A self-powered kinesin-microtubule system for smart cargo delivery†
Yi
Jia
a,
Weiguang
Dong
a,
Xiyun
Feng
a,
Jieling
Li
a and
Junbai
Li
*ab
aBeijing National Laboratory for Molecular Sciences, CAS Key Lab of Colloid, Interface and Chemical Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: jbli@iccas.ac.cn
bNational Center for Nanoscience and Technology, Beijing, 100190, China
First published on 10th September 2014
Abstract
A smart self-powered cargo delivery system that is composed of creatine phosphate kinase (CPK) microspheres, kinesins and microtubules is demonstrated. The CPK microsphere not only acts as an ATP generation and buffering system, but also as a carrier for cargo transport, thus realizing the easy loading and self-powered delivery of cargos at the same time.
Over the last two decades, advances in modern biology and nanotechnology have allowed us to develop diverse nanodevices for various applications. One important development is biomotor-based (kinesins, myosins, dyneins) vehicles, which is expected to lead to devices with multi-functions and may totally change the way we monitor, diagnose, and cure diseases. With adenosine triphosphate (ATP) as ‘fuel’, these biomotors could effectively and directly convert chemical energy into mechanical energy and move directionally. Due to their small size, biocompatibility and remarkable performance, these biomotors have attracted great attention and have been successfully applied in cargo transport,1–5 nanostructuring,6,7 surface imaging,8,9 sorting of protein assemblies,10 biosensors11,12 and optical devices.13 In the building of these nanodevices, the energy supply of ATP is essential. Many researchers have tried to control ATP supply by ultraviolet light,1,14 electric field15,16 or microfluidics.17 However, ultraviolet light and electric field may cause damage to the activity of proteins; meanwhile they require additional equipment and cannot realize continuous supply of ATP. Though microfluidic technology could continuously supply ATP to the system, repeated replacement of the ATP solution is required. For most systems, one problem should also be addressed that the increased concentration of adenosine diphosphate (ADP) released during the motion of biomotors has an inhibition effect on the activity of biomotors,18 accompanied by a decreased velocity and run length.19 Therefore, an ATP-regenerated system with an ability to supply and buffer the concentration of ATP in artificial devices is needed.
Creatine phosphate kinase (CPK), also known as creatine kinase, is an enzyme found mainly in the brain, skeletal muscles, heart and other tissues. In the tissues and cells that consume ATP rapidly, especially skeletal muscles, CPK serves as an energy generator for production of ATP in situ, as well as energy buffering.20 The strong buffering ability of CPK is essential to keep intracellular ATP constant during contraction cycles. Based on this, CPK microspheres were fabricated through a co-precipitation method and employed as an ATP generation and buffering system for biomotor kinesin, as shown in Fig. 1. Enzymatically induced ‘fuel’ production can be achieved under mild conditions and without the use of external fields. It is thus better suited for use in biomotor systems than most other ways on the small scale. In view of the advantages of the co-precipitation method, various cargos could be easily encapsulated into the microspheres, thus realizing the easy loading and self-powered delivery of cargos at the same time. This method present here would have great potential in intelligent self-powered cargo delivery systems for performing challenging tasks.21
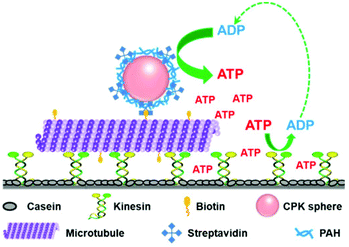 | ||
| Fig. 1 Schematic illustration of the self-powered MT-CPK complex (“train”) gliding on the kinesin-modified surface (“rail”). | ||
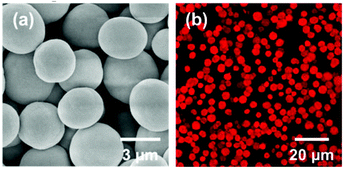
Spherical colloidal particles are found to be attractive candidates for cargo delivery applications as they are cost effective and easy to synthesize. Here, CPK microspheres for both energy generation and cargo delivery were prepared through a one-step protein–CaCO3 co-precipitation method.22,23 Briefly, 1 mL 0.33 M Na2CO3 solution and 1 mL 4 mg mL−1 CPK solution were first vigorously stirred in a round flask for 5 min. Then 1 mL 0.33 M CaCl2 solution was added quickly to the above mixture with intense agitation on a magnetic stirrer (1200 rm min−1). After 24 s, this system was allowed to stand for 2 min. The obtained microspheres were washed with deionized water thoroughly three times, and then incubated in 0.025 wt% glutaraldehyde (GA) solution for 2 h, followed by centrifugation and washing. Fig. 2a and 2b show scanning electron microscopy (SEM) image and confocal laser scanning microscopy (CLSM) image of CPK microspheres excited at 559 nm. It can be clearly seen that the CPK microspheres were obtained with an average diameter of 3.6 μm and displayed intriguing autofluorescence properties without conjugating to any external fluorochromes. This phenomenon can be attributed to the n–π* transition of C–N bonds in the Schiff's base bonds formed during the crosslinking reaction between amino groups of CPK and aldehyde groups of GA.24,25 The autofluorescence of these CPK microspheres would facilitate in monitoring the motion of the microtubule-CPK (MT-CPK) complex in the following gliding assay, avoiding the use of external fluorochromes.
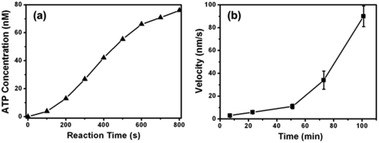
To confirm the generation of ATP from CPK microspheres, a bioluminescence luciferin–luciferase assay kit was used. Luciferin is rapidly oxidized by luciferase in the presence of ATP, producing light.26 Since the luminescence intensity is proportional to the amount of ATP in the sample, we have first derived a standard ATP curve by plotting the luminescence intensity as a function of the ATP concentration. To analyse the ATP concentration, the CPK microspheres were first dispersed into a BRB80 buffer supplement with ADP and creatine phosphate (CP). Then 5 μL of the CPK microsphere dispersion (2.85 mg mL−1) was added into a luminometer cuvette and mixed with 50 μL of the luciferin–luciferase reagent, immediately followed by registering the luminescence intensity on a recorder. ATP production within an 800 s reaction time was measured, as shown in Fig. 3a. It can be seen clearly that the concentration of ATP continuously increased with reaction time. The result proved that the CPK assembled in the microspheres retain their bioactivity and could catalyze the conversion of ADP and CP to ATP.
To further confirm the generation of ATP from CPK microspheres, an in vitro gliding assay of microtubules was conducted. A MT suspension containing CPK microspheres, ADP, CP and oxygen scavenger was flowed into the kinesin coated chamber, and the time-lapse images of microtubule gliding were obtained by CLSM. It can be seen from Fig. 3b (also see ESI, Mov. S1–S5†) that the time-average velocity of the MT increased exponentially with time, from 3 ± 1 nm s−1 to 90 ± 10 nm s−1. This result further demonstrated that ATP was continuously generated by catalysis of the CPK microspheres, and the concentration of ATP in the chamber increased with time. It should be mentioned that in the presence of a fixed concentration of ATP, the velocity of MT gliding was reported to be reduced exponentially due to gradual consumption of ATP and increased production of ADP.19,27 Here, our self-powered system successfully got over this problem and could maintain the ATP concentration on a nearly constant level, maintaining the velocity of MT gliding on a constant value at equilibrium (the average velocity of MT gliding is 92 ± 7 nm s−1 from 100 min to 260 min in the observation time), further confirming the CPK microspheres’ ability to buffer ATP. Compared with CPK solutions, the CPK microspheres could carry chemically active zones which convert species from their surroundings,28 thus they may produce concentration gradients without any extra force. For verification, the velocity of MT gliding versus different distances between MT and CPK microspheres was measured. It can be seen from Fig. S1† that the velocity of MT gliding is distance-dependent and that the gliding velocity decreased with the increased distance between MT and CPK microspheres, especially obvious when over 3000 μm. This demonstrates that the CPK microsphere may have great potential in local control of biomotors.29
For cargo delivery, glucose oxidase (GOD) loaded, fluorescent isothiocyanate-dextran (FITC-dextran, 20 kDa) loaded and doxorubicin (DOX) loaded CPK microspheres (Fig. S2 and S3†) were fabricated by the same procedure as for pure CPK microspheres, respectively. In order to achieve controlled release of the cargos, layer-by-layer (LbL) assembly of CPK and GA on the cargo loaded microspheres was conducted, and (FITC-dextran–CPK)/(GA/CPK)4 microspheres were obtained. Fig. S4† shows that the release of FITC-dextran exhibited pH-responsivity. This phenomenon was mainly due to the faster hydrolysis of Schiff's base bond (formed between CPK and GA) under acidic conditions, which makes the crosslinked multilayer structure loose and allows FITC-dextran to diffuse through the shells at pH 4.5.24,30 In consideration of the versatility of LbL assembly, various functional or responsive components could be easily assembled on the surface of CPK microspheres, thus rendering flexible, controllable release of cargos in a smart system.
For fabrication of the self-powered kinesin-microtubule cargo delivery system, the streptavidin–biotin system was applied to link cargo-loaded CPK microspheres with microtubules.3 GOD-loaded CPK microspheres were first modified with a layer of positively charged poly(allylamine hydrochloride) (PAH), and then incubated with negatively charged streptavidin (SA) to form a stable complex. The GOD–CPK/PAH/SA complex microsphere was subsequently linked to the biotinylated microtubule through the strong specific interaction between streptavidin and biotin. The obtained GOD-loaded CPK-MT complex was then applied to the in vitro motility assay. The time-lapse images in Fig. 4 (see also ESI, Mov. S6†) shows that the GOD-loaded CPK-MT complex successfully moved on the kinesin-modified surface in the presence of ADP and CP. The average velocity of the GOD-loaded CPK-MT complex is 62 nm s−1. The result demonstrated that the CPK microsphere coupled to MT here could successfully act both as a ‘fuel’ generation system for kinesin-MT movement and as a carrier for cargo delivery. This makes it extremely attractive toward the design of self-powered biomotor-based hybrid nano- and micro-devices for intelligent cargo delivery.
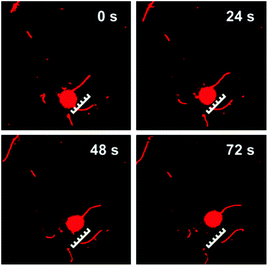 | ||
| Fig. 4 CLSM images of the GOD-loaded CPK-MT complex gliding on the kinesin-modified surface at different times using self-supplying ATP. The scale bars represent 5 μm. | ||
Conclusions
We have successfully prepared a smart self-powered cargo delivery system with CPK microspheres, kinesins and microtubules. The CPK microsphere fabricated by a co-precipitation method was employed both as a ‘fuel’ generation, buffering system for kinesin-MT movement and as a carrier for cargo delivery. CPK catalyzed ‘fuel’ production can be achieved under mild conditions and without the use of external fields. It is thus better suited for use in biomotor systems than most other ways on the small scale. Compared with CPK solutions, the CPK microspheres are able to produce concentration gradients without any external forces; therefore they may have great potential in local control of biomotors. Considering the advantages of a co-precipitation method, various cargos were easily encapsulated into the microspheres, thus realizing the easy loading and self-powered delivery of cargos at the same time. In view of the versatility of LbL assembly, various functional or responsive components could be easily assembled on the surface of CPK microspheres, thus rendering flexible, controllable release of cargos in a smart system. The method presented here would pave the way to develop self-powered intelligent machines for cargo delivery, material separation and certain biomedical applications.Acknowledgements
The authors acknowledge the financial support from the National Nature Science Foundation of China (project no. 91027045, 21303221, 21273053 and 21273055), the National Basic Research Program of China (973 program, 2013CB932800) and the Institute of Chemistry, Chinese Academy of Science (CMS-PY-201333).Notes and references
- H. Hess, J. Clemmens, D. Qin, J. Howard and V. Vogel, Nano Lett., 2001, 1, 235–239 CrossRef CAS.
- M. J. I. Mueller, S. Klumpp and R. Lipowsky, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 4609–4614 CrossRef CAS PubMed.
- W. Song, H. Moehwald and J. Li, Biomaterials, 2010, 31, 1287–1292 CrossRef CAS PubMed.
- K. J. Bohm, R. Stracke, P. Muhlig and E. Unger, Nanotechnology, 2001, 12, 238–244 CrossRef CAS.
- A. Goel and V. Vogel, Nat. Nanotechnol., 2008, 3, 465–475 CrossRef CAS PubMed.
- S. Diez, C. Reuther, C. Dinu, R. Seidel, M. Mertig, W. Pompe and J. Howard, Nano Lett., 2003, 3, 1251–1254 CrossRef CAS.
- H. Hess, J. Clemmens, C. Brunner, R. Doot, S. Luna, K. H. Ernst and V. Vogel, Nano Lett., 2005, 5, 629–633 CrossRef CAS PubMed.
- H. Hess, J. Clemmens, J. Howard and V. Vogel, Nano Lett., 2002, 2, 113–116 CrossRef.
- A. Agarwal and H. Hess, Prog. Polym. Sci., 2010, 35, 252–277 CrossRef CAS PubMed.
- L. Ionov, M. Stamm and S. Diez, Nano Lett., 2005, 5, 1910–1914 CrossRef CAS PubMed.
- G. D. Bachand, H. Hess, B. Ratna, P. Satir and V. Vogel, Lab Chip, 2009, 9, 1661–1666 RSC.
- T. Fischer, A. Agarwal and H. Hess, Nat. Nanotechnol., 2009, 4, 162–166 CrossRef CAS PubMed.
- S. Aoyama, M. Shimoike and Y. Hiratsuka, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 16408–16413 CrossRef CAS PubMed.
- A. Nomura, T. Q. P. Uyeda, N. Yumoto and Y. Tatsu, Chem. Commun., 2006, 3588–3590 RSC.
- B. D. Martin, L. M. Velea, C. M. Soto, C. M. Whitaker, B. P. Gaber and B. Ratna, Nanotechnology, 2007, 18, 055103 CrossRef.
- I. Dujovne, M. van den Heuvel, Y. Shen, M. de Graaff and C. Dekker, Nano Lett., 2008, 8, 4217–4220 CrossRef CAS PubMed.
- J. R. Wasylycia, S. Sapelnikova, H. Jeong, J. Dragoljic, S. L. Marcus and D. J. Harrison, Lab Chip, 2008, 8, 979–982 RSC.
- W. R. Schief, R. H. Clark, A. H. Crevenna and J. Howard, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1183–1188 CrossRef CAS PubMed.
- J. Yajima, M. C. Alonso, R. A. Cross and Y. Y. Toyoshima, Curr. Biol., 2002, 12, 301–306 CrossRef CAS.
- T. Wallimann, M. Tokarska-Schlattner and U. Schlattner, Amino Acids, 2011, 40, 1271–1296 CrossRef CAS PubMed.
- D. Patra, S. Sengupta, W. Duan, H. Zhang, R. Pavlick and A. Sen, Nanoscale, 2013, 5, 1273–1283 RSC.
- A. I. Petrov, D. V. Volodkin and G. B. Sukhorukov, Biotechnol. Prog., 2005, 21, 918–925 CrossRef PubMed.
- L. Duan, X. Yan, A. Wang, Y. Jia and J. Li, ACS Nano, 2012, 6, 6897–6904 CrossRef CAS PubMed.
- Y. Jia, J. Fei, Y. Cui, Y. Yang, L. Gao and J. Li, Chem. Commun., 2011, 47, 1175–1177 RSC.
- Y. Jia, Y. Cui, J. Fei, M. Du, L. Dai, J. Li and Y. Yang, Adv. Funct. Mater., 2012, 22, 1446–1453 CrossRef CAS.
- W. Qi, L. Duan, K. W. Wang, X. H. Yan, Y. Cui, Q. He and J. Li, Adv. Mater., 2008, 20, 601–605 CrossRef CAS.
- Y. Z. Du, Y. Hiratsuka, S. Taira, M. Eguchi, T. Q. P. Uyeda, N. Yumoto and M. Kodaka, Chem. Commun., 2005, 2080–2082 RSC.
- S. Gaspar, Nanoscale, 2014, 6, 7757–7763 RSC.
- K.-E. Byun, D. S. Choi, E. Kim, D. H. Seo, H. Yang, S. Seo and S. Hong, ACS Nano, 2011, 5, 8656–8664 CrossRef CAS PubMed.
- B. D. Wang, C. J. Xu, J. Xie, Z. Y. Yang and S. L. Sun, J. Am. Chem. Soc., 2008, 130, 14436–14437 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Fig. S1–S4, and Mov. S1–S6. See DOI: 10.1039/c4nr04454a |
| This journal is © The Royal Society of Chemistry 2015 |