Lignin: a nature-inspired sun blocker for broad-spectrum sunscreens†
Yong
Qian
ab,
Xueqing
Qiu
*b and
Shiping
Zhu
*a
aDepartment of Chemical Engineering, McMaster University, Hamilton, Ontario L8S 4L7, Canada. E-mail: shipingzhu@mcmaster.ca
bSchool of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640, People's Republic of China. E-mail: xueqingqiu66@163.com
First published on 1st September 2014
Abstract
We report the evaluation of lignin for the development of high-performance broad-spectrum sunscreens. Lignin is added into several commercial sunscreen products. Significant enhancements in ultraviolet (UV) absorbance are observed. The results show that the sunscreen effect of sun protection factor (SPF) 15 could reach that of SPF 30 with the addition of 2 wt% lignin. Adding 10 wt% lignin makes SPF 15 outperform SPF 50. It is also interesting to find that the sunscreen performance improves with UV-radiation time. After 2 h of UV radiation, the UV absorbance of the 10 wt% lignin SPF 15 lotion increases dramatically. This has been attributed to certain synergistic effects between lignin and other sunscreen actives in the lotions, as well as the antioxidant property of lignin. This nature-inspired lignin system may provide a green alternative to replace some synthetic sunscreen actives.
Introduction
Long-time exposure to ultraviolet (UV) radiation is hazardous and causes skin problems. In addition to wearing adequate clothing without impregnation,1 sunscreen provides protection.2,3 Commercial sunscreen products are divided into two categories according to their active ingredients, i.e., physical and chemical sunscreens. In physical sunscreens, the active ingredients are mainly zinc oxide and titanium dioxide, based on the reflection mechanism.4 Physical sunscreens are healthy but are not very comfortable, especially for people with dry skins. As compared to physical sunscreens, chemical sunscreens are easier to use but have their own drawbacks. The active ingredients in chemical sunscreens are often synthetic chemicals. Long-time use of such chemicals may cause unexpected side effects on skins.5 Therefore, natural sunscreens are receiving more and more attention recently. Natural products such as green coffee oil, extracts of carica papaya, rosa kordesii, and helichrysum arenarium are proven to have radiation protection functions.6–9 Usually, many natural sunscreens also have good antioxidant properties. However, most of the natural products are part-spectrum sun blockers and cannot block the full spectrum of UV light. In addition, the extraction of active ingredients from raw materials is expensive. Natural sun blockers are often expensive and their large volume commercial production is limited.Lignin is the only biomass rich in aromatic rings in nature due to its basic phenylpropane unit.10–14 It also contains UV-absorbing functional groups such as phenolic, ketone and other chromophores.15–17 Lignin is a natural broad-spectrum sun blocker.18 In addition to the sunscreen property, the free radical scavenging ability of phenolic groups gives lignin an excellent antioxidant property.19–22 Although 5 to 36 × 108 tons of lignin is produced in nature and over 50 million tons of industrial lignin is produced annually, lignin has never been used as sunscreen and antioxidant actives in cosmetics and/or pharmaceuticals due to some safety concerns.23–25 Except for a small amount of lignosulfonates used as low-end surfactants, such as concrete water reducers, water-coal-slurry dispersants, and dye stuff dispersants, most lignin is burned for energy.26–28 The high-end applications of industrial lignin at a large scale is still under exploration.
Recently, Ugartondo et al. tested the cell cytotoxicity of four types of industrial lignin and found that the products are safe.29 Lignin-based products, such as microcapsules, are not cytotoxic and can be incorporated into cells.30 These studies resolved the doubts regarding the safety of lignin for potential applications in cosmetics and pharmaceuticals. In this work, alkali lignin recovered from a black liquid is investigated for its sunscreen performance. Lignin is added to pure creams and commercial sun protection factor (SPF) 15 sun lotions as a broad-spectrum sunscreen active. The sunscreen results of SPF 15 sunscreen lotions containing various amounts of lignin are compared to those of SPF 30 and SPF 50 sunscreen products. The irradiation stabilities of the prepared lignin sunscreens are also examined.
Experimental section
Materials
Alkali lignin was obtained from Shuntai Technology Corp (Huaihua, Hunan, China). Before use, alkali lignin was purified again by alkali-acid treatment for at least three times and continuous washing with DI water. The particle sizes of dry alkali lignin ranged from nanometers to micrometers (observed from SEM images in ESI†). Pure creams were NIVEA refreshingly soft moisturizing cream (75 mL) (Cream-N) and LIFE glycerin hand cream (75 mL) (Cream-L). Sunscreen lotions were LIFE SPF 15, 30, and 50 sunscreen lotions (SPF15-L, SPF30-L, and SPF50-L, respectively) and BIOTHERM SPF 15, 30, and 50 sunscreen lotions (SPF15-B, SPF30-B, and SPF50-B, respectively). All these pure creams and sunscreen lotions were bought from SHOPPERS drug market (Hamilton, Ontario, Canada).Preparation of lignin sunscreen samples
All the lignin-based sunscreen creams and lotions were prepared by simple magnetic stirring. For example, 5 wt% lignin sunscreen was prepared by blending 0.10 g lignin and 1.90 g pure creams at 1000 rpm for 24 h. The whole blending process was performed under room temperature in a dark room. After blending, these lignin sunscreens were transferred onto a transpore tape surface for the measurement of UV transmittance. The dry alkali lignin used in this work was brown. The formulated lignin sunscreen lotions exhibited a light brown to brown color as its content increased.Sun protection factor (SPF) determination
3 M transpore tape was stickered on clean quartz slides of 2 mm thickness. A tape of 12.5 cm2 was used to assure measurements on at least five non-overlapping spots. For each sunscreen cream and lotion, a minimum of five samples were prepared for the UV transmittance measurement. At the same time, a reference sample of transpore tape was also prepared. In the preparation of the sunscreen samples, a 1 mL fine needle syringe was used to transfer the sample. For a 12.5 cm2 sample size, the slide was placed on an analytical balance and 2 mg cm−2 of lignin sunscreen sample was distributed on the tape by dotting sunscreen on the slide. The sunscreen was then distributed over the entire surface by slowly rubbing the slide surface with a cot coated finger. The sample was then placed in a dark room, drying 20 min prior to measurement.The UV transmittance of the lignin sunscreen was measured by Shimadzu UV-3600 with an integrating sphere (Shimadzu, Japan). Five spots were scanned for each sample. In each scan, transmittance (T) measurement per 1 nm was collected in the wavelength range from UVB (290–320 nm) to UVA (320–400 nm). The SPF value was calculated by using the following equation:
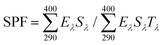 | (1) |
Photostability test
The photostability of the sunscreen creams with or without lignin was studied using a DU 800 UV/visible spectrophotometer (Beckman Coulter, USA). Specifically, the sunscreen samples were prepared according to the same procedure as that used for the SPF measurements and were exposed to UV radiation for two hours. The UV source was the deuterium light of the DU 800 UV/visible spectrophotometer. UV absorbance in the UVB and UVA areas (290–400 nm) before and after radiation was then determined for the photostability of these sunscreens.Results and discussion
Lignin added to pure creams
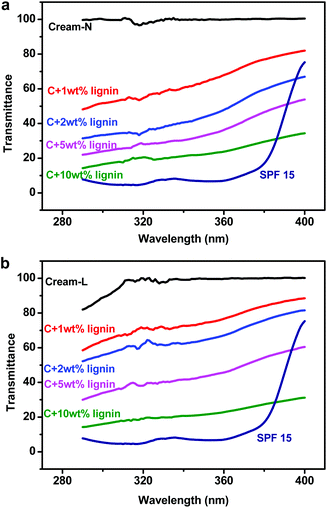
Two kinds of lignin sunscreens were prepared. One was the blends of lignin with Cream-N, and the other was lignin blended with Cream-L. Because Cream-L was too sticky, 10 wt% water was added. The pure Cream-N had no absorbance in the UVA and UVB areas, as shown in Fig. 1a, while Cream-L with 10 wt% water had marginal absorbance in 290–310 nm, as shown in Fig. 1b. It was negligible because its corresponding SPF was only 1.05. The SPF value of the pure Cream-N without UV absorbance was 1. When lignin was added into these pure creams, their UV transmittance decreased and SPF values increased. When the amount of lignin increased to 10 wt%, the SPF of the lignin + Cream-N increased to 5.72, while the SPF of the lignin + Cream-L increased to 5.33. Table 1 summarizes the SPF values of the pure creams blended with different amounts of lignin.| Lignin (wt%) | 0 | 1 | 2 | 5 | 10 |
|---|---|---|---|---|---|
| Cream-N | 0.99 ± 0.01 | 1.82 ± 0.13 | 2.74 ± 0.30 | 3.68 ± 0.30 | 5.72 ± 0.26 |
| Cream-L | 1.06 ± 0.01 | 1.48 ± 0.01 | 1.69 ± 0.03 | 2.68 ± 0.17 | 5.33 ± 0.47 |
As expected, the UV transmittance of the pure cream + 10 wt% lignin was higher than that of LIFE SPF 15 sunscreen lotion. The latter contained two kinds of active ingredients with the total weight percent over 10 wt% (refer to Table S1 in the ESI†). However, the transmittance of the lignin sunscreens in the UVA area, especially between 385–400 nm, was lower than that of SPF 15. This suggested that lignin is a very good natural candidate for broad spectrum sunscreens.
Lignin added to sunscreen lotions
It is known that the sunscreen effect of green coffee oil itself is not obvious, but the oil can help to improve the performance of synthetic sunscreen actives.6 This sunscreen enhancement has been attributed to antioxidants in the green coffee oil. Similarly, lignin is not only a natural sun blocker but it is also well known for its antioxidant activities due to the presence of phenolic hydroxyl groups. Therefore, the sunscreen enhancement of commercial SPF 15 sunscreen lotions with added lignin was investigated and compared with the sunscreen performance of SPF 30 and 50 sunscreen products.It can be seen in Fig. 2a that the UV transmittance of SPF 15-B + 2 wt% lignin was close to that of SPF 30, but it was lower than that of SPF 50 in the UVA area, especially in the range of 380–400 nm. The SPF values of SPF 15-B blended with different lignin amounts, as well as SPF 30-B and SPF 50-B, were measured and summarized in Table 2. Unfortunately, further increase in the lignin amount did not improve the sunscreen effect. When lignin was increased to 10 wt%, the sunscreen effect disappeared and the SPF level returned to that of the original SPF 15 lotion. This was mainly caused by the poor dispersion of lignin in SPF 15-B at such high loading. SPF 15-B with 10 wt% lignin dried quickly when it was distributed on the transpore tape.
The sunscreen effect enhancement in SPF 15-L was not so obvious with 2 wt% lignin addition. However, when the lignin amount was increased to 10 wt%, the sunscreen effect was enhanced dramatically. The blended lotion not only became much better than SPF 15-L but also significantly outperformed SPF 50-L, especially in the UVA area, as shown in Fig. 2b. The SPF value of SPF 15-L + 10 wt% lignin reached 89.58 (in Table 2). SPF 15-L and SPF 15-B contained different active ingredients. It was also easier to disperse lignin in SPF 15-L than in SPF 15-B.
Radiation-enhanced sunscreen effect
We then evaluated the photostability of the lignin-modified sunscreen lotions. There could be a concern about the service lifetime of lignin as a UV blocker in these lotions. To our surprise, when the lignin-modified sunscreens were exposed to UV radiation for two hours, the UV absorbance in the UVA and UVB areas increased several times, as shown in Fig. 3a. In comparison, there was a marginal change in the UV absorbance with the commercial sunscreen lotions without lignin, as shown in Fig. 3b and c. This result was unexpected and promising. How could the sunscreen performance of lignin lotions get better with UV radiation? We made an effort to elucidate the underlying mechanisms. The first suspect was the effect of radiation on lignin. The UV-radiated lignin somehow became a better UV blocker. To validate this assumption, the photostability of a pure cream was used as the reference. Different from these lotions, the cream contained no active sunscreen ingredients. Fig. 3d shows the radiation effect on the cream + 10 wt% lignin sample. Although there was a marginal increase in the UVB area after two hours of UV radiation, the absorbance in the UVA area was not obvious at all. Therefore, the radiation effect on lignin alone could not explain the observed results with the lignin lotions. Another possibility might be some synergistic effect of lignin and synthetic sunscreen actives in the lotions, such as homosalate, avobenzone, and so on. During the two hours of UV radiation, lignin might interact and/or react with some active ingredients, forming higher performance UV blockers. We hope this work provokes further studies on the actual molecular processes involved in these interactions and/or reactions. It should also be pointed out that lignin is an excellent antioxidant. Sunscreen actives such as avobenzone and octinoxate do not have good photostability and can easily form free radicals by photolysis.33 Lignin is an effective radical scavenger and stops further formation of free radicals.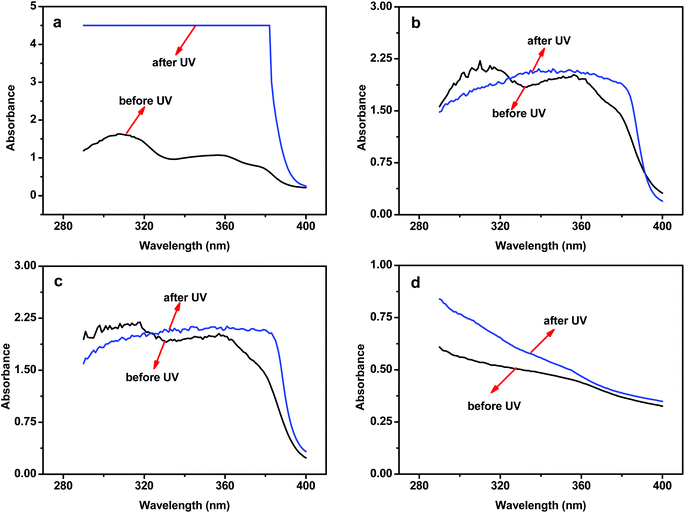 | ||
| Fig. 3 UV absorbance of the sunscreens before and after 2 h UV radiation: (a) SPF 15-L + 10 wt% lignin; (b) SPF 15-L; (c) SPF 30-L; (d) Cream-L + 10 wt% lignin. | ||
Conclusions
Lignin is evaluated as a nature-inspired broad-spectrum sun blocker in sunscreen lotions. As expected, lignin enhanced the sunscreen performance when it was added to commercial sunscreen lotions. However, the enhancement level was unexpectedly high. The UV absorbance of lignin-containing sunscreen lotions increased several times. It was particularly interesting to find that the performance improved with the UV radiation time. Although the underlying mechanism remained to be elucidated, it was attributed to a synergistic effect between lignin and other sunscreen actives, as well as the antioxidant property of lignin. This work demonstrated the potential of lignin in such value-added applications as cosmetics.Acknowledgements
Yong Qian would like to acknowledge the China Scholarship Council (CSC) for supporting his visit at McMaster. We also thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for supporting this research.Notes and references
- EPA, The burning facts, United States Air and Radiation EPA 430-F-06-013 Environmental Protection (6205J) September 2006; http://www.epa.gov/sunwise/doc/sunscreen.pdf.
- N. J. Lowe, Dermatol. Clin., 2006, 24, 9–17 CrossRef CAS PubMed.
- Sun protection clothing guide: Shield your skin without sunscreen; http://www.rodalenews.com/sun-protectionclothing.
- Z. A. Lewicka, A. F. Benedetto, D. N. Benoit, W. W. Yu, J. D. Fortner and V. L. Colvin, J. Neurosci. Res., 2011, 13, 3607–3617 CAS.
- S. T. Butt and T. Christensen, Radiat. Prot. Dosim., 2000, 91, 283–286 CrossRef CAS.
- B. G. Chiari, E. Trovatti, É. Pecoraro, M. A. Corrêa, R. M. B. Cicarelli, S. J. L. Ribeiro and V. L. B. Isaac, Ind. Crops Prod., 2014, 52, 389–393 CrossRef CAS PubMed.
- P. N. Shenekar, P. S. Ukirade, S. D. Salunkhe, S. T. Sutar, C. S. Magdum, S. K. Mohite, S. G. Lokapure and S. M. Metri, Eur. J. Exp. Biol., 2014, 4, 44–47 CAS.
- P. P. Maske, S. G. Lokapure, D. Nimbalkar, S. Malavi and J. I. D'souza, J. Pharm. Res., 2013, 7, 520–524 CrossRef CAS PubMed.
- A. Jarzycka, A. Lewinska, R. Gancarz and K. A. Wilk, J. Photochem. Photobiol., B, 2013, 128, 50–57 CrossRef CAS PubMed.
- S. S. Y. Tan, D. R. MacFarlane, J. Upfal, L. A. Edye, W. O. S. Doherty, A. F. Patti, J. M. Pringle and J. L. Scott, Green Chem., 2009, 11, 339–345 RSC.
- S. Nanayakkara, A. F. Patti and K. Saito, Green Chem., 2014, 16, 1897–1903 RSC.
- Z. J. Wei, Y. Yang, R. Yang and C. Y. Wang, Green Chem., 2012, 14, 3230–3236 RSC.
- E. Windeisen and G. Wegener, in Polymer Science: A Comprehensive Reference, ed. K. Matyjaszewski and M. Möller, Elsevier, Amsterdam, Oxford, Waltham, 2012, vol. 10, pp. 255–265 Search PubMed.
- E. Windeisen and G. Wegener, in Sustainable solutions for modern economies, ed. R. Höfer, RSC Publ., Cambridge, 2009, ch. 9.4, pp. 300–338 Search PubMed.
- O. Lanzalunga and M. Bietti, J. Photochem. Photobiol., B, 2000, 56, 85–108 CrossRef CAS.
- S. Barsberg, T. Elder and C. Felby, Chem. Mater., 2003, 15, 649–655 CrossRef CAS.
- S. I. Falkehag, J. Marton and E. Adler, in Lignin structure and reactions, ed. J. Marton, E-Publishing Inc., ACS, 1966, vol. 59, ch. 7, pp. 75–89 Search PubMed.
- M. Zimniewska, J. Batog, E. Bogacz and B. Romanowska, J. Fiber Bioeng. Inf., 2012, 5, 321–339 CrossRef.
- T. Dizhbite, G. Telysheva, V. Jurkjane and U. Viesturs, Bioresour. Technol., 2004, 95, 309–317 CrossRef CAS PubMed.
- X. J. Pan, J. F. Kadla, K. Ehara, N. Gilkes and J. N. Saddler, J. Agric. Food Chem., 2006, 54, 5806–5813 CrossRef CAS PubMed.
- M. P. Vinardell, V. Ugartondo and M. Mitjans, Ind. Crops Prod., 2008, 27, 220–223 CrossRef CAS PubMed.
- A. Garcíaa, M. G. Alriolsa, G. Spignob and J. Labid, Biochem. Eng. J., 2012, 67, 173–185 CrossRef PubMed.
- G. Gellerstedt and G. Henriksson, in Monomers, polymers and composites from renewable resources, ed. M. N. Belcagem and A. Gandini, Elsevier, Amsterdam, 2008, ch. 9, pp. 201–224 Search PubMed.
- R. J. A. Gosselink, E. de Jong, B. Guran and A. Abacherli, Ind. Crops Prod., 2004, 20, 121–129 CrossRef CAS PubMed.
- T. Saito, R. H. Brown, M. A. Hunt, D. L. Pickel, J. M. Pickel, J. M. Messman, F. S. Baker, M. Keller and A. K. Naskar, Green Chem., 2012, 14, 3295–3303 RSC.
- M. S. Zhou, X. Q. Qiu, D. J. Yang, H. M. Lou and X. P. Ouyang, Fuel Process. Technol., 2007, 88, 375–382 CrossRef CAS PubMed.
- X. P. Ouyang, L. X. Ke, X. Q. Qiu, Y. X. Guo and Y. X. Pang, J. Dispersion Sci. Technol., 2009, 30, 1–6 CrossRef CAS.
- H. R. Wu, F. G. Chen, Q. G. Feng and X. P. Yue, Bioresources, 2012, 7, 2742–2751 CAS.
- V. Ugartondo, M. Mitjans and M. P. Vinardell, Bioresour. Technol., 2008, 99, 6683–6687 CrossRef CAS PubMed.
- M. Tortora, F. Cavalieri, P. Mosesso, F. Ciaffardini, F. Melone and C. Crestini, Biomacromolecules, 2014, 15, 1634–1643 CrossRef CAS PubMed.
- A. F. McKinley and B. L. Diffey, CIE J., 1987, 6, 17–22 Search PubMed.
- Erythema reference action spectrum and standard erythema dose. Commission Internationale de l'Eclairage International (CIE) Central Bureau. Publ. CIE S007/E, Vienna, Austria, 1998.
- R. M. Sayre, J. C. Dowdy, A. J. Gerwig, W. J. Shields and R. V. Lloyd, Photochem. Photobiol., 2005, 81, 452–456 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Active and inactive ingredients of purchased sunscreen lotions and pure creams. See DOI: 10.1039/c4gc01333f |
| This journal is © The Royal Society of Chemistry 2015 |