Ultrastrong composites from dopamine modified-polymer-infiltrated colloidal crystals†
F.
Liaqat
a,
M. N.
Tahir
*a,
H.
Huesmann
a,
P.
Daniel
a,
M.
Kappl
b,
G. K.
Auernhammer
b,
D.
Schneider
b,
I.
Lieberwirth
b,
K.
Char
c,
G.
Fytas
bd,
H.-J.
Butt
b and
W.
Tremel
*a
aInstitut für Anorganische Chemie und Analytische Chemie, Johannes Gutenberg-Universität, Duesbergweg 10-14, 55099 Mainz, Germany. E-mail: tremel@uni-mainz.de; tahir@uni-mainz.de; Fax: +49-6131-39-25605; Tel: +49-6131-39-25135
bMax Planck-Institut für Polymerforschung, Ackermannweg 10, 55128 Mainz, Germany
cSchool of Biological and Chemical Engineering, Center for Functional Polymer Thin Films, Seoul National University, Seoul 151-744, South Korea
dDepartment of Materials Science, University of Crete and IESL/FORTH, 71110 Heraklion, Greece
First published on 26th May 2015
Abstract
Although strong and stiff synthetic composites have long been developed, the microstructure of today's most advanced composites has yet to achieve the sophisticated hierarchy of hybrid materials built up by living organisms. We have assembled hard and tough multilayered nanocomposites, which contain alternating layers of Fe3O4 nanoparticles and a 3-hydroxy-tyramine (dopamine) substituted polymer (dopamine modified polymer), strongly cemented together by chelation through infiltration of the polymer into the Fe3O4 mesocrystal. With a Young's modulus of 17 ± 3 GPa and a hardness of 1.3 ± 0.4 GPa the nanocomposite exhibits high resistance against elastic as well as plastic deformation. Key features leading to the high strength are the strong adhesion of the polymer to the inorganic nanoparticles and the layered assembly.
Conceptual insightsFinding pathways to synthetic analogs of nacre or bones represents a fundamental milestone in the development of composite materials. The brick and- mortar arrangement of inorganic and organic layers is believed to be the most essential strength- and toughness-determining structural feature of nacre. It has also been found that the crosslinking by tightly folded macromolecules is equally important. When the binding of this polymer is weak, flexible composites are obtained, whereas strongly binding polymers may lead to hard composites. Here we demonstrate that both structural features of nacre can be reproduced by sequential deposition of Fe3O4 nanoparticles and “sticky” polymers. This simple process leads to a nanoscale analogue of nacre with alternating inorganic and organic layers, where the polymer infiltrates the Fe3O4 mesocrystal, thereby cementing together the individual particles by chelation. The structural and functional resemblance makes the particle–polymer composite a close replica of natural biocomposites whose nanoscale nature enables elucidation of molecular processes occurring under stress. |
Introduction
Many biomaterials combine disparate properties such as exceptional strength, toughness, and extensibility with tenability, mutability, and a functionality that is unmatched by most man-made materials.1–3 This functionality emerges often from simple and abundant constituents that allow the adaptation to changing environmental conditions. Still, many constituents used in biology are in terms of their intrinsic properties inferior to modern synthetic materials, because energy as well as material quality and availability are limited. Nacre from seashells is a textbook example showing how evolution can lead to a high performance material by blending intrinsically weak constituents, CaCO3 (aragonite) and collagen-related biopolymers3,4 in a composite. The excellent performance of many biomaterials originates from a combination of hard and soft building blocks in a multi-level, hierarchical structure. A hard mineral component serves as the reinforcing part and the soft biopolymer allows dissipating energy. The morphology of the mineral blocks and chemical bonding between them provides a physicochemical basis for stiffness and flexibility at multiple length scales, leading to an increased robustness against catastrophic materials failure.5,6Several efforts have been made in the past to mimic nature in the fabrication of synthetic bio-nanomaterials7–11 but current materials still suffer from severe shortcomings. The fabrication of artificial shell materials with their intricate microstructure is a challenge that requires both the design of optimum microstructures and the development of fabrication procedures to implement these designs. Various methods have been employed to build these hetero-structures, e.g. atomic layer deposition to infiltrate the inner protein structures of biomaterials (e.g. spider silk) with metals,11 freeze-casting,12,13 or electrophoretic deposition.14 However, these synthetic engineering techniques are time-consuming, expensive, and plagued with problems of agglomeration of nanoparticles and complex fabrication. Ordered multilayered organic–inorganic hybrid structures have been obtained via a simple dip-coating approach15 or through layer-by-layer assembly of alternating hard and soft components16,17 to obtain composites based on repeated calcium carbonate crystallization onto a film of charged molecules. The structural and physical properties of composites can be modified by varying (i) the polymer, (ii) the hard–mineral components, (iii) the interactions between the components, or (iv) the architecture.18,19 The individual components in the hierarchy have to be held together by a binding force such as in nacre, where the inorganic constituents have a micro-scale brick wall architecture cemented by thin layers of protein holding the bricks together. When the binding of this “cement” is weak, flexible composites are obtained,18 whereas strongly binding polymers should lead to strong and hard composites.5
In order to have an efficient and stable assembly of nanoparticles in a polymer matrix, it is important for the polymer to have functional groups that bind strongly to the nanoparticle surface.20 Nature provides inspiration for such binding groups; marine mussels are able to stick to wet surfaces with the aid of specialized adhesion proteins with a high content of the catechole group containing molecule domanine.21–23 Both natural and synthetic adhesives containing catechol show strong adhesion properties.22–24 In this work, we highlight the concept of a bio-inspired strong composite with a multilayer structure based on Fe3O4 nanoparticles (ξ ≈ 4 nm) and a dopamine modified polymer (Mw = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).
000 g mol−1).
Experimental
General
The dopamine modified polymer–Fe3O4 multilayer composite was built up on glass substrates using a Laurell WS-400-6NPP-LITE spin-coater. The size of the metal oxide nanoparticles was determined by transmission electron microscopy (TEM) on a Phillips EM-420 equipped with a slow scan CCD detector (1 k × 1 k) and a LaB6 electron gun operated with an acceleration voltage of 120 kV. The TEM images were processed with the Gnu Image Manipulation Program GIMP Version 2.6.8 or with Image J Version 1.43u. TEM imaging of the multilayers of dopamine modified polymer–Fe3O4 was performed by preparing a lamella using Focused Ion Beam (FIB) (FEI Nova 600 Nanolab FIB instrument). The lamella was fixed on TEM grid and eventually analyzed by electron energy-dispersive X-ray analysis (EDX) using a transmission electron microscope (Technai G2 F20; FEI/Philips) equipped with an EDX detector. A Horiba Jobin Y LabRAM HR spectrometer with a frequency doubled Nd:YAG-laser was used for performing Raman spectroscopy of the multilayered films. The sample was prepared by cutting a small portion (1 cm × 1 cm) of the layered assemblies prepared on a glass substrate. The AFM images of the multilayers were taken using a commercial AFM instrument (Multimode Nanoscope IIIa controller, Veeco, California, USA) in tapping mode. A piezoelectric scanner allowed a maximum x, y-scan size of 17 μm and a maximum z-extension of 3.9 μm. All topography and phase contrast images were taken at room temperature under ambient conditions. The Young's modulus and hardness of the multilayer films was determined by nanoindentation using a MFP Nanoindenter (Asylum Research, Santa Barbara, CA) equipped with a diamond Berkovich indenter.The 16 bilayers of dopamine modified polymer–Fe3O4 coated on glass substrate were analyzed by Brillouin light scattering (BLS) to get a comparative simultaneous estimation of the elastic modulus. BLS is a non-destructive and non-contact technique to probe the propagation of thermally activated propagating phonons at hypersonic frequencies (GHz).25 BLS makes use of the photo-elastic interaction between incident photons with wave vector (ki) and thermally excited phonons, so that the scattering vector (q) is defined by q = ks− ki where ks is the wave vector of the scattered photon. The energy of the phonon is defined by the frequency shift of inelastically scattered light of monochromatic (532 nm) laser beam and the small Brillouin shifts are resolved by an actively stabilized tandem FP interferometer. The accumulation times for the spectra in thin composites range from 12 to 24 h. In this work, the transmission and reflection geometry are used to obtain frequency as a function of wave vector in plane (qpara) and out of plane (qperp) of the multilayer film, respectively.
Materials
Unless noted otherwise, the precursors for the synthesis of nanoparticles and dopamine modified polymer were purchased from Aldrich, Acros and other commercial suppliers and used as received.Synthesis of dopamine modified polymer
The poly(active ester) poly(pentafluoro-phenylacrylate) (PFA) was prepared as reported earlier.26 Gel permeation chromate-graphy (GPC) analysis of the obtained polymer (tetrahydrofuran, light scattering detection) gave the following values: number average molar mass, Mn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 390 g mol−1, with a poly dispersity index, PDI = 1.39, having 70 repeating units on average. This pre-polymer is used for the synthesis of multifunctional poly(acrylamide).
390 g mol−1, with a poly dispersity index, PDI = 1.39, having 70 repeating units on average. This pre-polymer is used for the synthesis of multifunctional poly(acrylamide).
The poly(active ester) PFA (700 mg, 2.94 mmol repeating units) was dissolved in a mixture of 12 mL of dry dimethylformamide (DMF) and 0.5 mL of triethylamine. The next step was adding 3-hydroxytyramine hydrochloride (555 mg) dissolved in 3 mL of DMF and 0.5 mL of trimethylamine to the mixture and the final contents were stirred for 6 hours at 50 °C. The solvent was evaporated using rotary evaporator and product was resolved in about 3 ml acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (3
water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and precipitated in water as illustrated in Scheme S2 (ESI†).
1) and precipitated in water as illustrated in Scheme S2 (ESI†).
The precipitated polymer was re-dissolved in a mixture of acetone (3 mL) and methanol (1 mL) and again precipitated in excess of water. The precipitated polymer was centrifuged (9000 rpm, 10 min and room temperature) and the solvent was decanted. The process was repeated until 800 mg of colorless solid polymer was obtained on drying.
Synthesis of iron oxide nanoparticles
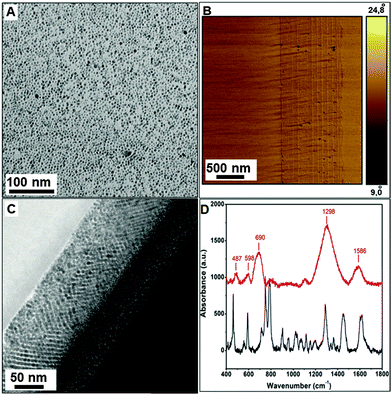
Iron oxide nanoparticles were synthesized following a procedure reported by Sun et al.27 with some alterations. Iron acetyl acetonate (2 mmol) was mixed in phenyl ether (20 mL), 1,2-hexadecanediol (10 mmol), oleic acid (6 mmol), and oleylamine (6 mmol) under argon. The reaction mixture was heated to 200 °C with a heating rate of 5 °C min−1. The contents were stirred at this temperature for 60 min followed by refluxing at 280 °C for another 30 min. The resulting dark brown mixture was cooled to room temperature and precipitated from the solution with ethanol. The resulting magnetite nanoparticles are monodisperse and have a size of ∼4 nm, as confirmed by transmission electron microscopy (TEM) (Fig. 2A).Fabrication of multilayers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The multilayers were assembled by consecutive spin coating of a dopamine modified polymer solution in dimethylacetamide (DMA) and a Fe3O4 nanoparticle dispersion in hexane, starting from the dopamine modified polymer layer due to its higher affinity to the substrate because of its adhesive character. The thickness of the layers was controlled by optimizing the concentration of the solutions to be spin-casted while the optimized spin-coating parameters were speed (5000 rpm), acceleration (5040 rpm s−1) and time (10 s). Each spin-coated layer was subjected to heat treatment at 120 °C for 20 min. The use of orthogonal solvents and an optimized heating rate is important to sustain the multilayered structure. The final multilayered structure of 12 alternating layers of polymer and Fe3O4 nanoparticles was considered for structural characterization and nanoindentation experiments (Fig. 2B and C). The characterization was carried out on several samples to ensure reproducibility of results. The multilayers showed structural colors (Fig. S7, ESI†) due to multilayer interference.
1). The multilayers were assembled by consecutive spin coating of a dopamine modified polymer solution in dimethylacetamide (DMA) and a Fe3O4 nanoparticle dispersion in hexane, starting from the dopamine modified polymer layer due to its higher affinity to the substrate because of its adhesive character. The thickness of the layers was controlled by optimizing the concentration of the solutions to be spin-casted while the optimized spin-coating parameters were speed (5000 rpm), acceleration (5040 rpm s−1) and time (10 s). Each spin-coated layer was subjected to heat treatment at 120 °C for 20 min. The use of orthogonal solvents and an optimized heating rate is important to sustain the multilayered structure. The final multilayered structure of 12 alternating layers of polymer and Fe3O4 nanoparticles was considered for structural characterization and nanoindentation experiments (Fig. 2B and C). The characterization was carried out on several samples to ensure reproducibility of results. The multilayers showed structural colors (Fig. S7, ESI†) due to multilayer interference.
Characterization
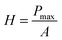 | (1) |
A model depicting nanoindentation of the multilayered polymer–Fe3O4 hybrid films is shown in Fig. S6 (ESI†). The strong crosslinking between the Fe3O4 nanoparticles and the multi-dentate polymer ligand help the matrix resist deformation and make the composites harder and stronger.
The multilayers of dopamine modified polymer–Fe3O4 nanoparticles prepared by dip-coating are measured by nanoindentation (Fig. S10, ESI†). However, these multilayers show a much lower elastic modulus of E ∼ 2 GPa compared to the spin-coated multilayers due to the unavoidable defects introduced during the fabrication procedure (Fig. S6B, ESI†).
Results and discussion
The multilayer nanocomposites were assembled by consecutive spin-coating a sol of Fe3O4 nanoparticles (600 mg in 8 mL hexane) and a 2% dopamine modified polymer solution in dimethyl-acetamide (DMA), respectively (Fig. 1). These multilayer Fe3O4–dopamine modified polymer nanocomposites consisting of six bilayers were deposited onto a cleaned (in acidic piranha solution of concentrated sulfuric acid and hydrogen peroxide, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
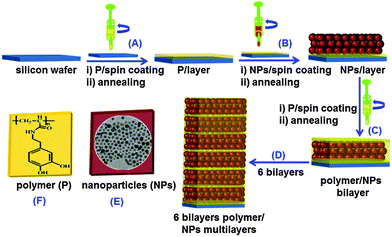
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) silicon wafer or glass substrate starting with the polymer layer (Fig. 1A). Spin coating of each layer was carried out for 10 s at a speed of 5000 rpm and an acceleration of 5040 rpm s−1. The dopamine modified polymer strongly binds to the Fe3O4 nanoparticles (Fig. 1B and C) due to the under coordinated surface atoms because of the redox-active behavior of the 1,2-dioxolene groups and the resulting covalency cause a high affinity of catechol groups to the Fe surface sites.29 Each layer was heated to 120 °C for 20 min to allow for solvent evaporation. The cycle was repeated until a nanocomposite consisting of 12 layers was obtained (Fig. 1D). The resulting multilayer nanocomposites were stable for extended periods of time (∼8 months). The polymer seeps through the interstices of the layers of close-packed Fe3O4 nanoparticles (Fig. 1C), where it cross-links different Fe3O4 nanoparticles thereby forming a highly coordinated network.
1 v/v) silicon wafer or glass substrate starting with the polymer layer (Fig. 1A). Spin coating of each layer was carried out for 10 s at a speed of 5000 rpm and an acceleration of 5040 rpm s−1. The dopamine modified polymer strongly binds to the Fe3O4 nanoparticles (Fig. 1B and C) due to the under coordinated surface atoms because of the redox-active behavior of the 1,2-dioxolene groups and the resulting covalency cause a high affinity of catechol groups to the Fe surface sites.29 Each layer was heated to 120 °C for 20 min to allow for solvent evaporation. The cycle was repeated until a nanocomposite consisting of 12 layers was obtained (Fig. 1D). The resulting multilayer nanocomposites were stable for extended periods of time (∼8 months). The polymer seeps through the interstices of the layers of close-packed Fe3O4 nanoparticles (Fig. 1C), where it cross-links different Fe3O4 nanoparticles thereby forming a highly coordinated network.
The Fe3O4 nanoparticles (Fig. 1E) were synthesized with little modifications as reported by Sun et al.27 Briefly, iron acetylacetonate (2 mmol) was mixed in phenyl ether (20 mL) with 1,2-hexadecanediol (10 mmol), oleic acid (6 mmol), and oleylamine (6 mmol) under argon atmosphere and heated to 200 °C with a heating rate of 5 °C min−1. The mixture was stirred at 200 °C for 60 min followed by increasing the temperature to 240 °C for another 30 min. The polymer was synthesized as described previously.30 It is based on a pre-polymer (active ester polymers based on pentafluorophenylacrylates)26 that contains active ester side groups which can be specifically substituted using primary amine group. This pre-polymer was transformed into the dopamine modified polymer (Fig. 1F and 2A) by a 100% replacement of the active ester groups with catechol anchor groups. A schematic representation of pre-polymer and dopamine modified polymer is shown in Schemes S1 and S2 (ESI†). The polymer poly(pentafluorophenyl acrylate) and the dopamine modified polymer were characterized using 1H and 19F NMR spectroscopy (Fig. S1–S3, ESI†).31 Various investigations have shown the dopamine modified polymer to bind strongly to 3d transition metals.32
An important factor is the solvent mismatch between the organic and inorganic films. Miscible solvents do not allow building-up a phase-separated multilayer but lead rather to a composite mixture. Therefore, the nanoparticles were dispersed in a low-boiling solvent (such as hexane) that is immiscible with DMA, which is the solvent required for a clear polymer solution. Another essential feature is the heat treatment after each coating. It has been observed that the spin coating procedure does not yield multilayers without any repetitive heat treatment but rather yields a blend. Additionally, fast heating or high temperatures lead to the formation of cracks and non-uniform structural films. These parameters were carefully optimized to ensure a smooth assembly of multilayer nanocomposites. For less than 6 BLs, it was difficult to perform nanoindentation measurments due to the low film thickness. In contrast more than 6 BLs the first defects in the layers appear, and therefore the mechanical properties deteriorate. For a multilayered (dopamine modified polymer–Fe3O4)6 nanocomposite (where the subscript 6 denotes the number of bilayers) atomic force microscopy (AFM) images (Fig. 2B) show the presence of uniform periodic layers of the soft organic (light) and hard inorganic material (dark). The thickness of the polymer layer was ≈4 nm. The polymer infiltrated into the Fe3O4 nanoparticle layers, leading to a dense cross-linked structure, as apparent from the high-resolution transmission electron microscope (HRTEM) image (Fig. 2C). A long range ordering was also observed from the TEM image, and it appears to contribute significantly to the remarkable mechanical properties of the nanocomposite. The complexing ability of the catechol ligand greatly enhances the mechanical properties and strength of the hybrids,33 and the choice of Fe3O4 nanoparticles as the inorganic constituent is a further strength booster owing to the well-documented coordination chemistry of iron with catechol.34
Raman studies of the multilayered composite confirmed that the binding of the catechol units to the metal oxide is the major binding force that lends strength to the hybrid material. The Raman spectrum of the as-prepared polymer (Fig. 2D) displays a strong band at 1629 cm−1 due to OH deformation modes of the hydroxyl groups and weak bands at 1752 cm−1 and 1600 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH bending vibrations of the amide bond of the polymeric ligand. The intense C–O stretch of the phenolic OH is located at 1285 cm−1. Moreover, bands at 852 cm−1, 814 cm−1, 791 cm−1 are assigned to the C–O groups of 1,2,4 tri-substituted aromatic rings and the C
O and NH bending vibrations of the amide bond of the polymeric ligand. The intense C–O stretch of the phenolic OH is located at 1285 cm−1. Moreover, bands at 852 cm−1, 814 cm−1, 791 cm−1 are assigned to the C–O groups of 1,2,4 tri-substituted aromatic rings and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretch appears at 1503 cm−1. For the dopamine modified polymer–Fe3O4 composite, the ν(O–H) stretching modes between 470–700 cm−1 indicate the catechol–iron coordination. The most prominent bands can be assigned to the interaction of the transition metal atoms with the catechol ligand.35 The bands at 487, 598 cm−1 and 690 cm−1 are assigned specifically to the bidentate chelation of the metal ion by the phenolic oxygen atoms of the catechol units indicating catechol–metal complexation. A strong broad band centered at 1298 cm−1 (containing most of the vibrations related to the aromatic ring) confirms the catechol iron complexation. For comparison, the FTIR spectrum of the hybrid Bragg stack shows almost all prominent bands. Only the C–O ring vibrations are slightly shifted to lower wave numbers, and the bands assigned to the phenolic OH groups (broad band extending up to 3600 cm−1 and 1320 cm−1) are absent. A strong band centered at 664 cm−1 confirms the complexation of Fe3+ by dopamine (Fig. S4, ESI†).
C ring stretch appears at 1503 cm−1. For the dopamine modified polymer–Fe3O4 composite, the ν(O–H) stretching modes between 470–700 cm−1 indicate the catechol–iron coordination. The most prominent bands can be assigned to the interaction of the transition metal atoms with the catechol ligand.35 The bands at 487, 598 cm−1 and 690 cm−1 are assigned specifically to the bidentate chelation of the metal ion by the phenolic oxygen atoms of the catechol units indicating catechol–metal complexation. A strong broad band centered at 1298 cm−1 (containing most of the vibrations related to the aromatic ring) confirms the catechol iron complexation. For comparison, the FTIR spectrum of the hybrid Bragg stack shows almost all prominent bands. Only the C–O ring vibrations are slightly shifted to lower wave numbers, and the bands assigned to the phenolic OH groups (broad band extending up to 3600 cm−1 and 1320 cm−1) are absent. A strong band centered at 664 cm−1 confirms the complexation of Fe3+ by dopamine (Fig. S4, ESI†).
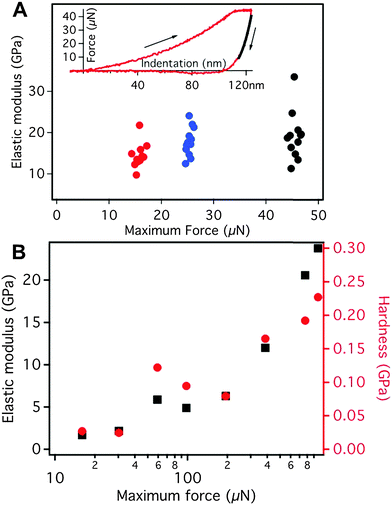
The dopamine modified polymer–Fe3O4 nanocomposite showed extraordinary mechanical strength with a Young's modulus of E = 17 ± 3 GPa and a hardness of H = 1.3 ± 0.4 MPa. It should be mentioned that the estimation of E requires the knowledge of the Poisson ratio ν, the value of which is assumed to be ν ∼ 0.3. However a variation of ν in the range of 0.2 to 0.4 had only a negligible effect (about 5% of the absolute value) on the elastic modulus and the hardness. Young's modulus and hardness were measured by nanoindentation using a MFP Nanoindenter (Asylum Research, Santa Barbara, CA) equipped with a diamond Berkovich indenter. Each series of indentations was done on a grid of 2 × 6 indents on a 90 × 90 μm2 area at three different positions on the sample, i.e., 36 indents per series. The Young's moduli and hardness were calculated by fitting the indentation curves according to the Oliver–Pharr method28 using the analysis software of the nanoindenter. The Young's modulus was obtained as the elastic response of the sample upon unloading, i.e., from the slope of the onset of the unloading curve. The hardness of the material was obtained as the ratio of maximum applied load divided by the indenter contact area at that load. It is indicative of the resistance of the material against plastic deformation. To exclude any contribution from the silicon substrate only indentation forces of less than 50 μN were used. In this range no measurable influence of the substrate on the elastic modulus could be observed (Fig. S5, ESI†).
In order to clarify the role of the dopamine-modified polymer in the polymer–particle interactions we conduct a control experiment with a composite film containing a polymer with a similar structure but without dopamine groups to compare the mechanical properties. To this end we prepared analogous samples using only iron oxide (Fe3O4) nanoparticles and iron oxide (Fe3O4) nanoparticles with PMMA (a polymer without any reactive sites) to see the effect of dopamine binding. After drying the sample the PMMA was in the glassy state. We performed a series of indentation experiments on this PMMA–Fe3O4 nanoparticle sample, varying systematically the load (from ∼10 μN to ∼80 μN) and time scale of the indentation (from 0.1 s to 10 s). Upon indentation, the PMMA-based sample showed a strong creeping behavior irrespective of the indenter shape (cube corner or Berkovic). This implies that even the smallest loads possible were beyond the yield stress of the PMMA-based sample. In the resulting for curves, no hint for the onset of the creeping behavior was found, i.e. the onset of creeping vanished in the noise of the instrument.
Several factors may explain the high mechanical strength of the dopamine modified polymer–Fe3O4 composites. (i) The catechol groups in the dopamine modified polymer form stable complexes with iron in natural environment with the degree of crosslinking varying with the dopamine and Fe3+ content and pH.36,37 It is worth mentioning that the multidentate nature of polymeric ligand, intermolecular surface interactions like π–π, and H-bonding of the same polymer cementing different nanoparticles surfaces play a major role for enhanced mechanical properties of the stacks. The possible intermolecular interactions of the polymeric ligand upon heating is also indicated by measuring the solubility of the polymeric ligand annealed under a similar set of conditions; it is insoluble in DMA even after long time ultrasonication and heating (Fig. S5, ESI†). A scheme illustrating the possible role of the polymeric ligand and surface of nanoparticles in enhanced mechanical properties is presented as Scheme S3 (ESI†). Hydrogen binding is assumed to play a minor role, because all samples were prepared under non-aqueous conditions. (ii) The high stability constant of the iron–catecholate complexes appears to be an important factor in describing the mechanical behavior of mussel cuticles containing both the catechol-containing amino acid, catechol and Fe3+ ions.38–40 In the multilayered composites reported here, the biopolymer acts like a strong “glue” for the Fe3O4 nanoparticles. For a particle diameter of 4 nm, each Fe3O4 particle has a surface area of approx. 5000 Å2. Assuming a surface segment of 25 Å2 per catechol anchor group, a 100% side group substitution of the polymer and full surface coverage, each particle will be bound to ≈200 dopamine groups of different polymer chains. The bonding at the interface and subsequent infiltration of the polymer into the nanoparticle layers leads to the formation of a tough dopamine modified polymer–Fe3O4 composite with the polymer acting as glue. (iii) This “glue” is responsible for the solidification through metal coordination36,37 and oxidative chemical crosslinking with catechols of neighboring the polymer chains, because catechols are oxidized to quinones at temperatures >90 °C, which undergo a Michael-type addition with adjacent catechol groups.41 At ambient temperature catechols do not exhibit strong chemical reactivity.
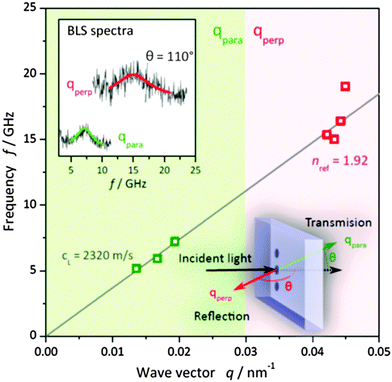
The dense and well-ordered packing of the nanoparticles in the nanocomposites (confirmed by TEM, Fig. 2C) is the second important structural parameter that affects their mechanical properties, similar as described for nacre.10 In the nanocomposite the Fe3O4 nanoparticles are close-packed (Fig. 2C), and the individual polymer chains can directly cross-link neighboring particles. This directly affects the strength of the nanocomposite. The hardness, i.e. the resistance against plastic deformation, is strongly enhanced if there is direct bridging between individual nanoparticles by the polymer. Such a configuration imposes the necessity to break many strong iron–catechol bonds (or the backbone of the polymer chain itself) to allow for plastic deformation as the indenter penetrates into the surface of the hybrid nanocomposite (Fig. S6, ESI†). To examine whether the strong interfacial bonding of the infiltrated close packed Fe3O4 nanoparticle layers is sufficient to account for the extraordinary strength of the 6 BL nanocomposite, we have addressed the role of defects and morphology. We increased the number of layers and hence the defect probability, while for the exemplification of the latter we considered a dopamine modified polymer–Fe3O4 blend of similar composition. For the thicker nanocomposite consisting of 16 BL, nanoindentation yields a much lower E = 11 ± 5 GPa using ν in the range 0.2–0.3. For this thicker stack, we could employ Brillouin light scattering (BLS) to obtain directly the longitudinal modulus M, which is related to E via the Poisson ratio.42 BLS, a non-destructive and non-contact optical technique, directly measures the frequency f of the thermally activated acoustic phonons due to the inelastic scattering of photons. From the BLS spectra (upper inset to Fig. 4) recorded at different phonon wave vectors q, the dispersion f(q) for homogenous samples is linear and leads to the phase sound velocity c = 2πf/q.43 Since the refractive index enters in the calculation of qperp only, the linear dispersion applies with neff = 1.92 for the 16 BL stack. Due to the inevitable infiltration of dopamine modified polymer in the much thicker Fe3O4 layers, the volume fraction of Fe3O4 is estimated from a linear composition dependence of neff to be ϕ ∼ 0.43 (nFe3O4 = 2.42, ndopamine = 1.55). For longitudinal polarization, the corresponding modulus is M = ρcL2 = 15 ± 2 GPa for a density ρ = 2.8 g cm−3 (ρFe3O4 = 5.1 g cm−3 and ρdopamine = 1 g cm−3). The two moduli, M and E from two different techniques are consistent for a Poisson's ratio ν ∼ 0.3. Using the latter value, the computed longitudinal modulus for the 6 BL film (E = 24 GPa) corresponds to M = 32 GPa.
Mammeri et al.44 suggested that important factors determining the mechanical properties of inorganic–organic hybrids are (i) the choice of solvent, (ii) the number of anchor groups and (iii) the nature of the interaction. Indeed, these factors can account for the increased hardness and Young's modulus of the Fe3O4–polymer composite discussed here. In addition, the structure of the particular nanostructure (Fig. 2C), which can be assumed as close packed, makes an important contribution to the mechanical properties. To address the role of structure, we measured the elastic E and M in a dopamine modified polymer–Fe3O4 mixture with a deliberately high particle filling ratio (0.6) prepared by drop-casting. For this dispersion, =12 ± 3 GPa assumes clearly a much lower value than in the 6 BL periodic structure (17 ± 3 GPa). A BLS experiment on the same homogeneous dispersion leads to a single cL = 2210 m s−1 and M = 18 ± 1 GPa being consistent with the E modulus using ν ∼ 0.3. Apparently, strong binding is a necessary, but not sufficient condition to boost the mechanical properties of polymer nanocomposites, and a defect-free structure should be present as well.
In dispersion-strengthened composites, the matrix is the major load-bearing component and therefore carries most of the load. Deformation occurs by slip and dislocation movement. The particles strengthen the material by impeding slip and dislocation, but do not react with the matrix. In particle–reinforced composites the particle size is on the order of a few microns, and the particles carry a major portion of the load. The particles are used to increase the modulus and decrease the ductility of the matrix, but in general particle–matrix interactions are of minor importance. Our approach to infiltrate a mesocrystal of hard metal oxide nanoparticles with “sticky” polymers cements the nanoparticles together, as many metal–catechol bonds have to be broken prior to mechanical failure. The individual nanoparticles are too small and too strong to break. In essence, the specific crosslinking between different nanoparticles and the multidentate polymer ligand helps the matrix resist deformation and make the composite harder and stronger.
Conclusions
In summary, by utilizing the strong surface complexation of Fe3O4 nanoparticles by a dopamine modified polymer, we have fabricated hard multi-layered nanocomposites with greatly enhanced and unprecedented mechanical properties. To our knowledge, the Young's modulus of E = 17 ± 3 GPa and a hardness of H = 1.3 ± 0.4 GPa measured for Fe3O4–dopamine modified polymer nanocomposites are among the highest values reported so far for inorganic nanoparticle–polymer composites.7–15,45 The multi-lamellar films may be used as robust, hard coatings with a high degree of flexibility depending on the template. Our approach is widely applicable for different types of polymers and inorganic building blocks. The multilayers can be used as a model to understand fundamental mechanisms responsible for mechanical properties of composites predicted theoretically. This may allow the rational design of new materials equally desirable for diverse industries from aviation to medicine.Acknowledgements
We thank Tobias Häger for acquisition of the Raman spectra and Ms Yeongseon Jang for assistance in dip-coating. This work was partially supported by the Deutsche Forschungsgemeinschaft (DFG) within the program 1404 (International Research Training Group for Opto-electronics), the priority program 1569 (Generation of multifunctional inorganic materials by molecular bionics), the priority program 1486 (Particles in contact), and by the state Excellence Center COMATT.Notes and references
- R. Lakes, Nature, 1993, 361, 511–515 CrossRef PubMed.
- G. Mayer, Science, 2005, 310, 1144–1147 CrossRef CAS PubMed.
- P. Fratzl and R. Weinkamer, Prog. Mater. Sci., 2007, 52, 1263–1334 CrossRef CAS PubMed.
- P.-Y. Chen, J. McKittrick and M. A. Meyers, Prog. Mater. Sci., 2012, 57, 1492–1704 CrossRef CAS PubMed.
- H. D. Espinosa, J. E. Rim, F. Barthelat and M. J. Buehler, Prog. Mater. Sci., 2009, 54, 1059–1100 CrossRef CAS PubMed.
- M. J. Buehler and S. Keten, Rev. Mod. Phys., 2010, 82, 1459–1487 CrossRef.
- Z. Tang, N. A. Kotov, S. Maganov and B. Ozturk, Nat. Mater., 2003, 2, 413–418 CrossRef CAS PubMed.
- P. Padsiadlo, A. K. Kaushik, E. M. Arruda, A. M. Waas, B. S. Shim, J. Xu, H. Nandivada, B. G. Pumplin, J. Lahann, A. Ramamoorthy and N. A. Kotov, Science, 2007, 318, 80–83 CrossRef PubMed.
- A. Walther, I. Bjurhager, J.-M. Malho, J. Ruokalainen and L. Berglund, Angew. Chem., Int. Ed., 2010, 49, 6448–6453 CrossRef CAS PubMed.
- A. Finnemore, P. Cunha, T. Shean, S. Vignolini, S. Guldin, M. Oyen and U. Steiner, Nat. Commun., 2012, 3, 1–6 Search PubMed.
- S.-M. Lee, E. Pippel, U. Gösele, C. Dresbach, Y. Qin, C. Vinod Chandran, T. Bräuniger, G. Hause and M. Knez, Science, 2009, 324, 488–492 CrossRef CAS PubMed.
- S. Deville, E. Saiz, R. K. Nalla and A. P. Tomsia, Science, 2006, 311, 515–518 CrossRef CAS PubMed.
- E. Munch, M. E. Launey, D. H. Alsem, E. Saiz, A. P. Tomsia and R. O. Ritchie, Science, 2008, 322, 1516–1520 CrossRef CAS PubMed.
- T. Lin, W. Huang, I. Jun and P. Jiang, in Advances in Biomimetics, ed. M. Cavrak, 2011, ISBN: 978-953-307-191-6 Search PubMed.
- A. Sellinger, P. M. Weiss, A. Nguyen, Y. F. Lu, R. A. Assink, W. L. Gong and J. Brinker, Nature, 1998, 394, 256–260 CrossRef CAS.
- Z. Burghard, L. Zini, V. Srot, P. Bellina, P. van Aken and J. Bill, Nano Lett., 2009, 9, 4103–4108 CrossRef CAS PubMed.
- L. J. Bonderer, A. R. Studart and L. J. Gaukler, Science, 2008, 319, 1069–1073 CrossRef CAS PubMed.
- F. Natalio, T. Coralles, M. Panthöfer, I. Lieberwirth, D. Schollmeyer, W. E. G. Müller, M. Kappl, H.-J. Butt and W. Tremel, Science, 2013, 339, 1298–1302 CrossRef CAS PubMed.
- J. Choi, M. J. A. Hore, J. S. Meth, N. Clarke, K. I. Winey and R. J. Composto, ACS Macro Lett., 2013, 2, 485–490 CrossRef CAS.
- R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239–2314 CrossRef CAS.
- E. Karabulut, T. Pettersson, M. Ankerfors and L. Wagberg, ACS Nano, 2012, 6, 4731–4739 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- M. J. Harrington, A. Masic, N. Holten-Andersen, J. H. Waite and P. Fratzl, Science, 2010, 328, 216–220 CrossRef CAS PubMed.
- H. Zeng, D. S. Hwang, J. N. Israelachvili and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 12850–12853 CrossRef CAS PubMed.
- R. S. Penciu, H. Kriegs, G. Petekidis, G. Fytas and E. N. Economou, J. Chem. Phys., 2003, 118, 5224 CrossRef CAS PubMed.
- M. Eberhardt, R. Mruk, R. Zentel and P. Theato, Eur. Polym. J., 2005, 41, 1569–1575 CrossRef CAS PubMed.
- S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204–8205 CrossRef CAS PubMed.
- W. C. Oliver and G. M. Pharr, J. Mater. Res., 1992, 7, 1564–1583 CrossRef CAS.
- C. G. Pierpont and C. W. Lange, Prog. Inorg. Chem., 1994, 41, 381–492 Search PubMed.
- J. Wang, M. N. Tahir, M. Kappl, W. Tremel, N. Metz, M. Barz, P. Theato and H.-J. Butt, Adv. Mater., 2008, 20, 3872–3876 CrossRef CAS PubMed.
- M. N. Tahir, M. Eberhardt, P. Theato, S. Faiss, A. Janshoff, T. Gorelik, U. Kolb and W. Tremel, Angew. Chem., Int. Ed., 2006, 45, 908–912 CrossRef CAS PubMed.
- M. I. Shukoor, F. Natalio, N. Metz, N. Glube, M. N. Tahir, H. A. Therese, V. Ksenofontov, P. Theato, P. Langguth, J. Paul Boissel, H. C. Schroeder, W. E. G. Mueller and W. Tremel, Angew. Chem., Int. Ed., 2008, 47, 4748–4752 CrossRef CAS PubMed.
- P. Podsiadlo, Z. Lin, D. Paterson, P. B. Messersmith and N. A. Kotov, Adv. Mater., 2007, 19, 949–955 CrossRef CAS PubMed.
- M. J. Sever, J. T. Weisser, J. Monahan, S. Srinivasana and J. J. Wilker, Angew. Chem., Int. Ed., 2004, 43, 448–450 CrossRef CAS PubMed.
- N. Holten-Andersen, M. J. Harrington, H. Birkedal, B. P. Lee, P. B. Messersmith, K. Y. C. Lee and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651–2655 CrossRef CAS PubMed.
- D. G. Barrett, D. E. Fullenkamp, L. He, N. Holten-Andersen, K. Y. C. Lee and P. B. Messersmith, Adv. Funct. Mater., 2013, 23, 1111–1119 CrossRef CAS PubMed.
- S. W. Taylor, D. B. Chase, M. H. Emptage, M. J. Nelson and J. H. Waite, Inorg. Chem., 1996, 35, 7572–7577 CrossRef CAS.
- N. Holten-Andersen, T. E. Mates, M. S. Toprak, G. D. Stucky, F. W. Zok and J. H. Waite, Langmuir, 2009, 25, 3323–3326 CrossRef CAS PubMed.
- L. D. Loomis and K. N. Raymond, Inorg. Chem., 1991, 30, 906–911 CrossRef CAS.
- M. J. Sever and J. J. Wilker, Dalton Trans., 2006, 813–822 RSC.
- J. H. Waite, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 1990, 97, 19–29 CrossRef CAS.
- T. Still, M. Oudich, G. K. Auerhammer, D. Vlassopoulos, B. Djafari-Rouhani, G. Fytas and P. Sheng, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 094102 CrossRef.
- K. L. Kearns, T. Still, G. Fytas and M. D. Ediger, Adv. Mater., 2010, 22, 39–42 CrossRef CAS PubMed.
- M. Mammeri, E. L. Bourhis, L. Rozes and C. Sanchez, J. Mater. Chem., 2005, 15, 3787–3811 RSC.
- F. Liaqat, M. N. Tahir, E. Schechtel, M. Kappl, D. Schneider, K. Char, H.-J. Butt and W. Tremel, Macromol. Rapid Commun., 2015 DOI:10.1002/marc.201400706.
Footnote |
| † Electronic supplementary information (ESI) available: These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data. See DOI: 10.1039/c5mh00016e |
| This journal is © The Royal Society of Chemistry 2015 |