Efficient 4,5-dihydro-1H-imidazol-5-one formation from amidines and ketones under transition-metal free conditions†
Yanjun
Xie
a,
Xiufang
Cheng
a,
Saiwen
Liu
a,
Hui
Chen
a,
Wang
Zhou
b,
Luo
Yang
a and
Guo-Jun
Deng
*ac
aKey Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: gjdeng@xtu.edu.cn; Fax: (+86)0731-5829-2251; Tel: (+86)0731-5829-8601
bCollege of Chemical Engineering, Xiangtan University, Xiangtan 411105, China
cKey Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, China
First published on 26th September 2014
Abstract
An efficient procedure for 4,5-dihydro-1H-imidazol-5-one preparation from aryl amidines and ketones under transition-metal free conditions is described. When cyclic ketones were employed, various spiro-fused 4,5-dihydro-1H-imidazol-5-ones were formed in high yields via rearrangement reaction.
Nitrogen-containing heterocycles are frequently found in natural alkaloids, functional materials, agrochemicals and pharmaceutical drugs.1 Therefore, development of efficient methods for the synthesis of heterocycles with multi-nitrogen atoms is of great importance for drug development.2 Traditionally, imidazole and pyrimidine derivatives were prepared by the condensation of 1,3-dicarbonyl compounds and 1,3-diamines such as ureas, thioureas, amidines and guanidines under acidic or basic conditions.3 In recent years, transition-metal catalyzed cross-coupling reactions were successfully employed for the construction of nitrogen-containing heterocycles.4
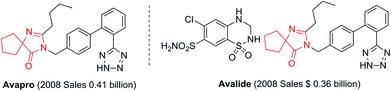
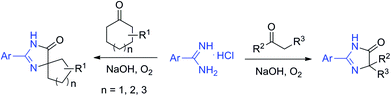
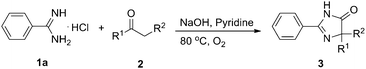
Spirans which contain at least two rings and share one spirocarbon atom widely exist in natural products and pharmaceutical drugs.5 For example, natural and synthetic compounds such as acorenone B,6 β-vetivone,7 isocomene,8 triangulanes9 and coronane10 all contain spirocarbon atoms. Due to their unique structural features, the synthesis of spirocycles has attracted much attention from organic chemists and great progress has been made in the past few decades.11 Compared to the extensively investigated preparation of spirocarbocycles, methods for the synthesis of nitrogen-containing spiro-heterocycles were much less developed. A number of substituted 4,5-dihydro-1H-imidazol-5-ones which contain a highly strained spiro structure belong to selective and non-toxic herbicides.12 For example, Avapro and Avalide (both contain a spiro structure produced by Bristol-Myers Squibb) are angiotensin II receptor blockers used to treat hypertension (Scheme 1).13 The limited methods for spiro-fused 4,5-dihydro-1H-imidazol-5-ones are mainly based on the cyclization reaction of the five-membered rings, such as the acylation of amide or nitrile of 1-aminocyclopentancarboxylic acid with pentanoic acid chloride followed by ring closure reaction,14 the condensation of ethyl 1-aminocyclopentancarboxylate with ethyl pentanimidate,15 and the reaction of 1-aminocyclopentanecarboxamide with trimethyl orthopentanoate.16 Dehydrogenation of the corresponding saturated spiro-heterocycles can afford an alternative route for the preparation of 4,5-dihydro-1H-imidazol-5-ones.17 Oxidative rearrangement of tetrahydrobenzimidazoles with dimethyldioxirane can provide spiro-fused 5-imidazolones with good selectivity.18 However, these methods require highly functionalized five-membered heterocycles usually prepared by several steps which severely limit the reaction scope and their further application. Due to the importance of 4,5-dihydro-1H-imidazol-5-ones in pharmaceuticals, it is highly desirable to develop efficient methods to prepare them using readily available raw materials. Herein, we describe a strategy for the preparation of 4,5-dihydro-1H-imidazol-5-ones from commercially available amidines and ketones via a rearrangement reaction strategy under transition-metal free conditions (Scheme 2).
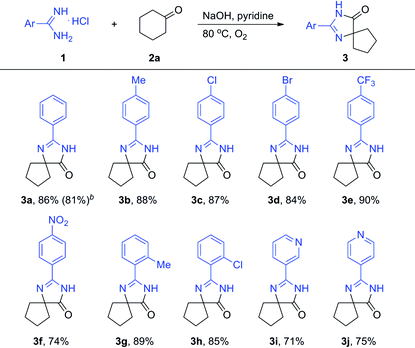
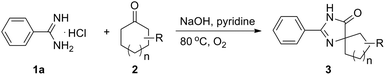
To obtain the optimized reaction conditions, the reaction of benzamidine hydrochloride hydrate (1a) with cyclohexanone (2a) was chosen as the model reaction in the absence of the metal catalyst under an oxygen atmosphere (for details, see Table S1 in ESI†). The desired product 3a was observed in 5% yield when the reaction was carried out in pyridine (entry 2). Basic conditions are favorable to this kind of reaction, and the reaction yield could be improved to 33% when NaOH was used as the base (entry 4). The amount of base is very important to the reaction yield, and the desired product was obtained in 93% GC yield when 4.5 equiv. of NaOH was used (entry 8). Besides pyridine, another nitrogen-containing solvent quinoline was also proved to be a good reaction medium for this kind of reaction (entry 11). A slightly lower yield was obtained when the reaction was carried out in other organic solvents such as NMP and toluene (entries 10 and 12). Other solvents such as DMSO, DMF, DMA, 1,4-dioxane and 1,2-dichlorobenzene were less effective for this reaction. High yield still could be obtained when the reaction temperature was decreased to 60 °C (entry 14). Oxygen was necessary and a much lower yield was obtained when the reaction was carried out in air (entry 16).
With the optimized reaction conditions established, the substrate scope with respect to amidines was examined, and the results were summarized in Table 1. The isolated yield of 3a was 86%. When the same reaction was carried out on a 5 mmol scale, 3a was obtained in 81% isolated yield. The desired product 3b was obtained in 88% yield when 4-methylbenzimidamide (1b) was treated with cyclohexanone (2a) under the optimized conditions. Halogen functional groups such as chloro, bromo and trifluoromethyl were well tolerated to give the corresponding spiro-fused heterocycles (3c–3e) in high yields. A nitro substituent was also compatible, and the corresponding product 3f was obtained in 74% yield. The position of the methyl substituent did not show much impact on the reactivity (3b and 3g, 3c and 3h). To our delight, nitrogen-containing hetero amidines such as 1i and 1j smoothly reacted with 2a to give the desired products 3i and 3j in good yields. Unfortunately, aliphatic amidines were not active under the current optimized reaction conditions.
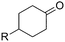
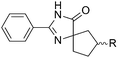
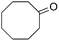
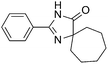
The scope of the reaction with symmetrical cyclic ketones is outlined in Table 2. Cyclohexanones bearing alkyl substituents at the para position were able to smoothly react with 1a to give the corresponding spiro-fused heterocycles in high yields (entries 1–5). Other cyclic ketones such as cycloheptanone (2h) and cyclooctanone (2i) also could be used for this kind of reaction, and the corresponding products 3q and 3r were obtained in 81% and 89% yields, respectively (entries 7 and 8). In all cases, spiro-fused heterocycles were selectively formed in good to high yields when cyclic ketones were used.
| Entry | Substrate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2 (0.3 mmol), NaOH (4.5 equiv.), pyridine (0.8 mL), 80 °C, 24 h. b Isolated yield based on 1a. | ||||
|
|
|||
| 1 | R = Me | 2b | 3k | 80 |
| 2 | R = Et | 2c | 3l | 84 |
| 3 | R = iso-C3H7 | 2d | 3m | 83 |
| 4 | R = n-C5H11 | 2e | 3n | 85 |
| 5 | R = tert-C5H11 | 2f | 3o | 71 |
| 6 | R = Ph | 2g | 3p | 76 |
| 7 |
|
2h |
|
81 |
| 8 |
|
2i |
|
89 |
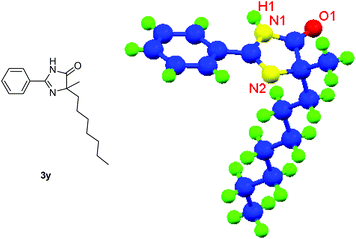
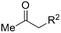
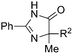
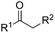
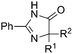
Besides cyclic ketones, linear ketones were also investigated to form non-spiro-fused heterocycles and the results are summarized in Table 3. The reaction showed good selectivity when various methyl–alkyl ketones were reacted with 1a, and only the longer alkyl group selectively shifted while the methyl group remained (entries 1–7). When 2-decanone (2p) was used, the desired product 3y was obtained in 73% yield. The structure of 3y was confirmed by X-ray crystallography (Fig. 1). Besides methyl–alkyl ketones, several other symmetrical alkyl–alkyl ketones were also employed for this reaction to give the corresponding products in good yields (entries 8–10). To our delight, non-symmetrical aromatic ketones such as propiophenone (2t) and 1,2-diphenylethanone (2u) also could be used for this reaction and the desired products 3ac and 3ad were obtained in 85% and 86% yields (entries 11 and 12).
| Entry | Substrate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Conditions: 1a (0.2 mmol), 2 (0.3 mmol), NaOH (4.5 equiv.), pyridine (0.8 mL), 80 °C, 24 h. b Isolated yield based on 1a. | ||||
|
|
|||
| 1 | R2 = C2H5 | 2j | 3s | 68 |
| 2 | R2 = n-C3H7 | 2k | 3t | 71 |
| 3 | R2 = iso-C3H7 | 2l | 3u | 61 |
| 4 | R2 = n-C4H9 | 2m | 3v | 80 |
| 5 | R2 = n-C5H11 | 2n | 3w | 77 |
| 6 | R2 = n-C6H13 | 2o | 3x | 75 |
| 7 | R2 = n-C7H15 | 2p | 3y | 73 |
|
|
|||
| 8 | R1 = C2H5, R2 = CH3 | 2q | 3z (3s) | 74 |
| 9 | R1 = n-C3H7, R2 = n-C2H5 | 2r | 3aa | 70 |
| 10 | R1 = n-C4H9, R2 = n-C3H7 | 2s | 3ab | 81 |
| 11 | R1 = Ph, R2 = CH3 | 2t | 3ac | 85 |
| 12 | R1 = Ph, R2 = Ph | 2u | 3ad | 86 |
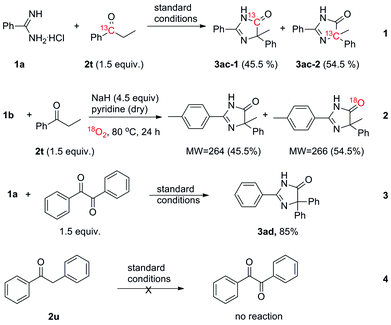
To gain insight into the reaction mechanism, several control experiments were conducted (Scheme 3). The reaction result of 1a with 13C labeled 2t showed that the 13C-labeled carbon atom in 2t appeared at two different sites in the product 3ac (eqn (1)). The result of 18O labeling reaction showed that part of the carbonyl oxygen atom in the product came from the molecular oxygen (eqn (2)). Benzamidine hydrochloride hydrate (1a) could smoothly react with benzil to afford the desired product 3ad in 85% yield (eqn (3)). However, 2u could not be converted into benzil in the absence of 1a (eqn (4)). This means the CH2 group ortho to the carbonyl group in ketone might be oxidized to the carbonyl group and serves as a key intermediate under the standard reaction conditions with the aid of the amidine substrate.
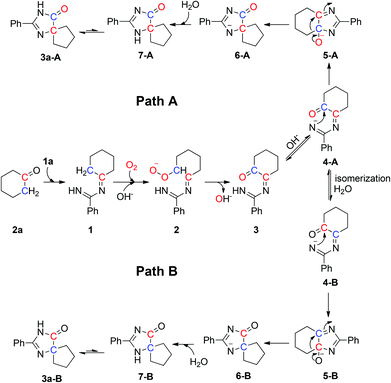
Based on our observations and the literature, a plausible mechanism with two pathways is outlined in Scheme 4. The reaction of 2a with 1a generates intermediate 1, which converts into intermediate 2 under an oxygen atmosphere in the presence of NaOH. Chemical 2 can be converted into carbonyl intermediate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 and affords 4-A by protonation. The nitrogen anion of 4-A attacks the carbon atom of the carbonyl group to form 6-A,20 and 6-A traps a hydrogen ion to yield 7-A which can be further resonated to give the final product 3a-A (path A). In another pathway, 4-A is converted into 4-B by isomerization and hydrolysis reaction. The nitrogen anion of 4-B attacks the carbon atom of the carbonyl group to form 6-B,20 and 6-B traps a hydrogen ion to yield 7-B which can be further resonated to give the final product 3a-B (path B).
19 and affords 4-A by protonation. The nitrogen anion of 4-A attacks the carbon atom of the carbonyl group to form 6-A,20 and 6-A traps a hydrogen ion to yield 7-A which can be further resonated to give the final product 3a-A (path A). In another pathway, 4-A is converted into 4-B by isomerization and hydrolysis reaction. The nitrogen anion of 4-B attacks the carbon atom of the carbonyl group to form 6-B,20 and 6-B traps a hydrogen ion to yield 7-B which can be further resonated to give the final product 3a-B (path B).
Conclusions
In summary, we have developed a base-promoted approach for the synthesis of 4,5-dihydro-1H-imidazol-5-ones using amidines and ketones under transition-metal free conditions. Various amidines and ketones are suitable substrates for this reaction to give the corresponding products in good yields. When cyclic ketones were employed, various spiro-fused 4,5-dihydro-1H-imidazol-5-ones were selectively formed in good to high yields. This method affords an efficient approach especially for spiro-fused 4,5-dihydro-1H-imidazol-5-ones using readily available starting materials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21172185, 21372187), the New Century Excellent Talents in University from Ministry of Education of China (NCET-11-0974) and the Hunan Provincial Innovative Foundation for Postgraduate (CX2013B269).Notes and references
- (a) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, 2003 CrossRef; (b) A. R. Kattrizky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, Pergamon, Amsterdam, 2nd edn, 2000 Search PubMed; (c) J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell, Oxford, 4th edn, 2000 Search PubMed; (d) A. R. Katritzky, C. A. Ramsden, J. A. Joule and V. V. Zhdankin, Handbook of Heterocyclic Chemistry, Elsevier, Oxford, 3rd edn, 2010 Search PubMed.
- For selected reviews on the preparation of nitrogen-containing heterocycles, see: (a) E. El Ashry and A. El Nemr, Synthesis of Naturally Occurring Nitrogen Heterocycles from Carbohydrates, Blackwell, Oxford, 2005 Search PubMed; (b) K. Nylund and P. Johansson, Heterocyclic Compounds: Synthesis, Properties and Applications, Nova Science Publishers, Inc., New York, 2010 Search PubMed; (c) K. C. Majumdar, S. Muhuri, R. U. Islam and B. Chattopadhyay, Heterocycles, 2009, 78, 1109 CrossRef CAS PubMed; (d) D. J. Brown, The Pyrazines, John Wiley & Sons, Inc., New York, 2002 CrossRef.
- (a) J. Alvarez-Builla, J. J. Vaquero and J. Barluenga, Modern Heterocyclic Chemistry, Wiley-VCH, Weinheim, 2011, ch. 10, 11, and 19 Search PubMed; (b) J. C. J. Roberts, Chem. Soc., 1952, 3065 RSC; (c) V. L. Alifanov and E. V. Babaev, Synthesis, 2007, 263 CAS.
- (a) G. W. Gribble and J. A. Joule, Progress in Heterocyclic Chemistry, Elsevier, Oxford, 2012 Search PubMed; (b) J. J. Li and G. W. Gribble, Palladium in Heterocyclic Chemistry, Elsevier, Oxford, 2007 Search PubMed.
- (a) A. Baeyer, Ber. Dtsch. Chem. Ges., 1900, 33, 3771 CrossRef; (b) G. P. Moss, Pure Appl. Chem., 1999, 71, 531 CAS.
- (a) W. Oppolzer and K. K. Mahalanabis, Tetrahedron Lett., 1975, 16, 3411 CrossRef; (b) J. F. Ruppert, M. A. Avery and J. D. White, J. Chem. Soc., Chem. Commun., 1976, 978 RSC.
- G. H. Posner and T. G. Hamill, J. Org. Chem., 1988, 53, 6031 CrossRef CAS.
- (a) M. C. Pirrung, J. Am. Chem. Soc., 1979, 101, 7130 CrossRef CAS; (b) L. A. Paquette and Y. K. Han, J. Am. Chem. Soc., 1981, 103, 1835 CrossRef CAS.
- (a) A. de Meijere, S. I. Kozhushkov and H. Schill, Chem. Rev., 2006, 106, 4926 CrossRef CAS PubMed; (b) S. Zöllner, H. Buchholz, R. Boese, R. Gleiter and A. de Meijere, Angew. Chem., Int. Ed., 1991, 30, 1518 CrossRef; (c) H. D. Beckhaus, C. Rüchardt, S. I. Kozhushkov, V. N. Belov, S. P. Verevkin and A. de Meijere, J. Am. Chem. Soc., 1995, 117, 11854 CrossRef CAS.
- (a) D. Wehle and L. Fitjer, Angew. Chem., Int. Ed., 1987, 30, 130 CrossRef; (b) L. Fitjer and U. Quabeck, Angew. Chem., Int. Ed., 1987, 30, 1023 CrossRef; (c) L. Fitjer, M. Giersig, W. Clegg, N. Schormann and G. M. Schldrick, Tetrahedron Lett., 1983, 24, 5351 CrossRef CAS.
- (a) V. A. D'yakonov, O. A. Trapeznikova, A. de Meijere and U. M. Dzhemilev, Chem. Rev., 2014, 114, 5775 CrossRef PubMed; (b) A. P. Krapcho, Synthesis, 1978, 77 CrossRef; (c) S. F. Martin, Tetrahedron, 1980, 36, 419 CrossRef CAS; (d) M. Sannigrahi, Tetrahedron, 1999, 55, 9007 CrossRef CAS; (e) R. Pradhan, M. Patra, A. K. Behere, B. K. Mishrab and R. K. Behera, Tetrahedron, 2006, 62, 779 CrossRef CAS PubMed; (f) F. A. Kang and Z. Sui, Tetrahedron Lett., 2011, 52, 4204 CrossRef CAS PubMed; (g) R. Rios, Chem. Soc. Rev., 2012, 41, 1060 RSC; (h) I. R. Ramazanov, A. V. Yaroslavora and U. M. Dzhemilev, Russ. Chem. Rev., 2012, 81, 700 CrossRef CAS PubMed.
- (a) P. J. Wepplo, Pestic. Sci., 1990, 31, 293 CrossRef; (b) J. E. Harris, J. A. Gagne, J. E. Fischer, R. R. Sharma, K. A. Traul, J. D. Scot and F. G. Hess, in The Imidazole Herbicides, ed. D. L. Shaner and S. L. O'Connor, CRC Press, Boca Raton, FL, 1991, p. 179 Search PubMed.
- M. J. O'Neil, in The Merck Index-An Encyclopedia of Chemicals, Drugs, and Biologicals, ed. M. J. O'Neil, A. Smith and P. E. Hackelman, Merck & Co., Rahway, NJ, 13th edn, 2001, p. 914 Search PubMed.
- (a) C. V. Kavitha, S. L. Gaonkar, J. N. Narendra Sharath Chandra, C. T. Sadashiva and K. S. Rangappa, Bioorg. Med. Chem., 2007, 15, 7391 CrossRef CAS PubMed; (b) N. Cousaert, N. Willand, J. C. Gesquiére, A. Tartar, B. Deprez and R. Deprez-Poulain, Tetrahedron Lett., 2008, 49, 2743 CrossRef CAS PubMed.
- J. Y. Xu, N. G. Li, X. M. Wu, W. Y. Hua, J. Zhang and Q. J. Wang, Zhongguo Huaxue Zazhi, 2003, 13, 311 CAS.
- C. Huszar, A. Kis-Tamas, A. Nemeth, A. Gajary and L. Pali, PCT Int. Appl, WO 9905120 A1 19990204, 1999 Search PubMed.
- I. Panov, P. Drabina, Z. Padělková, J. Hanusek and M. Sedlák, J. Heterocycl. Chem., 2010, 47, 1356 CrossRef CAS.
- C. J. Lovely, H. W. Du, Y. He and H. V. R. Dias, Org. Lett., 2004, 6, 735 CrossRef CAS PubMed.
- (a) Y. F. Liang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 548 CrossRef CAS PubMed; (b) C. Zhang, Z. J. Xu, L. R. Zhang and N. Jiao, Tetrahedron, 2012, 68, 5258 CrossRef CAS PubMed.
- (a) M. C. Pankaskie and L. Small, J. Chem. Educ., 1986, 63, 650 CrossRef CAS; (b) R. C. Hayward, J. Chem. Educ., 1983, 60, 512 CrossRef CAS; (c) G. H. Lu, A. Katoh, Z. Q. Zhang, Z. Z. Hu, P. Lei and M. Kimura, J. Heterocycl. Chem., 2010, 47, 932 CrossRef CAS; (d) M. Kimura, G. H. Lu, H. Iga, M. Tsunenaga, Z. Q. Zhang and Z. Z. Hu, Tetrahedron Lett., 2007, 48, 3109 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1017138. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4gc01515k |
| This journal is © The Royal Society of Chemistry 2015 |