H | 91 | 4550 | Trace |
| 2e | p-COCH3 | H | 96 | 4800 | Trace |
| 3f | p-COCH3 | H | 99 | 4950 | Trace |
| 4 | p-COH | H | 95 | 4750 | <0.1 |
| 5 | p-OH | H | 90 | 4500 | 0.3 |
| 6 | p-OCH3 | H | 97 | 4850 | 0.2 |
| 7 | p-COOH | H | 93 | 4650 | 0.3 |
| 8 | H | p-CH3 | 90 | 4500 | — |
| 9 | H | Naphg | 95 | 4750 | — |
| 10 | p-CH3 | H | 91 | 4550 | 0.2 |
| 11 | p-CN | H | 98 | 4900 | 0.3 |
| 12 | o-CH3 | H | 87 | 4350 | 0.3 |
| 13 | p-NO2 | H | 93 | 4650 | <0.1 |
| 14 | Naphh | H | 95 | 4750 | <0.1 |
| 15 | H | 2,6-Dimethyl | 61 | — | — |
Byproduct was another important factor in the evaluation of the catalytic properties of the corresponding catalysts for the Suzuki–Miyaura reaction because of the high requirements for its purity in the fields of pharmaceutical synthesis and functional materials. The homocoupling of phenylboronic acid always decreased the purity of products because of its similar structures, which are difficult to be isolated. When some palladium nanoparticles were used as the catalyst, the byproducts were difficult to be avoid and the yield of the byproduct, under certain conditions, even exceeded 11%.56 This was the reason why we preferred to use the palladium complex rather than palladium nanoparticles as the catalytic center in our work. In Table 3, it was found that the yields of byproducts were not more than 0.3% when the Suzuki–Miyaura reaction was catalyzed by this magnetic catalyst. These results are rather inspirational and similar results were only found in Slaughter's work.57
| Run | 1 | 3 | 5 | 8 | 12 | 15 | 18 | 21 |
|---|---|---|---|---|---|---|---|---|
a Aryl halide (2 mmol), phenylboronic acid (2.4 mmol), catalyst 1 (1 mol%) and K2CO3 (3 mmol) were added to ethanol–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h. 1, 20 mL). The reaction temperature was set to 70 °C, and the reaction time was 12 h.
|
||||||||
| Yield (%) | 96 | 97 | 99 | 99 | 93 | 98 | 96 | 97 |
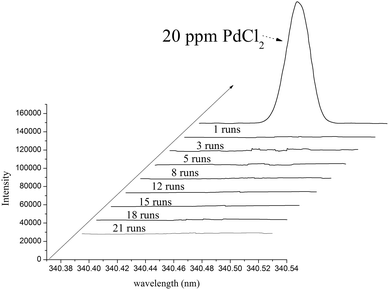
Another important advantage of this magnetic catalyst was that there was no Pd leaking. In Fig. 4, the palladium concentration in the products was always lower than 0.1 ppm measured by ICP-AES (detection limit 5 ppb Pd). Even after 21 runs of the recycling, the palladium content of the catalyst changed little, so that more than 99.8% of palladium was still retained on the magnetic supporters (analyzed by ICP-AES). It meant, under these conditions, the palladium atom was completely immobilized by the NHC ligand and the aryl bromides could not break the chelated structure of the NHC ligand with palladium ions.
The experiment of “hot filtration” was also carried out. After the coupling reaction of 1-bromo-4-methoxybenzene and phenylboronic acid had proceeded for 1 h (with conversion at 73%), the magnetic catalyst was immediately separated under the hot conditions. Then, the reaction was continued for another 12 h. It was found that the final conversion (73.8%) changed little. It meant that no palladium was leaked into the solution and the catalytic activity of the magnetic catalyst mainly depended on the palladium complex. The reaction nature was the heterogeneous catalysis.
The palladium complex was easily converted into palladium black in the process of catalysis, resulting in the decrease of catalytic efficiency. Thus, the structure of the magnetic catalyst was measured by XRD before and after use. The results are shown in Fig. 5. It was found that there were no characteristic peaks of palladium particles, which is likely to prove that no palladium complex was reduced to palladium black during the reaction. This good stability might be attributable to the bulky NHC structure, which could efficiently prevent the aggregation or agglomeration of palladium atoms through the spatial restrictions and electrostatic interactions of the NHC ligands and palladium atoms on the surface of magnetic nanoparticles.
Because triphenyl phosphine palladium and palladium nanoparticles are usually sensitive to oxygen, the comparison of using oxygen and air as the reaction atmosphere was also made. The result (entry 3 in Table 3) indicated that the catalytic activity of this magnetic catalyst had no evident difference in these atmospheres. It meant that the palladium atom in this magnetic catalyst was not easily oxidized. It was also found that the catalytic activity of this catalyst decreased little even after it was exposed to air for several days. In this aspect, the developed catalyst is stable enough for its application and storage.
The catalytic activity for the Suzuki–Miyaura reaction of aryl chlorides and phenylboronic acid
From a practical point of view, aryl chlorides become more attractive than aryl bromides as the substrates of the Suzuki–Miyaura reaction because they are inexpensive and readily available.25 But, the activation of the C–Cl bond is more difficult compared with C–Br and C–I, so that harsher reaction conditions are usually required for the corresponding catalysis. It is seen in Table 3 that the activity of magnetic catalyst 1 decreased greatly when aryl chlorides were used. When the reaction temperature increased to 100 °C, the reaction conversion improved. However, the Pd leaking of catalyst 1 was drastically increased when aryl chlorides were introduced.In Organ's work, the “throw-away” ligand was always used to stabilize the corresponding homogeneous catalysts.58 Therefore, a “throw-away” ligand (3-chloropyridine) was employed to stabilize the catalyst 1. The results are shown in Table 5. It was found that the palladium leaking of the magnetic catalyst 2 was completely avoided at 100 °C. The conversion of this reaction could reach 82%.
From the results in Table 5, it is found that the aryl chlorides had a great impact on the stability of catalyst 1. At 70 °C, the catalyst 1 could not activate the aryl chlorides, resulting in the much lower reaction conversion. When the temperature was increased to 100 °C, this catalyst could activate the C–Cl bond, resulting in the enhancement of reaction conversion. However, the Pd leaking of catalyst 1 increased greatly when the reaction of aryl chlorides and phenylboronic acid was carried out at 100 °C. According to the reaction mechanism of the Suzuki–Miyaura reaction, it was believed that the aryl chlorides could coordinate with the palladium leading to the disintegration of the chelated structure of the NHC ligand with palladium in the step of oxidative addition. However, after the chelated structure of the NHC ligand with palladium was further stabilized with 3-chloropyridine, the structure of catalyst 2 was stable enough to prevent the interference of aryl chlorides. Thus, no Pd leaking was found in the catalysis of catalyst 2.
Conclusion
In conclusion, we report an efficiently recoverable catalyst which combines the high catalytic activity of the palladium complex for the Suzuki–Miyaura reaction with the sensitive magnetic separation of polymer magnetic carriers. The high catalytic efficiency of this magnetic catalyst even exceeded that of some homogeneous catalysts. Furthermore, its excellent reusability brings great potential for the magnetic catalysts in some large scale applications. In particular, no palladium was leaked under the experimental conditions, which indicated the good stability of these magnetic catalysts.Acknowledgements
This research was supported by the program of the Fundamental Research Funds for HuaQiao University (2014KJTD06); the Natural Science Foundation of Fujian Province, China (grant no. 2013J01046); and the Special Foundation for Young Scientists of Fujian Province (grant 2011J05131).Notes and references
- K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6173–6177 CrossRef CAS PubMed.
- B. Saito and G. C. Fu, J. Am. Chem. Soc., 2007, 129, 9602–9603 CrossRef CAS PubMed.
- Z. Zhao, Y. L. Fang, P. J. J. Alvarez and M. S. Wong, Appl. Catal., B, 2013, 140–141, 468–477 CrossRef CAS PubMed.
- K. L. Billingsley and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 120, 4773–4776 CrossRef.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889–900 CrossRef CAS.
- D. Astruc, Inorg. Chem., 2007, 46, 1884–1894 CrossRef CAS PubMed.
- Á. Molnár, Chem. Rev., 2011, 111, 2251–2320 CrossRef PubMed.
- O. Aksin, H. Türkmen, L. Artok, B. Cetinkaya, C. Ni, O. Bueyuekguengoer and E. Oezkal, J. Organomet. Chem., 2006, 691, 3027–3036 CrossRef CAS PubMed.
- R. Sayah, K. Glegola, E. Framery and V. Dufaud, Adv. Synth. Catal., 2007, 349, 373–381 CrossRef CAS.
- E. Tyrrell, L. Whiteman and N. Williams, J. Organomet. Chem., 2011, 696, 3465–3572 CrossRef CAS PubMed.
- X. Li, X. Y. Yan, H. H. Chang, L. C. Wang, Y. Zhang, W. W. Chen, Y. W. Li and W. L. Wei, Org. Biomol. Chem., 2012, 10, 495–497 CAS.
- J. Yang, D. Wang, W. Liu, X. Zhang, F. Bian and W. Yu, Green Chem., 2013, 15, 3429–3437 RSC.
- S. Ogasawara and S. Kato, J. Am. Chem. Soc., 2010, 132, 4608–4613 CrossRef CAS PubMed.
- G. M. Pawar and M. R. Buchmeiser, Adv. Synth. Catal., 2010, 352, 917–928 CrossRef CAS.
- G. Nan, F. Ren and M. Luo, Beilstein. J. Org. Chem., 2010, 6, 70 Search PubMed.
- M. K. Bhunia, S. K. Das, P. Pachfule, R. Banerjee and A. Bhaumik, Dalton Trans., 2012, 41, 1304–1311 RSC.
- C. R. LeBlond, A. T. Andrews, Y. Sun and J. R. Sowa, Org. Lett., 2001, 3, 1555–1557 CrossRef CAS.
- R. K. Arvela and N. E. Leadbeater, Org. Lett., 2005, 7, 2101–2104 CrossRef CAS PubMed.
- A. Garcia-Bernabe, C. C. Tzchucke, W. Bannwarth and R. Haag, Adv. Synth. Catal., 2005, 347, 1389–1394 CrossRef CAS.
- A. K. Diallo, C. Ornelas, L. Salmon, J. R. Aranzaes and D. Astruc, Angew. Chem., Int. Ed., 2007, 46, 8644–8648 CrossRef CAS PubMed.
- S. Berardi, M. Carraro, M. Iglesias, A. Sartorel, G. Scorrano, M. Albrecht and M. Bonchio, Chem. – Eur. J., 2010, 16, 10662–10666 CrossRef CAS PubMed.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- D. H. Lee, J. H. Kim, B. H. Jun, H. Kang, J. Park and Y. S. Lee, Org. Lett., 2008, 10, 1609–1612 CrossRef CAS PubMed.
- U. Laska, C. G. Frost, G. J. Price and P. K. Plucinski, J. Catal., 2009, 268, 318–328 CrossRef CAS PubMed.
- M. J. Jin and D. H. Lee, Angew. Chem., Int. Ed., 2010, 49, 1119–1122 CrossRef CAS PubMed.
- H. Yang, G. Li and Z. Ma, J. Mater. Chem., 2012, 22, 6639–6648 RSC.
- Y. L. Chen, H. Qian, F. Wu and J. Zhou, Chemosphere, 2011, 83, 1214–1219 CrossRef CAS PubMed.
- Z. Y. Lin, Y. X. Zhang, Y. L. Chen and H. Qian, Chem. Eng. J., 2012, 200, 104–112 CrossRef PubMed.
- C. Marshall, M. F. Ward and W. T. A. Harrison, Tetrahedron Lett., 2004, 45, 5703–5706 CrossRef CAS PubMed.
- Ö. Doğan, N. Gürbüz, İ. Özdemir, B. Çetinkaya, O. Şahinc and O. Büyükgüngörc, Dalton Trans., 2009, 7087–7093 RSC.
- X. Zhang, Y. Qiu, B. Rao and M. Luo, Organometallics, 2009, 28, 3093–3099 CrossRef CAS.
- E. A. B. Kantchev, C. J. O'Brien and M. G. Organ, Angew. Chem., Int. Ed., 2007, 46, 2768–2813 CrossRef CAS PubMed.
- W. A. Herrmann, Angew. Chem., Int. Ed., 2002, 41, 1290–1309 CrossRef CAS.
- M. G. Organ, S. Çalimsiz, M. Sayah, K. H. Hoi and A. J. Lough, Angew. Chem., Int. Ed., 2009, 121, 2419–2423 CrossRef.
- C. Valente, S. Çalimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef CAS PubMed.
- S. Díez-González and S. P. Nolan, Coord. Chem. Rev., 2007, 251, 874–883 CrossRef PubMed.
- G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151–5169 RSC.
- A. Chartoire, X. Frogneux, A. Boreux, A. M. Z. Slawin and S. P. Nolan, Organometallics, 2012, 31, 6947–6951 CrossRef CAS.
- W. Zheng, F. Gao and H. Gu, J. Magn. Magn. Mater., 2005, 288, 403–410 CrossRef CAS PubMed.
- J. Liu, J. Chen, J. Zhao, Y. Zhao, L. Li and H. Zhang, Synthesis, 2003, 17, 2661–2666 Search PubMed.
- S. Ding, Y. Xing, M. Radosz and Y. Shen, Macromolecules, 2006, 39, 6399–6405 CrossRef CAS.
- H. Tsukamoto, M. Sato and Y. Kondo, Chem. Commun., 2004, 1200–1201 RSC.
- K. Manabe, K. Nakada, N. Aoyama and S. Kobayashi, Adv. Synth. Catal., 2005, 347, 1499–1503 CrossRef CAS.
- H. Ohmiya, Y. Mikida, T. Tanaka and M. Sawamura, J. Am. Chem. Soc., 2008, 130, 17276–17277 CrossRef CAS PubMed.
- H. Ohmiya, Y. Mikida, D. Li, T. Tanaka and M. Sawamura, J. Am. Chem. Soc., 2010, 132, 879–889 CrossRef CAS PubMed.
- H. Yang, X. Han, Z. Ma, R. Wang, J. Liu and X. Ji, Green Chem., 2010, 12, 441–451 RSC.
- S. MacQuarrie, B. Nohair, J. H. Horton, S. Kaliaguine and C. M. Crudden, J. Phys. Chem. C, 2010, 114, 57–64 CAS.
- A. Corma, D. Das, H. García and A. Leyva, J. Catal., 2005, 229, 322–331 CrossRef CAS PubMed.
- C. M. Crudden, M. Sateesh and R. Lewis, J. Am. Chem. Soc., 2005, 127, 10045–10050 CrossRef CAS PubMed.
- M. J. Jin, A. Taher, H. J. Kang, M. Choi and R. Ryoo, Green Chem., 2009, 11, 309–313 RSC.
- M. Lemay, V. Pandarus, M. Simard, O. Marion, L. Tremblay and F. Beland, Top. Catal., 2010, 53, 1059–1062 CrossRef CAS.
- C. Diebold, J. M. Becht, J. Lu, P. H. Toy and C. L. Drian, Eur. J. Org. Chem., 2012, 893–896 CrossRef CAS.
- M. Chtchigrovsky, Y. Lin, K. Ouchaou, M. Chaumontet, M. Robitzer, F. Quignard and F. Taran, Chem. Mater., 2012, 24, 1505–1510 CrossRef CAS.
- Y. M. A. Yamada, S. M. Sarkar and Y. Uozumi, J. Am. Chem. Soc., 2012, 134, 3190–3198 CrossRef CAS PubMed.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685–4696 CrossRef CAS PubMed.
- D. A. Alonso, C. Nájera and M. C. Pacheco, J. Org. Chem., 2002, 67, 5588–5594 CrossRef CAS PubMed.
- A. I. Moncada, S. Manne, J. M. Tanski and L. M. Slaughter, Organometallics, 2006, 25, 491–505 CrossRef CAS.
- J. Nasielski, N. Hadei, G. Achonduh, E. A. B. Kantchev, C. J. O'Brien, A. Lough and M. G. Organ, Chem. – Eur. J., 2010, 16, 10844–10853 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc00574k |
| This journal is © The Royal Society of Chemistry 2015 |