Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Opportunities for greener alternatives in chemical formulations
P. G.
Jessop
*a,
F.
Ahmadpour
b,
M. A.
Buczynski
c,
T. J.
Burns
d,
N. B.
Green II
e,
R.
Korwin
f,
D.
Long
g,
S. K.
Massad
h,
J. B.
Manley
i,
N.
Omidbakhsh
j,
R.
Pearl
k,
S.
Pereira
l,
R. A.
Predale
m,
P. G.
Sliva
n,
H.
VanderBilt
o,
S.
Weller
p and
M. H.
Wolf
q
aDept. of Chemistry, Queen's University, Kingston, ON, Canada K7L 3N6. E-mail: jessop@chem.queensu.ca
bVirox Technologies Inc., 2770 Coventry Road, Oakville, Ontario L6H 6R1, Canada. E-mail: faraz@virox.com; Tel: +(905) 813-0110 ext. 123
cChurch & Dwight Co., Inc., now with Reckitt Benckiser, 399 Interpace Parkway, Parsippany, NJ 07054-0225, USA. E-mail: michael.buczynski@rb.com; Tel: +1-973 404 2484
dNovozymes North America, Inc., 77 Perry Chapel Church Road, Franklinton, NC 27525, USA. E-mail: tjb@novozymes.com; Tel: +919-494-3043
eRochester Midland Corporation, 155 Paragon Drive, Rochester, NY 14624, USA. E-mail: ngreen@rochestermidland.com; Tel: +585 336 2405
fState Industrial Products, 5915 Landerbrook Drive, Mayfield Heights, OH 44124, USA. E-mail: rkorwin@stateindustrial.com; Tel: +440-544-5113
gEnvironmental Sustainability Solutions, LLC, 5551 Lake Ridge Trail, Frankfort, MI 49635, USA. E-mail: dlong.ess@gmail.com; Tel: +231-352-6907
hZep Inc., 1420 Seaboard Industrial Blvd, Atlanta, GA 30318, USA. E-mail: suhail.massad@zep.com; Tel: +404-603-7592
iGuiding Green LLC, 457 E. Mier Rd., Sanford, MI 48657, USA. E-mail: juliemanley@GuidingGreen.com; Tel: +815-325-4974
jFormerly of Virox Technologies Inc., 2770 Coventry Road, Oakville, Ontario L6H 6R1, Canada. E-mail: navid@virox.com; Tel: +(905) 813-0110 ext. 123
kFlorida Chemical Company, Inc., 1785 King Road, Hinckley, Ohio 44233-9775, USA. E-mail: rpearl@flotekind.com; Tel: +330-278-2564
lRug Doctor, 415C Axminister Drive, Fenton, MO 63026, USA. E-mail: schubert.pereira@rugdoctor.com
mJohnson & Johnson Group of Consumer Companies, 199 Grandview Road, Skillman, NJ 08558, USA. E-mail: rpredal@its.jnj.com; Tel: +908-874-2079
nAmway Corporation, 7575 Fulton Street East, Ada, Michigan 49355, USA. E-mail: phil.sliva@amway.com; Tel: +616-787-8448
oFormerly of Bissell Homecare, Inc., 2345 Walker NW, Grand Rapids, MI 49544, USA
pZep Inc., 1420 Seaboard Industrial Blvd, Atlanta, GA 30318, USA. E-mail: stan.weller@zep.com; Tel: +404-350-6284
qSeventh Generation, Inc., 60 Lake Street, Burlington, VT 05401, USA. E-mail: mhw@seventhgeneration.com; Tel: +802 658 3773 Ext. 791
First published on 23rd February 2015
Abstract
Formulated products, including household care and personal care products, contain some components that need to be replaced because of identified or suspected negative effects on health or the environment. The creativity and expertise of the academic green chemistry community could contribute to the identification of more desirable replacements for such components, if the community were aware of the needs. The formulator's industry, through the ACS Formulator's Roundtable, has identified 10 classes of components that are particularly in need of replacement. These classes are described, as are the characteristics that ideal replacements should possess.
Introduction
Greener or more environmentally benign replacements for key problematic processes or materials are a priority for both the chemical industry and the green chemistry academic community. Identification of key needs by industry players can help focus research efforts by those in the academic community who desire to tackle such problems. Needs of the pharmaceutical industry were identified in a 2007 paper by a collaboration of pharmaceutical companies;1 that paper has been cited in many journal articles and, more importantly, in research proposals as a justification for a choice of research area. Such communication between industry and academia helps to bring the creativity and resources of the academic community to bear on real green chemistry needs.The formulated products industry also seeks greener technologies and materials. This industry produces household care products (over US$80 billion worldwide p.a.) such as detergents, cleansers, polishes, air fresheners, and insecticides, plus personal care products (over US$200 billion worldwide p.a.) such as deodorants, cosmetics, fragrances, toothpaste, and shampoo.2 The industry has also identified key needs,3 which are presented here in a format convenient for the academic community. Each of these identified problems involves materials that are needed for their function but are recognized or suspected of having undesirable health or environmental impacts. Due to their manner of use, many of these materials end up in the sewer system or directly dispersed into the environment. Therefore greener replacements are actively sought.
The formulation industry uses hundreds of chemicals to formulate consumer cleaning products and consumer personal care products. Each ingredient in the formulation is selected to provide a specific benefit or function. As more environmental health and safety data becomes available, some ingredients have to be replaced because they do not have the environmental, health or safety profile desired by the industry. The industry would like to identify replacements that have a significantly better profile. In addition, these replacements must perform as well or better than the ingredients that they replace. The cost of replacement chemicals must be competitive. Today the formulation industry expects a full set of environmental, health and safety data before they consider substituting a current chemical. The industry must be sure that the new chemical has a better environmental, health and safety profile before the chemical will be used. No company wants to substitute a chemical with one they think is better but later find that it has some negative characteristics that may impact their customers, the environment, or their reputation.
The ACS Green Chemistry Institute® (GCI) Formulators’ Roundtable is a partnership between the GCI and 13 companies in the formulated products industry designed to be the driving force for the use of green chemistry in creating innovative products that are environmentally sustainable throughout the entire product life cycle and safer to make and use. A desire for reducing the environmental impact of formulated products has inspired the members of the Formulator's Roundtable to identify 10 specific needs for greener replacements, in the hope that this will trigger research activity in these areas. To initiate progress towards informing and influencing suppliers and academia to develop greener alternatives, the Roundtable believed it was imperative to define the top areas for opportunities for greener alternatives as identified from a formulator's perspective. The components of existing formulated products are considered safe and effective; however, the words “green” and “sustainable” are best defined as relative terms (i.e. having less risk than known alternatives) so that further improvement is always possible and desired. It is the intention of the Roundtable to foster the development of innovative greener components to enhance the overall sustainability of formulated products.
The following list was developed with input and review from all member companies. These ten opportunities are common to the industry and do not represent one particular company's interests. They were selected by the Roundtable members because the current best performing options in each category were found, by several member companies, to have potential health or environment concerns and because the existing “greener” replacements do not provide adequate performance.
General requirements
The following are general recommendations for greener alternatives and are applicable to most or all of the categories of materials discussed in this paper. These should be considered to be requirements in addition to those specified for each class of components.• Replacement ingredients should be cost effective and as efficacious as those ingredients that they are replacing
• Ingredients should be stable and should function in a pH range of 2 to 11.5.
• Ingredients should not be hazardous air pollutants (HAPs),4 or chemicals listed on the U.S. Toxics Release Inventory.5
• Ingredients preferably should not be Volatile Organic Compounds (VOCs). There is, unfortunately, no agreement on the definition of a VOC. In the context of solvents, the EU defines a VOC as “any organic compound having at 293.15 K a vapour pressure of 0.01 kPa or more”6 while the US EPA considers a solvent not a VOC if it has a vapour pressure <0.013 kPa (1 mmHg), has 12 or more carbons, or is a non-subliming solid at 20 °C.7 However, in the broader context of emission limits, the EU define VOCs as “organic compounds arising from human activities, other than methane, which are capable of producing photochemical oxidants by reactions with nitrogen oxides in the presence of sunlight”,8 while the EPA defines a VOC as an organic compound “which participates in atmospheric photochemical reactions”.9 Thus, bizarrely, it is possible for a compound to be both organic and volatile and yet not legally be considered a Volatile Organic Compound. Such exemptions include a fair number of halogenated compounds and a few nonhalogenated volatile organics (methane, ethane, acetone, methylated siloxanes, methyl acetate, methyl formate, dimethylcarbonate and propylene carbonate).10
• Ingredients shall not be Ozone Depleting Agents as defined by the Montreal Protocol.11
• Ingredients shall not contain particularly toxic elements such as heavy metals.
• Ingredients shall not be classified as carcinogens, mutagens or reproductive toxins by established authorities such as the International Agency for Research on Cancer12 or the US National Toxicology Program.13 Ingredients should not be in the GHS category 1 (known or presumed human carcinogen) or category 2 (suspected human carcinogens). If an ingredient contains a contaminant which is classified as a carcinogen, mutagen or reproductive toxin, it must be below an established “no effects level”.
• Ingredients shall not be classified as Persistent Organic Pollutants (POP) as defined by the United Nations Environment Programme (UNEP).14
• Ingredients shall not be classified as persistent, bioaccumulative, or toxic (PBT) by the US EPA. The EPA considers a compound in the PBT category if it has a transformational half-life (persistence) of >2 months, a fish BCF or BAF of ≥1000, a molar mass of <1000 g mol−1, and toxicity of concern.15,16 The EPA Design for the Environment (DfE) recommendations are stricter for biodegradation if the ecotoxicity is high (and vice versa), as shown in Table 1. Section 4.1.2.14 of the GHS specifies similar but not identical requirements.17 The requirements are even more stringent if the ingredient will be used in a direct release product, meaning one that is released directly into the environment rather than via a sewage system (Table 2). Note that the persistence and bioaccumulation limits do not apply to inorganic compounds.
| Acute aquatic toxicity value (LC/EC/IC50) | Persistence (results of biodegradation tests) | Bioaccumulation values (BAF/BCF) |
|---|---|---|
| a As measured in a ready biodegradation test without degradation products of concern. A degradation product of concern would be one which has LC/EC/IC50 ≤ 10 ppm and degrades <60% in 28 days.18 | ||
| ≤1 ppm | May be acceptable if the chemical meets the 10-day windowa | <1000 |
| >1 and ≤10 ppm | The chemical must meet the 10-day windowa | <1000 |
| >10 and <100 ppm | The chemical must reach the pass level within 28 daysa | <1000 |
| ≥100 ppm | The chemical need not reach the pass level within 28 daysa if its half-life is <60 days | <1000 |
| Acute aquatic toxicity value (LC/EC/IC50) | Persistence (results of biodegradation tests) | Bioaccumulation values (BAF/BCF) |
|---|---|---|
| a As measured in a ready biodegradation test without degradation products of concern. A degradation product of concern would be one which has LC/EC/IC50 ≤ 10 ppm and degrades <60% in 28 days.19 | ||
| ≤10 ppm | Not acceptable | — |
| >10 and <100 ppm | >60% degradation in 10-daysa | <1000 |
| ≥100 ppm | >60% degradation in 28 daysa | <1000 |
• Ingredients should, where possible, be non-sensitizing and non-irritating. Standard tests for this are OCSPP 870.2600 (guinea pig skin sensitization) and OCSPP 870.2400 (acute eye irritation).20 Chemicals that should be avoided include those listed as category 1A or 1B respiratory or skin sensitizers in the GHS21 or H317, H334, R42 or R43 by the EU.
• While every ingredient (including pure water)22 has some toxicity, and in that sense no ingredient can be “nontoxic” in the absolute sense, the ingredients in all formulations should have as little toxicity as possible. The GHS (Globally Harmonized System of Classification17 and Labelling of Chemicals) and DfE18 recommend that ingredients should have:
- LD50 (oral, mammal) > 2000 mg kg−1,
- LD50 (dermal, mammal) > 2000 mg kg−1,
- LC50 (inhalation of vapours, 4 h, mammal) > 20 mg L−1 (20 ppm), and
- LC50 (inhalation of dust or mist, 4 h, mammal) > 5 mg L−1.
Standard tests for quantifying acute toxicity are OECD 420 (acute oral toxicity in rats),23 OCSPP 870.1200 (acute dermal toxicity in rats) and OCSPP 870.1300 (acute inhalation toxicity in rats).20 The evaluation of toxicity should also include, if possible, chronic effects.
Specific opportunities
Greener antimicrobials
Many consumer products can become contaminated by bacteria or fungi, often during manufacture or filling of the product. In order to prevent the growth of such microorganisms, preservatives need to be a part of the formulation.Antimicrobial preservatives, by their very nature, are designed to kill microbes. By definition, most are stable compounds and potent toxicants to microorganisms. Specifically, they work by killing cells and preventing them from multiplying and are intended to prevent the growth of bacteria and fungi in commercial products – mainly Candida albicans, Pseudomonas aeruginosa, Escherichia coli, Aspergillus niger and Staphylococcus aureus – which could potentially cause serious infections on the skin and in the body. Unfortunately, these ingredients are often similarly toxic to aquatic organisms such as Daphnia species (water fleas).
All of the most commonly used preservatives (Scheme 1)24 have health or toxicity concerns.25–27 Some are sensitizers or cause dermatitis or other skin reactions.28,29 Some preservatives, known as formaldehyde donors, form formaldehyde, a carcinogen, when challenged with bacteria. Some pose toxicity,30–35 endocrine disruption,30,36,37 or other risks38 to aquatic fauna or flora or have slow biodegradation, at least at some concentrations, due presumably to their toxicity to soil or sludge bacteria.39–41 However, it is important to weigh the risks of including small amounts of antimicrobials into formulas which come into human contact versus the risk of leaving formulas less protected.
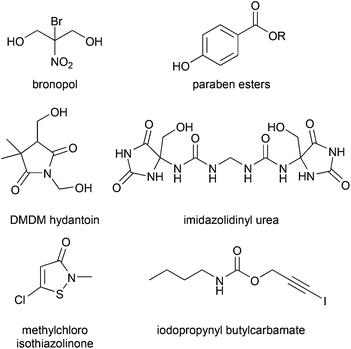 | ||
| Scheme 1 The structures of some antimicrobials used in cosmetic formulations.24 Other agents not shown include benzyl alcohol, benzoic acid, salicylic acid, sorbic acid, and phenoxyethanol. Note that the structure of imidazolinyl urea has recently been corrected in the literature.42 A different suite of antimicrobials is used in hand-washing, body-washing and shampoo formulations.43 | ||
Human exposure can take place at the workplace, via consumer products, or via food products from animals fed or exposed to antimicrobials.44,45 Humans also may be at risk from bacteria that have become anti-microbial resistant due to use of antimicrobials in farms44,45 or aquaculture,46 although the level of risk is controversial.47–50
Currently there are few “greener” alternatives for preservatives, although there has been some work towards finding new compounds or mixtures (such as essential oils51) that may avoid the above issues.52 However, to obtain a “Green Certification”, such as DfE, GreenSeal or EcoLogo, for a retail, consumer or industrial/institutional product, the antimicrobial must be pre-approved. The ACS GCI Formulators’ Roundtable is seeking new antimicrobial preservatives that have the following characteristics and that are designed considering the principles of green chemistry. Each should also, preferably, meet the general requirements mentioned above. To be used in certified green products, these would need to be submitted to the certifying bodies and approved.
Preferred characteristics of greener antimicrobial preservatives:
• Rapid acting at the first sign of contamination,
• In-container sanitization of gram positive bacteria, gram negative bacteria, yeast, and mold preferably in less than 2 days, at least less than 7 days after challenge,
• Broad spectrum, effective on multiple bacteria or fungi or both,
• Non-sensitizing, non-irritating, and having low toxicity to humans,
• Biodegradable and having low aquatic toxicity at likely concentrations in sewage,
• Not prone to causing antimicrobial resistance,
• Stable – does not break down during storage, stays active in a wide pH range (for fabric softener, pH 2.5–4.0; for dish detergent, pH 6.0–8.0; for laundry detergent, pH 7.0–9.5),
• Chemically and biologically inactive, except as an antimicrobial; will not interact with other ingredients (the chemical nature of the other ingredients is highly application dependent but would normally include common solvents, surfactants and chelants),
• Soluble in whatever solvent (water or oil) it will be used in, and
• Acceptable in odour and colour; will not impact on the aesthetics of the finished product (colour, odour, viscosity).
Greener solvents
The term “solvent” encompasses many classes of chemicals: alcohols, amides, amines, esters, glycols, glycol ethers, hydrocarbons, oxygenated hydrocarbons, terpenes, etc. The broad functionality of traditional solvents, such as petroleum distillates, makes them necessary ingredients in many product applications. Many formulators find these materials crucial to formulating high performance products that deliver concentrated cleaning. It is their varied attributes that make them indispensable in cleaning and personal care formulations. Solvents are used for many purposes such as dissolving raw materials (e.g., resins and waxes), dissolving various soils (e.g., adhesives, grease and inks) for removal, and as a carrier for essential oils. Solvents can be fossil-based or biobased and can be water-soluble or oil-soluble. Formulators need an assortment of solvents to meet the variety of applications required for green cleaning and personal care products.Unfortunately fossil-based solvents have disadvantages. The traditional hydrocarbon solvents and oxygenated hydrocarbons, such as petroleum distillates, glycol ethers, and isopropyl alcohol, are fossil-based and as such can cause a greater global warming contribution than some biomass-derived solvents (see Muñoz et al.53 for a comparison of biobased versus fossil-fuel derived ethanol). All of the most commonly used organic solvents have health, safety and environmental concerns.54,55 Most petroleum distillates are non-carcinogenic hydrocarbon blends, but because they are distillates, can contain small amounts of carcinogens such as benzene or HAPs such as xylene. Petroleum distillates are a safety concern for many reasons: some have inhalant/respiratory issues, and most cause defatting of the skin, dermatitis and other skin reactions. Most fossil-based solvents are VOCs or LVP VOCs (low vapour-pressure VOCs, usually with Tb > 216 °C)56 and some carry larger risks such as flammability. Because they are made from non-renewable sources, they can compare poorly against biobased solvents in terms of sustainability and resource depletion.
Many formulators are looking to biobased solvents57 from renewable feedstocks. Ethanol and ethyl lactate can be derived from fermentation of a food substance (cellulosic ethanol has not yet been commercialized). Others, such as soy methyl esters (“methyl soyate”),57–59 fusel oil esters,60 levulinic acid derivatives (levulinic ketal esters,61 2-methyltetrahydrofuran,62 and γ-valerolactone63,64), N-methylpyrrolidone,65 glycerol,66–68 glycerol derivatives,69–71 and propanediols can be chemically synthesized from bio-derived compounds. Such biobased solvents can have lower global warming potentials than their fossil-based counterparts,65 depending on their method of manufacture, but their production may in some cases impact food crops. Others, such as citrus oils72,73 and conifer (e.g. pine) derivatives, are expressed or steam-distilled from waste biomass without a chemical reaction and without impacting food crops; these could have less environmental impact. Life cycle assessments have been published for citrus oils74 and methyl soyate,75 while a partial LCA (energy only) has been published for pine derivatives.76 Another potential negative impact of biobased solvents is the reduction in biodiversity of the area due to monoculture or cutting of natural areas and replanting with the crop of choice. The conversion of a biomass feedstock into a biobased solvent can require both energy and reagents, increasing the environmental impact and the GWP of the solvent. Eutrophication of surface waters is an undesirable side effect of the production of some biobased chemicals.77 When renewable feedstocks are used, both a life cycle assessment and an environmental impact assessment are recommended.
New solvents that are petroleum-based are not likely to be as sustainable as bio-based or renewably sourced solvents but would still be welcome by the formulator's industry if the new solvents can be shown by LCA to be significantly greener than the solvents that are currently used for specific formulations. The LCA should include the impact of the solvent manufacture, use, and disposal or recycling.
Several review and perspective papers have been published recently about the design and selection of greener and/or biobased solvents,57,78,79 although many academic papers focus on solvents as reaction media rather than in formulations. Kerton and Marriott's book is recommended as an introduction for researchers new to the topic of green solvents.80
The ACS GCI Formulators’ Roundtable is seeking greener alternatives for commonly used solvents. In addition to meeting as many of the 12 Principles of Green Chemistry as possible, the following summarizes some of the key characteristics of suitable alternatives.
• Sourced from renewable raw materials avoiding petroleum feedstocks where possible.
• Non-sensitizing, non-irritating, and having low toxicity to humans. The toxicity requirement for solvents is often more strict than for other ingredients because of the higher quantities of solvents used in some applications. According to the EPA DfE,81 solvents should have oral and dermal mammalian LD50 of >2000 mg kg−1 and inhalation LC50 of >5000 ppm.
• Not showing reproductive toxicity. Standard tests are OECD 415 and 416.82,83
• Having minimal odour and colour, thus minimal impact on the finished product aesthetic
• Life cycle assessments (LCA) of cradle-to-gate, cradle-to-grave, or cradle-to-cradle are crucial and far more useful than studies that compare the impacts of solvents without regard to the impact of their manufacture.54,55,78 Researchers who lack the expertise to do an LCA should at least map out the entire manufacturing process from mined raw materials to determine whether obvious problems exist.78
• Having cleaning benefits such as grease-cutting and solubilizing. However, standard tests for cleaning benefits (e.g. ASTM G122-96, ASTM D5343, CSPA DCC-17) are typically done on finished formulations rather than the pure solvent, and address needs specific to each application. The solutes to be dissolved also depend on the application: for cleaning kitchen surfaces, “greasy kitchen soil” (a combination of Crisco® shortening, Wesson® cooking oil and bacon grease) is a standard testing material, while for laundry, test materials include lipstick, bacon grease, and motor oil.
• Modifying physical properties of finished formulations (e.g. reduced viscosity, freeze-thaw recovery, and freeze point depression for winter, high temperature stability for summer). Typically the properties of the solvent itself are not as important as the effect of the solvent on the properties of the formulation.
• Stabilizing formulations by keeping solids in solution and preventing precipitates
• Meeting the EPA DfE criteria for acceptable formulation ingredients.18
Greener small amines
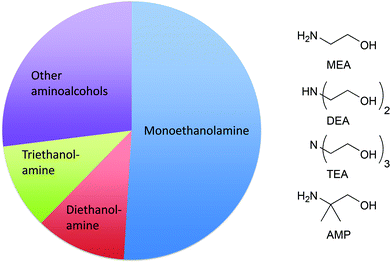
The broad functionality of small amines (such as monoethanolamine (MEA), diethanolamine (DEA), triethanolamine (TEA), and 2-amino-2-methylpropanol (AMP)) – alkalinity at low pH, corrosion protection,84 grease removal, film/streak inhibition, storage stability and dissolution in water without phase behaviour issues – makes them necessary ingredients in many product applications. Many formulators find these materials to be crucial to formulating high solids-content products to deliver concentrated cleaning. In concentrated formulations, small amines serve to lower viscosity, increase the solubility of the surfactant,85 and maintain uniform solids distribution. Laundry detergents typically have high solids content in comparison to other cleaning products. It is particularly difficult to formulate above a solids content of 30% and maintain physical properties without the use of small amines. Of the total production of the ethanolamines in 2008, 32% was used in detergents.86 The total production of aminoalcohols and simple derivatives was 476![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 million tonnes in the EU in 2013 (Fig. 1).
000 million tonnes in the EU in 2013 (Fig. 1).
 | ||
| Fig. 1 Production of MEA, DEA, TEA, and other aminoalcohols and their salts, ethers and esters in 2013 in the EU.87 | ||
Unfortunately small amines are a safety concern primarily because of the potential to form nitrosamines (eqn (1)).88 Nitrosamines have been shown to be carcinogenic, although the amines themselves are not carcinogenic. In addition, it has been shown that secondary amines and their salts89 form nitrosamines somewhat easily with nitrite, which is present in many natural materials such as saliva and vegetables, with nitrates in tap water, and nitrogen oxides in the air. Additionally, nitrosamine formation can be promoted by nitrate byproduct from the breakdown of preservatives in the formulation.90 Amongst the secondary amines, changes that reduce the basicity91,92 and increase the steric bulk around the nitrogen93 tend to lower the rate of nitrosation. Primary and tertiary amines do not usually form nitrosamines, unless they are contaminated with secondary amines. However, aryldialkylamines94–97 and certain other tertiary amines92,94,98 can react at significant rates.
While pure TEA is not problematic, the amine is rarely pure. TEA does not form nitrosamine at significant rates, but TEA contains DEA as a contaminant and as a degradation product,99 and DEA does form nitrosamines. A similar situation occurs with skin sensitization. TEA does not cause skin sensitization but MEA and DEA do.100,101 MEA also contains DEA as an impurity. Thus, replacement amines must not be contaminated with secondary amines and must not decompose to secondary amines under conditions of storage or use.
R2NH + NO2− + H+ → R2N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O + H2O O + H2O | (1) |
Formulating regulations in the EU102 have been established to minimize secondary amine and nitrosamine content in formulations containing tertiary amines; however, a safer/greener replacement is preferred.
The ACS GCI Formulator's Roundtable is seeking greener alternatives for small amines. In addition to meeting as many of the 12 Principles of Green Chemistry as possible, the following summarizes some of the key characteristics of suitable alternatives:
• Sourced from renewable raw materials rather than petroleum feedstocks,
• Non-sensitizing and non-irritating when used in the formulation,
• Having low toxicity to humans,
• Minimal odour and colour, having minimal impact on the finished product aesthetics,
• Alkalinity at relatively low pH values (such as 8 to 9), neutralizing (providing a counter-ion for) anionic detergents, neutralizing fatty acids, etc.,
• Able to supply alkalinity at high concentrations without causing phase separation of other components,†
• Corrosion protection (primarily for steel and aluminium),
• Cleaning benefits such as grease-cutting and solubilizing. AMP is particularly effective,
• Modifying physical properties (i.e. reduced viscosity, freeze-thaw recovery, freeze point depression) more effectively than inorganic bases,
• Preventing scale or film formation. TEA is effective in hard surface applications, and
• Meet the EPA DfE criteria for acceptable formulation ingredients.18
Greener chelants and sequestering agents
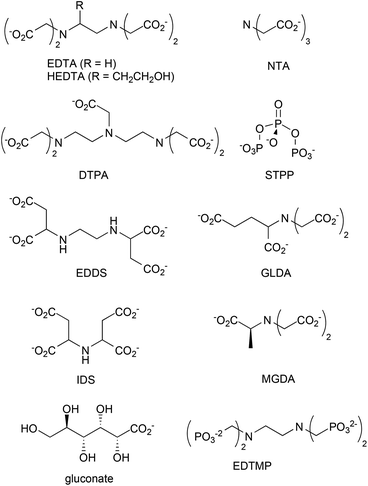
Chelants or sequestering agents are used in products to bind metals such as hard water cations. They can be used industrially as scale inhibitors or in cleaning products to bind calcium, magnesium, iron and other metals to improve cleaning performance. Chelants, according to ASTM A380,103 are “chemicals that form soluble, complex molecules with certain metal ions, inactivating the ions so that they cannot normally react with other elements or ions to produce precipitates or scale”. Of the many chelants in production (Scheme 2 and Fig. 2), the most widely consumed group is the aminopolycarboxylates, exemplified by the classical chelant EDTA, ethylenediaminetetraacetic acid tetrasodium salt; it is a colorless, water-soluble solid, widely used to dissolve scale by chelating metal ions such as Ca2+ and Fe3+. After being bound by EDTA, metal ions remain in solution but exhibit diminished reactivity.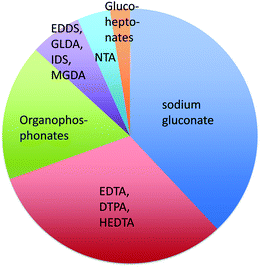 | ||
| Fig. 2 The share of world consumption of chelating agents in 2013.104 | ||
EDTA and other chelants have been linked to toxicity to internal organs such as the kidneys and the liver.105–107 This is an expected effect at high doses if the chemical is a good chelant. Because that problem may be insurmountable, a higher priority should be placed on environmental concerns related to the lack of biodegradability.108,109 If the chelant does not rapidly degrade, then there is a risk that it may bind heavy metals in sewage sludge110 or river and lake sediment111,112 and resuspend those metals into the water, so that the exposure of aquatic species to these metals is increased. STPP (sodium triphosphate) and organophosphonate chelants are problematic because of their high eutrophication potentials. Newer aminopolycarboxylates, such as EDDS (ethylenediamine-N,N′-disuccinic acid or its salts), GLDA (glutamic acid diacetic acid), IDS (iminodisuccinic acid), and MGDA (methylglycine diacetic acid), have greater rates of biodegradation.113 Sodium gluconate, which contains no nitrogen, is now up to about 1/3 of the market;104 it has a low eutrophication potential and low toxicity.
The EPA DfE program has unofficially rated chelants, although the ratings are not provided here to avoid any unintended preferential identification of specific chelants. Many of the new more biodegradable chelants that have been developed over the past several years have one of two problems. The backbones of some of the new chelant molecules113 look very much like NTA, trisodium nitrilotriacetate monohydrate, a suspected carcinogen.114 Thus, there is a concern that the replacement chelants may share this disadvantage. The second issue with several of the new chelants is they are not as effective on the most commercially important ions, Ca2+, Mg2+ or Fe3+, because the stability constants are lower (much lower in the case of gluconate115). This means a higher concentration of chelant needs to be used to obtain the same efficacy. The final issue is price; new chelants usually can not compete against the low cost of EDTA.
The topic of greener chelants has recently been reviewed.116
Preferred characteristics for greener chelants:
• Should be able to meet the chelation capacities listed in Table 3.
| Metal | Chelant capacity (g chelant per g metal) |
|---|---|
| a Better chelants would have lower values. | |
| Ca2+ | 16–20 |
| Mg2+ | 25–35 |
| Fe3+ | 10–20 |
| Cu2+ | 10–15 |
| Mn2+ | 10–15 |
• Should be active from a neutral pH to a pH of 12 or from a neutral pH to a pH of 2. A chelant effective over the full pH range would be ideal but would be technically very difficult to achieve.
• Should meet the EPA DfE criteria for chelants.117
Greener boron alternatives
Boron compounds useful in cleaning products include boric acid,118,119 borates119 and perborates.119 Boric acid acts as a non-alkali buffer and an enzyme stabilizer in liquid cleaning products. Borate (commonly known as borax) is used in many cleaning/laundry products to impart alkalinity, to provide buffering and deodorizing and to aid in emulsification and removal of oily soils. In addition, it is used as a gentle abrasive in some powdered cleaning products.119 Of the total world production of borates (4 million tonnes in 2010), 4% is used in detergents and soaps.120 Perborates are employed as stable sources of oxygen bleach. Boron is one of the least abundant light elements in the earth's crust and does not occur in the free state in nature. Boron, in its oxygenated compounds, constitutes only 950 ppm by weight of the earth's crust.121While boron compounds are effective and more benign than many alternatives, there are still some issues of concern. Boron is an essential element necessary for plant growth,122,123 but excess levels can be phytotoxic.124 While human safety studies have shown that perborate (as the sodium salt) is neither irritating nor sensitizing to human skin,125 boron is toxic to mammals in relatively low doses, with a NOAEL (No Observed Adverse Effect Level) for boron of 9.6 mg per kg bw per d (i.e. 55 mg kg−1 of boric acid) set by the critical effect of reduced fetal weight in a developmental toxicity study.126 Sodium perborate has recently been included in annex XIV of REACH, suggesting that its phase-out is just a matter of time. Because cleaning products contribute boron into the sewage system, greener and safer alternatives are needed. No truly suitable alternative for boric acid for enzyme stabilization has been found. The standard perborate replacement, sodium percarbonate, has many issues most important of which are poor stability127 and very high alkalinity.
The ACS GCI Formulators Roundtable is seeking greener alternatives for these boron compounds. In addition to meeting as many of the 12 Principles of Green Chemistry as possible, the following summarizes some of the key characteristics of suitable alternatives.
(a) Preferred characteristics for greener peroxygen compounds:
• Non-sensitizing, non-irritating, and having low toxicity to humans.
• Minimal odour and white in colour, thus having minimal impact on the finished product aesthetics.
• Active available oxygen (wt% of oxygen that is available for oxidation as measured by redox titration) at least 10% by weight in the neat dry form. As raw material and in powder finished product – shelf life 3 years. Both chemical and physical stability (flow, colour, odour).
• Very high water solubility with a complete release of all available active oxygen within 2 min in cold water (10 °C) as measured by redox titration.
• Safe during handling and shipping before formulation
• Synthesized from renewable materials (if the compound is organic).
(b) Preferred characteristics for greener replacements for boric acid (i.e. greener stabilizers of enzymes or peroxygen compounds):
• Non-sensitizing, non-irritating, and having low toxicity to humans
• Minimal odour and colour, thus having minimal impact on the finished product aesthetics
• Provide enzyme stability in aqueous based cleaning products for 3 years (ideal)
• Synthesized from renewable materials (if the compound is organic).
Greener fragrance raw materials
While many fragrances are natural materials, they can nevertheless cause health problems, including respiratory and dermal sensitivity. Amongst synthetic fragrances, musks are of concern because of their high volume of usage and potential for bioaccumulation.35,128Preferred characteristics for greener fragrance raw materials:
• Fragrances must meet the International Fragrance Association (IFRA) Standards.129
• All fragrance raw materials present at 100 ppm (or 0.01 percent by weight) or greater in the fragrance should be screened for toxicity following the guidelines in the EPA DfE Human Health criteria.130
• Fragrance ingredients present at or above 0.01% in the cleaning product should be screened to meet the DfE Environmental Toxicity and Fate (ETF) Criteria (Table 1).18
• Fragrance ingredients should be non-sensitizing and not listed on the EU list of 26 allergens.131,132
• Fragrance ingredients should not be derived from unsustainable sources (e.g. ambergris from sperm whales) or sources which will endanger another species.
• Non-aroma ingredients such as solvents should be ready biodegradable and either non-volatile or have low vapour pressure.
Greener corrosion inhibitors
Corrosion is the destruction, degradation or deterioration of substrate material at its interface with the environment, due to chemical reaction between the material and its environment. Corrosion can be prevented or inhibited by (A) coating the substrate with a non-reactive medium, (B) passivating the substrate, and (C) using chemical corrosion inhibitors. Corrosion inhibitors can delay or prevent metal corrosion rate. They are broadly divided by their electrochemical theoretical mechanisms as anodic inhibitors (e.g. nitrates, molybdates, phosphates, silicates), cathodic inhibitors (e.g. Mg, Zn, Ni, phosphonates, tannins) and mixed inhibitors or those that can serve as both anodic and cathodic (e.g. amines, urea, and nitrogen heterocycles).133 The annual economic cost of corrosion in the US alone is $276 billion.134 Replacing corroded steel consumes a large fraction of steel production,135 and therefore is responsible for a similar proportion of that industry's environmental impact. Thus, corrosion inhibitors support sustainability by the very nature of their function.Unfortunately, many corrosion inhibitors are manufactured using energy intensive methods, have environmentally unfavorable life-cycles and are made from nonrenewable resources. Many are corrosive, toxic, not biodegradable and can bioaccumulate.
To increase sustainability, manufacturers and formulators need to evaluate alternative strategies. One strategy is to determine how to reduce the negative effects of these corrosion inhibitors; another is to try to improve activity of corrosion inhibitors, and consequently use less. The field of corrosion inhibitors encompasses too many classes of chemicals and individual chemicals to list here. Even though sustainability development in this class of chemicals is at the nascent stage, several new classes of compounds useful in corrosion inhibition were introduced recently. Amino acid salts from renewable resources, natural soy-based polymers, casein-based polymers, and marine polysaccharides have demonstrably outperformed traditional corrosion inhibitors in various corrosion tests. The use of plant extracts as corrosion inhibitors has recently been reviewed.136 The availability of recently introduced “green” corrosion inhibitors has weakened the old argument that corrosion inhibitors help sustainability so their harmful and non-desirable effects should be acceptable. Development of acceptability criteria is perhaps the best way to confer a “sustainable” or green tag to an inhibitor chemical. For example, the North Sea Standard137 (primarily minimizing marine toxicity) acceptability criteria are as follows:
• Biodegradability: >60% in 28 days
• Marine toxicity: Effective Concentration, 50% (EC50)/Lethal Concentration, 50% (LC50) > 10 mg L−1 to North Sea species
• Bioaccumulation: Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) octanol/water partition coefficient (log
octanol/water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) < 3.
Kow) < 3.
However, because the Roundtable is primarily concerned with formulations of household, industrial and institutional (HI&I) products, the acceptability criteria are different from those of the North Sea and may vary from application to application. Limits are currently lacking, but need to be established for:
• Corrosivity
• Skin/Eye Irritation
• Toxicity (of all kinds)
• Biodegradability
• Life cycle analysis (energy used to manufacture, store, use concentration, length of useful life, post-use disposal)
• Renewability (for example 50% or more of the raw materials need to be from renewable sources).
Greener replacements for alkanolamides
Alkanolamides138 have traditionally been used by cleaning product formulators to increase viscosity and/or stabilize foam139 (the main purpose of alkanolamides in shampoos and dish wash detergents). They also provide solubilization of oily components, thanks to the low HLB (hydrophile–lipophile balance) values of some alkanolamides. In the product itself, this can aid the incorporation of fragrance and other non-polar ingredients. In end use applications such as laundry detergent, they can improve the removal of an oily soil from a substrate. In addition, they are virtually 100% “active” (meaning that they are stored and sold as pure compounds, with no water or other materials added). These attributes have made them valuable components in shampoos, dishwashing liquid, laundry hand wash detergents and other products that are enhanced by stable foam, increased viscosity or high concentrations.In recent years, alkanolamides have been identified as needing safer alternatives. A common preparation for alkanolamides, using bio-derived fatty acid methyl esters, is shown in eqn (2).140 These amides contain residual small secondary or primary amine molecules, which are a safety concern primarily because of their potential to form nitrosamines, as described above in the section on amines. Removal of the secondary amine does not solve the problem because slow hydrolysis will regenerate the secondary amine.
 | (2) |
The Formulators’ Roundtable is therefore seeking greener alternatives for alkanolamides. In addition to meeting as many of the 12 Principles of Green Chemistry as possible, the alternatives should possess the following key characteristics:
• Sourced from renewable raw materials rather than petroleum feed stocks
• Non-sensitizing, non-irritating, and having low toxicity to humans
• Minimal odour and colour, thus reducing impact on the finished product aesthetics
• High activity (alkanolamides are essentially 100% active)
• Compatible with anionic and nonionic surfactants
• Cleaning benefits such as oil solubilizing (low HLB)
• Able to modify the physical properties of finished formulations (e.g. increase viscosity, freeze-thaw recovery (i.e. if it separates on freezing, will easily remix upon thawing), enhance freeze point depression, or improve high temperature stability)
• Meet the EPA DfE criteria for acceptable formulation additives.18
Greener surfactants
Surfactants (Fig. 3) have a wide range of applications such as personal care, detergents, lubricants, fuels, environmental remediation, paints, inks, polishes, pharmaceutical dosage forms (i.e. inclusion in formulations to ensure delivery of the pharmaceutical to the target organ), pesticides, textiles, and mining. In these applications, surfactants serve a wide range of functions, such as reducing static, cleansing, emulsifying, solubilizing, foaming, or hair conditioning.141 The existence of so many applications for surfactants explains their high volume consumption and the resulting wide distribution in the environment. Thus formulators are looking for greener surfactants. The ideal green surfactant should have the least impact on the environment; therefore, it should preferably come from a sustainable source (not petroleum based). The source should preferably not have any food value nor have a negative impact on eco-diversity. Surfactants that are produced from renewable resources may be plant based, animal fats or even derived from microorganisms.142–144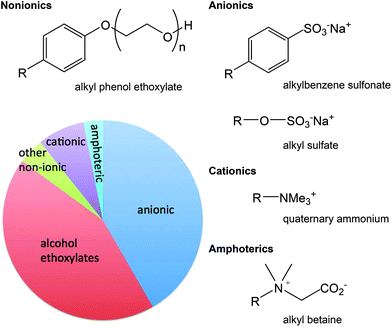 | ||
| Fig. 3 The classes of surfactants, with common examples, represented in terms of their portion of surfactant production in Europe in 2012.145 | ||
Plant based surfactants can have a negative impact on the environment even though they coming from a renewable resource. Often the environmental impact is reduced biodiversity of the area due to monoculture or cutting of natural areas and replanting with the crop of choice. Another negative impact is on the food supply if the crop is more valuable for its chemical value than its food value. A Life Cycle Assessment should be considered to determine whether use of renewable feedstocks is resulting in less environmental impact.
Biosurfactants146–148 are produced on living surfaces such as microbial cell surfaces or on their secretions. Several biosurfactants have high surface activity and low critical micelle concentration (CMC) and are, therefore, promising substitutes for synthetic surfactants but are currently constrained by the high cost of production.
The variety of surfactants from renewable feedstocks is limited in their physical properties when compared to petroleum-derived surfactants. Formulators need a variety of surfactants to meet the large number of applications needed for cleaning and personal care products. Many surfactants from renewable feedstocks are anionic surfactants including sulfates, sulfonate, esters, phosphate esters, and carboxylates.142 Therefore, formulators are looking for nonionic and cationic surfactants from renewable feedstocks.
Depending on the chemical structure, and specifically the hydrophobic moiety, surfactants can have varying toxicity and environmental fate. Most of today's commonly used surfactants are readily biodegradable during wastewater treatment, which leads to very low environmental concentrations. In evaluating the environmental impact of surfactants, their environmental concentration should be compared to their aquatic toxicity levels, and if their concentrations, due to their biodegradation speed, are significantly lower than the aquatic toxic levels, then they do not pose an environmental risk. Biodegradation products of surfactants should also be considered for their environmental fate evaluations. Biodegradation products of some surfactants are more toxic (e.g. alkyl phenol ethoxylates) than the surfactant itself; such surfactants are considered water pollutants149,150 and must be abandoned. Nonyl phenol ethoxylates are, as of 2014, candidates for being banned by the EU under the REACH regulation Annex XIV.
Minor levels of surfactants can also end up in aquatic sediments, due to their sorption to organic solids. It is therefore preferred that surfactants have fast anaerobic biodegradability to reduce their environmental impact. Some green certification programs such as the European “Flower” Eco-label now require ready anaerobic biodegradability to address this concern. This further narrows down the list of green surfactants.
Green surfactants should not pose any health concerns to the users. They must not be carcinogenic or mutagenic, and should not have developmental toxicity risks.
The physical properties of the ideal surfactant are varied because different uses will dictate a wide range in physical properties. It is therefore impossible to specify what physical characteristic a new surfactant should have without knowing exactly what application it will be used for. We therefore recommend that new surfactants have their physical properties measured; once that data is available for a new surfactant, it is possible to determine what application(s) might benefit from the discovery of this new surfactant. Thus, apart from issues of sustainability and environmental impact, any new surfactant needs to be tested to determine its physical properties, include the following, so that its suitability for various applications can be assessed:
• Cloud Point (the temperature at which a 1 wt% solution of a nonionic surfactant in water will cloud due to the onset of precipitation) or, for ionic surfactants, the Krafft point,
• HLB (the hydrophile-lipophile balance),
• Pour Point (the lowest temperature at which a neat liquid surfactant will still pour),151,152
• Moles of EO (the number of ethylene oxide units in the structure of the surfactant molecule),
• CMC in water, in ppm at 25 °C,
• Viscosity of the neat liquid surfactant, at 25 °C (cP),152
• Density of the neat liquid surfactant at 20 °C (g ml−1)
• Flash Point, Closed Cup, ASTM D93153
• Surface Tension (dynes cm−1) at 1% at 25 °C,
• Ross–Miles foam heights in mm at 0.1% actives at 25 °C, initial and after 5 minutes.154,155
Preferred characteristics for greener surfactants:
• Ready biodegradability in freshwater, seawater, and anaerobic (soil) conditions.
• Low aquatic toxicity for fish, algae and invertebrates (i.e. LC50 > 10 mg l−1)
• Derived from a feedstock that has no food value and that will not have a negative impact on eco-diversity.
• Manufactured by a process designed considering the 12 Principles of Green Chemistry.
Greener UV screens
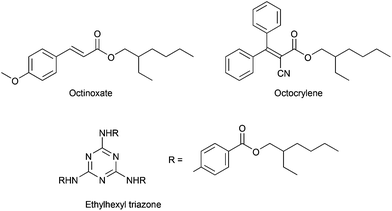
Sunscreens contain one or more ultraviolet (UV) filters. UV filters absorb potentially harmful ultraviolet rays, preventing those rays from penetrating the skin. UV screens include both organic compounds (e.g., octinoxate, octocrylene, ethylhexyl triazone, Scheme 3) and inorganics (e.g., zinc oxide, titanium dioxide). The inorganic UV screens are often used in the form of nano-scale particles. Few data exist to characterize the persistence, bioaccumulation potential,156–158 and aquatic toxicity159 of organic UV screens. According to widely used predictive models, nearly every UV screen is a potent aquatic toxicant. Decomposition products from UV screens may also present toxicity risks.160 Model predictions indicate that many UV screens are also expected to be persistent and/or bioaccumulative because of their lipophilicity.128,156 Recent research161,162 indicates that certain UV screens have the potential to cause chronic reproductive effects to aquatic life at low exposure levels. A desirable alternative would be well-characterized as readily biodegradable, of low toxicity to aquatic organisms, and not endocrine active.Preferred characteristics for greener UV screens:
• Readily biodegradable
• Low octanol-water partition coefficient (Kow) (e.g., log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow less than 3.5)
Kow less than 3.5)
• Low acute toxicity to aquatic organisms (e.g., lethal and adverse effects concentrations to 50% of a test population – LC50 and EC50 values – greater than 100 mg L−1)
• Does not elicit a positive response in endocrine disruption screening tests (e.g., in vitro estrogen receptor binding assay).163 Ideally, endocrine disruption tests with fish (OECD 229,164 OECD 230,165 and OECD 234166) would also be performed but this may be outside the budget of academic researchers.
• Non-sensitizing, non-irritating, and having low toxicity to humans
• Minimal odour and white in colour, thus a minimal impact on the finished product aesthetics
• Safe handling and shipping as a raw material
• Sourced from renewable raw materials
• Meet the EPA DfE criteria for acceptable formulation additives.18
Conclusions
The academic green chemistry community has the time, resources, and creativity to greatly contribute to the green chemistry needs of the formulators’ industry. Because many formulations are dispersed into the environment when they are used, and because consumers are exposed to the contents of many formulations, it is particularly important that the environmental and health impacts of formulation ingredients be minimized by careful molecular design. We have presented ten classes of ingredients which are particularly in need of replacement, in the hope that the academic community will be able to design greener alternatives.Abbreviations
| ACS | American Chemical Society |
| AMP | 2-Amino-2-methylpropanol |
| ASTM | American Society for Testing and Materials |
| BAF | Bio-accumulation factor |
| BCF | Bio-concentration factor |
| CMC | Critical micelle concentration |
| CSPA | Consumer Specialty Products Association |
| DEA | Diethanolamine |
| DfE | Design for the Environment |
| DTPA | Diethylene triamine pentaacetic acid or its salts |
| EC50 | Half-maximum effective concentration |
| EDDS | Ethylenediamine-N,N′-disuccinic acid or its salts |
| EDTA | Ethylenediaminetetraacetic acid or its salts |
| EDTMP | Ethylenediamine tetra(methylene phosphonic acid or its salts |
| EO | Ethylene oxide number |
| EPA | U.S. Environmental Protection Agency |
| ETF | Environmental Toxicity and Fate (DfE) |
| GCI | Green Chemistry Institute |
| GHS | Globally Harmonized System |
| GLDA | Glutamic acid diacetic acid or its salts |
| HEDTA | N-(Hydroxyethyl)-ethylenediaminetriacetic acid or its salts |
| HI&I | Household, industrial & institutional |
| HLB | Hydrophile/lipophile balance |
| IDS | Iminodisuccinic acid or its salts |
| IFRA | International Fragrance Association |
| K ow | 1-Octanol/water partition coefficient |
| LC50 | Concentration lethal for 50% of population |
| LCA | Life cycle assessment |
| LD50 | Dosage lethal for 50% of population |
| LVP | Low vapour pressure |
| MEA | Monoethanolamine |
| MGDA | Methylglycine diacetic acid or its salts |
| NTA | Nitrilotriacetic acid or its salts |
| OCSPP | Office of Chemical Safety and Pollution Prevention (EPA) |
| OECD | Organization for Economic Cooperation and Development |
| PBT | Persistent, bioaccumulative, or toxic |
| POP | Persistent organic pollutant |
| STPP | Sodium triphosphate |
| T b | Boiling temperature |
| TEA | Triethanolamine |
| UNEP | United Nations Environment Programme |
| VOC | Volatile organic compound |
Acknowledgements
The authors acknowledge the great assistance of the ACS Green Chemistry Institute. PGJ acknowledges funding from the Canada Research Chairs program and from Landolt & Cie for a Landolt & Cie “Innovative Strategies for a Sustainable Future” Chair for 2013-4 at the Ecole Polytechnique Fédérale de Lausanne.Representatives of the following companies contributed to this document: Amway, Bissell Homecare, Inc., Church & Dwight Co. Inc., The Clorox Company, Ecolab, Florida Chemical Company, Inc., Johnson & Johnson Consumer Companies, Novozymes North America, Rochester Midland Corporation, Rug Doctor, Inc., S.C. Johnson & Son, Inc., Seventh Generation, Inc., State Industrial Products Corp., Virox Technologies Inc., and Zep Inc.
Notes and references
- D. J. C. Constable, P. J. Dunn, J. D. Hayler, G. R. Humphrey, J. Johnnie, L. Leazer, R. J. Linderman, K. Lorenz, J. Manley, B. A. Pearlman, A. Wells, A. Zaksh and T. Y. Zhang, Green Chem., 2007, 9, 411–420 RSC.
- K. Peyre, in happi Magazine, Household and Personal Products Industry, July 2004, http://www.happi.com/contents/view_features/2005-11-15/household-personal-care-a-look-at-the-big-pi/.
- Opportunities for Greener Alternatives from a Formulator's Perspective, ACS GCI Formulators’ Roundtable, 2010, http://www.acs.org/content/acs/en/greenchemistry/industry-business/formulators.html.
- The Clean Air Act Amendments of 1990 List of Hazardous Air Pollutants, http://www.epa.gov/ttn/atw/orig189.html, Accessed 23 October 2013, 2013.
- Toxics Release Inventory (TRI) Program, http://www2.epa.gov/toxics-release-inventory-tri-program, Accessed 23 October 2013, 2013.
- Council Directive 1999/13/EC of 11 March 1999 on the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain activities and installations, The Council of the European Union, 1999, http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31999L0013&from=EN.
- 40 CFR 59.203 - Standards for consumer products, U. S. Government Printing Office, http://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&sid=0ed93c4b29b06ccaa1e65ca7a1691768&rgn=div5&view=text&node=40:6.0.1.1.7&idno=40#40:6.0.1.1.7.3.6.3.
- Directive 2001/81/EC of the European Parliament and of the Council of 23 October 2001 on national emission ceilings for certain atmospheric pollutants, 2001, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2001:309:0022:0030:EN:PDF.
- Definition of Volatile Organic Compounds (VOC), U. S. EPA, http://www.epa.gov/ttn/naaqs/ozone/ozonetech/def_voc.htm, Accessed 26 March 2014, 2014.
- 40 CFR 51.100(s) Definitions, U.S. Government Printing Office, http://www.ecfr.gov/cgi-bin/text-idx?rgn=div8&node=40:2.0.1.1.2.3.8.1.
- United Nations Environment Programme Ozone Secretariat, http://ozone.unep.org/new_site/en/montreal_protocol.php, Accessed 23 October 2013, 2013.
- International Agency for Research on Cancer, World Health Organization, http://www.iarc.fr, Accessed 23 October 2013, 2013.
- National Toxicology Program, National Institutes of Health, http://ntp.niehs.nih.gov, Accessed 23 October 2013, 2013.
- Persistent Organic Pollutants, United Nations Environment Programme, http://www.chem.unep.ch/pops/, Accessed 23 October 2013, 2013.
- S. H. Wayland, Fed. Regist., 1999, 64, 60194–60204 Search PubMed.
- TSCA New Chemicals Program (NCP) Chemical Categories, U.S. Environmental Protection Agency, 2010, http://www.epa.gov/oppt/newchems/pubs/npcchemicalcategories.pdf.
- Globally Harmonized System of Classification and Labelling of Chemicals, 5th Edition, United Nations, New York and Geneva, ST/SG/AC.10/30/Rev.5, http://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev05/English/ST-SG-AC10-30-Rev5e.pdf, 2013.
- Design for the Environment Program: Master Criteria for Safer Ingredients, Office of Pollution Prevention & Toxics, U.S. Environmental Protection Agency, 2012, http://www.epa.gov/dfe/pubs/projects/gfcp/dfe_master_criteria_safer_ingredients_v2_1.pdf.
- Design for the Environment: Criteria for Environmental Toxicity and Fate for Chemicals in Direct Release Products, U.S. Environmental Protection Agency, http://epa.gov/dfe/pubs/projects/gfcp/index.htm - Toxicity, Accessed 26 March 2014, 2014.
- OCSPP Harmonized Test Guidelines, U.S. Environmental Protection Agency, http://www.epa.gov/ocspp/pubs/frs/publications/Test_Guidelines/series870.htm, Accessed 6 March 2014, 2014.
- UN Economic Commission for Europe, Globally Harmonized System of Classification and Labelling of Chemicals (GHS), http://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html, Accessed 6 March 2014, 2014.
- D. J. Farrell and L. Bower, J. Clin. Pathol., 2003, 56, 803–804 CrossRef CAS.
- OECD Guidelines for Testing of Chemicals, Acute Oral Toxicity - Fixed Dose Procedure, http://www.oecd-ilibrary.org/environment/test-no-420-acute-oral-toxicity-fixed-dose-procedure_9789264070943-en, Accessed 6 March 2014, 2014.
- G. Bognolo, Chim. oggi, 2005, 23(6), 20–25 CAS.
- J. Van Miller, S. Hermansky, P. Losco and B. Ballantyne, Toxicology, 2002, 175, 177–189 CrossRef CAS.
- E. Zeiger, B. Gollapudi and P. Spencer, Mutat. Res., Rev. Mutat. Res., 2005, 589, 136–151 CrossRef CAS PubMed.
- J. Michałowicz, Pol. J. Environ. Stud., 2007, 16, 347–362 Search PubMed.
- A. C. DeGroot, I. R. White, M.-A. Flyvholm, G. Lensen and P.-J. Coenraads, Contact Dermatitis, 2010, 62, 2–17 CrossRef CAS PubMed.
- A. C. DeGroot, I. R. White, M.-A. Flyvholm, G. Lensen and P.-J. Coenraads, Contact Dermatitis, 2010, 62, 18–31 CrossRef CAS PubMed.
- A. B. Dann and A. Hontela, J. Appl. Toxicol., 2010, 31, 285–311 CrossRef PubMed.
- L. Sano, A. Krueger and P. Landrum, Aquat. Toxicol., 2005, 71, 283–296 CrossRef CAS PubMed.
- H. Leung, Ecotoxicol. Environ. Saf., 2001, 49, 26–39 CrossRef CAS PubMed.
- T. Tišler and J. Zagorc-Končan, Water, Air, Soil Pollut., 1997, 97, 315–322 Search PubMed.
- J. Cooper, Ecotoxicol. Environ. Saf., 1988, 16, 65–71 CrossRef CAS.
- J. M. Brausch and G. M. Rand, Chemosphere, 2011, 82, 1518–1532 CrossRef CAS PubMed.
- M. M. Schultz, S. E. Bartell and H. L. Schoenfuss, Arch. Environ. Contam. Toxicol., 2012, 63, 114–124 CrossRef CAS PubMed.
- E. J. Routledge, J. Parker, J. Odum, J. Ashby and J. P. Sumpter, Toxicol. Appl. Pharmacol., 1998, 153, 12–19 CrossRef CAS PubMed.
- T. Kawanai, Environ. Toxicol. Pharmacol., 2011, 32, 417–422 CrossRef CAS PubMed.
- M. Garcia, E. Campos, J. Sanchez-Leal and I. Ribosa, Chemosphere, 1999, 38, 3473–3483 CrossRef CAS.
- M. Qu and S. Bhattacharya, Biotechnol. Bioeng., 1997, 55, 727–736 CrossRef CAS.
- R. Boethling, Water Res., 1984, 18, 1061–1076 CrossRef CAS.
- S. V. Lehmann, U. Hoeck, J. Breinholdt, C. E. Olsen and B. Kreilgaard, Contact Dermatitis, 2006, 54, 50–58 CrossRef CAS PubMed.
- L. V. Dunkerton, E. J. Fendler and R. A. Williams, in Chemistry and Manufacture of Cosmetics, ed. M. L. Schlossman, 3rd edn, 2002, vol. 3Bk. 1, pp. 171–241 Search PubMed.
- L. Tollefson and B. E. Karp, Méd. Mal. Infect., 2004, 34, 514–521 CrossRef PubMed.
- H. L. DuPont and J. H. Steele, Clin. Infect. Dis., 1987, 9, 447–460 CrossRef CAS PubMed.
- O. E. Heuer, H. Kruse, K. Grave, P. Collignon, I. Karunasagar and F. J. Angulo, Clin. Infect. Dis., 2009, 49, 1248–1253 CrossRef PubMed.
- R. G. Ledder, P. Gilbert, C. Willis and A. J. McBain, J. Appl. Microbiol., 2006, 100, 1132–1140 CrossRef CAS PubMed.
- R. A. Carnevale, Méd. Mal. Infect., 2005, 35, 105–106 CrossRef PubMed.
- T. M. Wassenaar, Crit. Rev. Microbiol., 2005, 31, 155–169 CrossRef CAS PubMed.
- P. M. Shah, V. Schäfer and H. Knothe, Vet. Microbiol., 1993, 35, 269–274 CrossRef CAS.
- R. Tsao and T. Zhou, in New Biocides Development, ACS Symposium Series 967, 2008, pp. 364–387 Search PubMed.
- A. Varvaresou, S. Papageorgiou, E. Tsirivas, E. Protopapa, H. Kintziou, V. Kefala and C. Demetzos, Int. J. Cosmet. Sci., 2009, 31, 163–175 CrossRef CAS PubMed.
- I. Muñoz, K. Flury, N. Jungbluth, G. Rigarlsford, L. M. i. Canals and H. King, Int. J. Life Cycle Assess., 2014, 19, 109–119 CrossRef.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC.
- C. Capello, G. Wernet, J. Sutter, S. Hellweg and K. Hungerbühler, Int. J. Life Cycle Assess., 2009, 14, 467–479 CrossRef CAS PubMed.
- LVP Coalition White Paper: Low Vapor Pressure (LVP) Compounds, http://www.nationalaerosol.com/wp-content/uploads/2013/03/LVP-Coalition-White-Paper-2012.pdf, Accessed 1 April 2014, 2014.
- E. M. Petrie, Met. Finish., 2011, 109, 33–36 CrossRef CAS.
- S. K. Spear, S. T. Griffin, K. S. Granger, J. G. Huddleston and R. D. Rogers, Green Chem., 2007, 9, 1008–1015 RSC.
- K. Srinivas, T. M. Potts and J. W. King, Green Chem., 2009, 11, 1581–1588 RSC.
- M. Bandres, P. d. Caro, S. Thiebaud-Roux and M.-E. Borredon, C. R. Chim., 2011, 14, 636–646 CrossRef CAS PubMed.
- C. Leibig, B. Mullen, T. Mullen, L. Rieth and V. Badarinarayana, in Renewable and Sustainable Polymers, ACS Symposium Series 1063, ed. G. F. Payne and P. B. Smith, 2011, pp. 111–116 Search PubMed.
- V. Pace, P. Hoyos, L. Castoldi, P. D. d. María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef CAS PubMed.
- D. Fegyverneki, L. Orha, G. Láng and I. T. Horváth, Tetrahedron, 2010, 66, 1078–1081 CrossRef CAS PubMed.
- I. T. Horváth, H. Mehdi, V. Fábos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238–242 RSC.
- T. M. Lammens, J. e. Potting, J. P. M. Sanders and I. J. M. D. Boer, Environ. Sci. Technol., 2011, 45, 8521–8528 CrossRef CAS PubMed.
- A. Wolfson, C. Dlugy and Y. Shotland, Environ. Chem. Lett., 2007, 5, 67–71 CrossRef CAS.
- Y. Gu and F. Jérôme, Green Chem., 2010, 12, 1127–1138 RSC.
- A. E. Diaz-Alvarez, J. Francos, P. Croche and V. Cadierno, Curr. Green Chem., 2014, 1, 51–65 CrossRef CAS.
- J. I. García, H. García-Marín, J. A. Mayoral and P. Pérez, Green Chem., 2010, 12, 426–434 RSC.
- A. E. Diaz-Alvarez, J. Francos, B. Lastra-Barreira, P. Crochet and V. Cadierno, Chem. Commun., 2011, 47, 6208–6227 RSC.
- J. I. Garcia, H. Garcia-Marin and E. Pires, Green Chem., 2014, 16, 1007–1033 RSC.
- B. Mira, M. Blasco, A. Berna and S. Subirats, J. Supercrit. Fluids, 1999, 14, 95–104 CrossRef CAS.
- A. Farhat, A.-S. Fabiano-Tixier, M. E. Maataoui, J.-F. Maingonnat, M. Romdhane and F. Chemat, Food Chem., 2011, 125, 255–261 CrossRef CAS PubMed.
- G. Roberts, Life Cycle Assessment - Renewable and Sustainable Citrus Oils Final Report, Renewable Citrus Products Association, 2012, http://renewablecitrus.org/LCA.
- Life Cycle Impact of Soybean Production and Soy Industrial Products, prepared for The United Soybean Board by Omni Tech International, 2010, http://www.nescaum.org/documents/stakeholder-comments-on-the-economic-analysis-of-the-northeast-mid-atlantic-low-carbon-fuel-standard-draft-data-and-assumptions-part-i/comments-from-national-biodiesel-board/soy-life-cycle-profile_report.pdf/view.
- Greenhouse Gas and Energy Life Cycle Assessment of Pine Chemicals Derived from Crude Tall Oil and Their Substitutes, American Chemical Council and Franklin Associates, 2013, http://www.americanchemistry.com/Media/PressReleasesTranscripts/ACC-news-releases/New-Study-Says-Diverting-Pine-Chemistry-Co-Products-to-Biofuel-Does-Not-Further-Reduce-GHG.html.
- S. A. Miller, A. E. Landis and T. L. Theis, Environ. Sci. Technol., 2007, 5176–5182 CrossRef CAS.
- P. G. Jessop, Green Chem., 2011, 13, 1391–1398 RSC.
- Y. Gu and F. Jerome, Chem. Soc. Rev., 2013, 42, 9550–9570 RSC.
- F. M. Kerton and R. Marriott, Alternative Solvents for Green Chemistry, RSC Publishing, Cambridge, UK, 2nd edn, 2013 Search PubMed.
- DfE Screen for Solvents in Cleaning Products, U.S. Environmental Protection Agency, Washington, 2009, http://www.epa.gov/dfe/pubs/projects/gfcp/dfe_screen_for_solvents_in_cleaning_products_february2009.pdf.
- OECD Guidelines for Testing of Chemicals, Test No. 415: One-Generation Reproduction Toxicity Study, http://www.oecd-ilibrary.org/environment/test-no-415-one-generation-reproduction-toxicity-study_9789264070844-en, Accessed 6 March 2014, 2014.
- OECD Guidelines for Testing of Chemicals, Test No. 416: Two-Generation Reproduction Toxicity Study, http://www.oecd-ilibrary.org/environment/test-no-416-two-generation-reproduction-toxicity_9789264070868-en, Accessed 6 March 2014, 2014.
- Anonymous, Amine selection for metalworking applications, in CMF Plus, Society of Tribologists and Lubrication Engineers, 2003, vol. 59(9), pp. 24–26 Search PubMed.
- The Complete Technology Book on Detergents, NIIR Board of Consultants Engineers, 2013, http://www.niir.org/books/book/complete-technology-book-on-detergents-2nd-revised-edition-niir-board-consultants-engineers/isbn-9789381039199/zb,5c,a,0,0,a/index.html.
- H. A. Wittcoff, B. G. Reuben and J. S. Plotkin, Industrial Organic Chemicals, John Wiley & Sons, Hoboken, NJ, 2013 Search PubMed.
- PRODCOM - Statistics by Product, Eurostat, European Commission, http://ec.europa.eu/eurostat/web/prodcom/overview, Accessed 12 Jan 2015, 2015.
- SCCS, (Scientific Committee on Consumer Safety), Opinion on Nitrosamines and Secondary Amines in Cosmetic Products, 2012, http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_090.pdf.
- SCCNFP, Scientific Committee for Cosmetics and Non-Food Products of the European Commission: Opinion concerning Dialkyl- and Dialkanolamines and their salts in cosmetic products adopted by the SCCNFP during the 17th plenary meeting of 12 June 2001, http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/sccnfp_opinions_97_04/sccp_out144_en.htm, Accessed 9 March 2014, 2014.
- A survey of cosmetic and certain other skin-contact products for N-nitrosamines, Department of Trade and Industry, UK, 1998.
- G. Eisenbrand, in The Significance of N-Nitrosation of Drugs, ed. G. Eisenbrand, G. Bozler and H. v. Nicolai, Gustav Fischer Verlag, Stuttgart, 1990, pp. 47–68 Search PubMed.
- S. S. Mirvish, Toxicol. Appl. Pharmacol., 1975, 31, 325–351 CrossRef CAS.
- A. R. Jones, W. Lijinsky and G. M. Singer, Cancer Res., 1974, 34, 1079–1081 CAS.
- D. L. H. Williams, Nitrosation reactions in the chemistry of nitric oxide, Elsevier, Amsterdam, 2004 Search PubMed.
- R. N. Loeppky, S. P. Singh, S. Elomari, R. Hastings and T. E. Theiss, J. Am. Chem. Soc., 1998, 120, 5193–5202 CrossRef CAS.
- R. N. Loeppky, R. Hastings, J. Sandbothe, D. Heller, Y. Bao and D. Nagel, in Relevance of Human Cancer of N-Nitroso Compounds, Tobacco Smoke, and Mycotoxins, ed. I. K. O'Neill, J. Chen and H. Bartsch, International Agency for Research on Cancer, Lyon, France, 1991, pp. 244–252 Search PubMed.
- R. N. Loeppky and W. Tomasik, J. Org. Chem., 1983, 48, 2751–2756 CrossRef.
- R. N. Loeppky, G. Shevlin and L. Yu, in Significance of N-Nitrosation of Drugs, ed. G. Eisenbrand, G. Bolzer and H. V. Nicolai, Gustav Fischer Verlag, New York, 1990 Search PubMed.
- H. Yano, A. Noda, T. Hukuhara and K. Miyazawa, J. Am. Oil Chem. Soc., 1997, 74, 891–893 CrossRef CAS PubMed.
- B. Scheuer, Hautarzt., 1983, 34, 126–129 CAS.
- H. Lessmann, W. Uter, A. Schnuch and J. Geier, Contact Dermatitis, 2009, 60, 243–255 CrossRef PubMed.
- Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products, European Union, 2009, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF.
- ASTM A380/A380M - 13: Standard Practice for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems, ASTM International, West Conshohocken, PA.
- Chemical Economics Handbook: Chelating Agents, IHS, 2013, https://www.ihs.com/products/chelating-agents-chemical-economics-handbook.html.
- Cosmetic Ingredient Review Expert Panel, Int. J. Toxicol., 2002, 21, 95–142 CrossRef PubMed.
- M. D. Reuber, J. Pathol., 1969, 97, 335–338 CrossRef CAS PubMed.
- R. C. Srivastava, P. P. Dwivedi, J. R. Behari and M. Athar, Toxicol. Lett., 1986, 32, 37–40 CrossRef CAS.
- H. Bolton, S. W. Li, D. J. Workman and D. C. Girvin, J. Environ. Qual., 1993, 22, 125–132 CrossRef CAS.
- A.-S. Allard, L. Renberg and A. H. Neilson, Chemosphere, 1996, 33, 577–583 CrossRef CAS.
- A. C. Alder, H. Siegrist, W. Gujer and W. Giger, Water Res., 1990, 24, 733–742 CrossRef CAS.
- B. Nowack, H. Xue and L. Sigg, Environ. Sci. Technol., 1997, 31, 866–872 CrossRef CAS.
- J. G. Lin and S. Y. Chen, Toxicol. Environ. Chem., 1998, 67, 511–529 CrossRef CAS.
- D. Kołodyńska, in Expanding Issues in Desalination, ed. R. Y. Ning, InTech, 2011, DOI:10.5772/21180, ch. 17.
- R. A. Goyer, H. L. Falk, M. Hogan, D. D. Feldman and W. Richter, J. Natl. Cancer Inst., 1981, 66, 869–880 CAS.
- A. E. Martell and R. M. Smith, Critical Stability Constants, Plenum Press, New York, 1977 Search PubMed.
- N. J. Dixon, in Handbook of Green Chemistry, Vol. 9: Designing Safer Chemicals, ed. R. Boethling and A. Voutchkova, Wiley-VCH, Weinheim, Germany, 2012 Search PubMed.
- Design for the Environment Program Criteria for Chelating and Sequestering Agents, Office of Pollution Prevention & Toxics, U.S. Environmental Protection Agency, 2010.
- B. J. Brotherton, Boron: Inorganic Chemistry, in Encyclopedia of Inorganic Chemistry, ed. R. B. King, John Wiley & Sons, 1994 Search PubMed.
- Household Products Database, U.S. Department of Health & Human Services, Bethesda, Maryland, http://householdproducts.nlm.nih.gov, Accessed 27 July 2014, 2014.
- M. A. Angulo and J. Robert and D. Crangle, 2010 Minerals Yearbook: Boron (Advanced Release), U.S. Geological Survey, 2011 Search PubMed.
- J. Emsley, The Elements, Oxford University Press, Oxford, 3rd edn, 1998 Search PubMed.
- T. Matoh, Plant Soil, 1997, 193, 59–70 CrossRef CAS.
- P. H. Brown, N. Bellaloui, M. A. Wimmer, E. S. Bassil, J. Ruiz, H. Hu, H. Pfeffer, F. Dannel and V. Römheld, Plant Biol., 2002, 4, 205–223 CrossRef CAS.
- R. Reid, Plant Sci., 2010, 178, 9–11 CrossRef CAS PubMed.
- Opinion on sodium perborate and perboric acid, Scientific Committee on Consumer Safety (SCCS), 2010, http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_031.pdf.
- C. J. Price, P. L. Strong, M. C. Marr, C. B. Myers and F. J. Murray, Fundam. Appl. Toxicol., 1996, 32, 179–193 CrossRef CAS.
- F. Zonfrilli, S. Germanà and V. Guida, Chem. Eng. Trans., 2009, 17, 705–710 Search PubMed.
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107, 907–938 CrossRef CAS.
- Anonymous, “In every sense”, IFRA (International Fragrance Association) http://www.ifraorg.org, Accessed 29 March 2014.
- Design for the Environment: Screen for Fragrances Human Health Criteria, EPA, 2009, http://www.epa.gov/dfe/pubs/projects/gfcp/dfe_screen_for_fragrances_human_health_criteria_version_1.pdf.
- SCCS (Scientific Committee on Consumer Safety), Opinion on fragrance allergens in cosmetic products, European Commission, 2012, http://ec.europa.eu/health/scientific_committees/opinions_layman/perfume-allergies/en/about-perfume-allergies.htm.
- Perfume Allergies, European Commission, http://ec.europa.eu/health/scientific_committees/opinions_layman/perfume-allergies/en/index.htm - il1, Accessed 28 March 2014, 2014.
- C. G. Dariva and A. F. Galio, in Developments in Corrosion Protection, ed. M. Aliofkhazraei, INTECH, 2014, ch. 16 Search PubMed.
- G. H. Koch, P. H. Brongers, N. G. Thompson, Y. P. Virmani and J. H. Payer, FHWA-RD-01-156 Corrosion Costs and Prevention Strategies in the United States, Federal Highway Administration, Washington, DC, 2002, http://trid.trb.org/view.aspx?id=707382 Search PubMed.
- H. A. Webster, Can we afford to ignore corrosion?, http://www.westcoastcorrosion.com/Papers/10102 Ignore Corrosion.pdf.
- P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113–116 CrossRef CAS PubMed.
- S. Papavinasam, Corrosion Control in the Oil and Gas Industry, Gulf Professional Publishing, Amsterdam, 2013 Search PubMed.
- H. L. Sanders, J. Am. Oil Chem. Soc., 1958, 35, 548–551 CrossRef.
- K.-Y. Lai and N. Dixit, in Foams: Theory, Measurements, and Applications, CRC Press, 1995, ch. 8, pp. 315–338 Search PubMed.
- B. Gutsche and A. Behler, in Handbook of Detergents, Part F: Production, ed. U. Zoller and P. Sosis, CRC Press, Boca Raton, FL, 2008, pp. 239–246 Search PubMed.
- Z. U. Ed, Handbook of Detergents, Part E: Applications, CRC Press, Boca Raton, FL, 2008 Search PubMed.
- P. Foley, A. Kermanshahipour, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518 RSC.
- Surfactants from Renewable Resources, ed. M. Kjellin and I. Johansson, Wiley, Chichester, UK, 2010 Search PubMed.
- J. Guilbot, S. Kerverdo, A. Milius, R. Escola and F. Pomrehn, Green Chem., 2013, 15, 3337–3354 RSC.
- CESIO Statistics 2012, CESIO, Cefic Sector Group, http://www.cefic.org/Documents/Industrysectors/CESIO/CESIO-Statistics-2012.pdf.
- F. Md, J. Pet. Environ. Biotechnol., 2012, 3, 124 CAS.
- I. M. Banat, A. Franzetti, I. Gandolfi, G. Bestetti, M. G. Martinotti, L. Fracchia, T. J. Smyth and R. Marchant, Appl. Microbiol. Biotechnol., 2010, 87, 427–444 CrossRef CAS PubMed.
- Y. Xu, T. Zhang, X. Li, L. Chen and H. Wang, Adv. Mater. Res., 2012, 550–553, 1124–1127 CAS.
- A. Lani, Nonylphenol and Nonylphenol Ethoxylates; Chapter 883 Basis Statement, Bureau of Remediation and Waste Management, Maine Department of Environmental Protection, 2010, http://www.maine.gov/dep/safechem/rules/nonylphenol%20_support_document_final.pdf.
- A. C. Nimrod and W. H. Benson, Crit. Rev. Toxicol., 1996, 26, 335–364 CrossRef CAS PubMed.
- ASTM D97 - 12. Standard Test Method for Pour Point of Petroleum Products, ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants.
- G. D. Smith, J. Am. Oil Chem. Soc., 1979, 56, 87–90 CrossRef CAS.
- ASTM Standard D39, Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester, ASTM International, West Conshohocken, PA.
- J. Ross and G. D. Miles, Oil Soap, 1941, 18, 99–102 CAS.
- E. J. Burcik, J. Colloid Sci., 1950, 5, 421–436 CrossRef CAS.
- K. Radke, S. Champ, E. Pfrommer and S. Schulte, SOFW J., 2008, 134, 2–6 CAS.
- E. Gomez, M. Bachelot, C. Boillot, D. Munaron, S. Chiron, C. Casellas and H. Fenet, Environ. Sci. Pollut. Res., 2012, 19, 2561–2569 CrossRef CAS PubMed.
- N. Bluthgen, N. Meili, G. Chew, A. Odermatt and K. Fent, Sci. Total Environ., 2014, 476–477, 207–217 CrossRef CAS PubMed.
- D. Kaiser, A. Sieratowicz, H. Zielke, M. Oetken, H. Hollert and J. Oehlmann, Environ. Pollut., 2012, 163, 84–90 CrossRef CAS PubMed.
- V. A. Sakkas, D. L. Giokas, D. A. Lambropoulou and T. A. Albanis, J. Chromatogr., A, 2003, 1016, 211–222 CrossRef CAS.
- P. Y. Kunz and K. Fent, Aquat. Toxicol., 2006, 79, 305–324 CrossRef CAS PubMed.
- K. Fent, P. Y. Kunz and E. Gomez, Chimia, 2008, 62, 368–375 CrossRef CAS.
- R. M. Blair, H. Fang, W. S. Branham, B. S. Hass, S. L. Dial, C. L. Moland, W. Tong, L. Shi, R. Perkins and D. M. Sheehan, Toxicol. Sci., 2000, 54, 138–153 CrossRef CAS PubMed.
- OECD Guidelines for the Testing of Chemicals: Test No. 229: Fish Short Term Reproduction Assay, OECD, 2012, http://www.oecd-ilibrary.org/environment/test-no-229-fish-short-term-reproduction-assay_9789264185265-en;jsessionid=1krntpaepsfp1.x-oecd-live-01.
- OECD Guidelines for the Testing of Chemicals, Test No. 230: 21-day Fish Assay, OECD, 2009, http://www.oecd-ilibrary.org/environment/test-no-230-21-day-fish-assay_9789264076228-en.
- OECD Guidelines for the Testing of Chemicals, Test No. 234: Fish Sexual Development Test, OECD, 2011, http://www.oecd-ilibrary.org/environment/test-no-234-fish-sexual-development-test_9789264122369-en.
Footnote |
| † Common inorganic bases such as NaOH are kosmotropes and therefore tend to decrease the solubility of organic components in an aqueous formulation unless the formulation is dilute. Decreased solubility can cause phase separations if the temperature varies during shipping or storage. Organic bases like TEA are chaotropes and therefore can supply the required basicity at high concentrations without causing unwanted phase separations. |
| This journal is © The Royal Society of Chemistry 2015 |