Functional importance of the sugar moiety of jasmonic acid glucoside for bioactivity and target affinity†
Minoru
Ueda
*,
Gangqiang
Yang
,
Yuuki
Nukadzuka
,
Yasuhiro
Ishimaru
,
Satoru
Tamura
and
Yoshiyuki
Manabe
Laboratory of Organic Chemistry, Department of Chemistry, Tohoku University, 6-3 Aramaki-Aza Aoba, Aoba-ku, Sendai 980-8578, Japan. E-mail: ueda@m.tohoku.ac.jp; Fax: +81-22-795-6553; Tel: +81-22-795-6553
First published on 15th October 2014
Abstract
12-O-β-D-Glucopyranosyljasmonic acid (JAG, 1) induces nyctinastic leaf-folding of Samanea saman. The SAR studies of 1 revealed the unique role of its glycone moiety. Biological activity and the target affinity of 1 were affected by the stereochemistry of the glycone moiety. JAG belongs to a unique class of ligands in which the structure of the glycone moiety is involved in the molecular recognition by the target protein.
Jasmonic acid (JA) and its derivatives, collectively referred to as jasmonates, play important roles in the life of higher plants.1–4 We identified 12-O-β-D-glucopyranosyljasmonic acid, also referred to as jasmonic acid glucoside (JAG, 1), as the bioactive metabolite that induces nyctinastic leaf-folding of Samanea saman.5–8 Nyctinastic leaf-folding of plants has been a historic matter of interest among biologists since Darwin's era9 and S. saman has been used as a standard plant material for the biology of nyctinasty.10,11 Thus, 1 is a key compound for the molecular understanding of this intriguing biological phenomenon.
The mode of action of JA has been verified through identification of the COI1-JAZ signalling module12–14 in which a complex of cytosolic COI1 and JAZ proteins functions as the jasmonate receptor. However, the leaf-folding induction by 1 was revealed to be independent of the COI1-JAZ signalling pathway.15 Instead, the membrane target protein referred to as MTJG (membrane target of jasmonic acid glucoside)15,16 is believed to be involved. Considering that most glycosides are regarded as biologically inactive derivatives of secondary metabolites, this is a rare example in which a glucoside of a plant hormone functions as a ligand totally different from the parent ligand. Here, we report that 1 belongs to a unique class of ligands in which the D-glucopyranosyl moiety participates in the molecular recognition by the target protein.
Three possible roles of the D-glucopyranosyl moiety can be proposed for the target selectivity of 1. First, the conjugation of the D-glucopyranosyl moiety could decrease the lipophilicity and membrane permeability of the jasmonate and then a membrane permeable jasmonate targeting COI1-JAZ could be converted to a membrane-impermeable 1 targeting membrane protein MTJG. Second, the D-glucopyranosyl moiety in 1 could provide steric hindrance to inhibit binding with the COI1-JAZ receptor complex. In the crystal structure of the COI1-JAZ-jasmonoyl L-Ile (JA-Ile) complex,14 JA-Ile is packed tightly between COI1 and JAZ, and no free space is left for accommodating additional D-glucopyranosyl moieties. Third, the D-glucopyranosyl moiety in 1 could enhance the affinity with the target protein as shown in the case of a steroid glycoside, ouabain. Recently, Cornelius et al. reported an intriguing example in which the conjugation of a glycone moiety strongly enhanced the affinity of a ligand with its target protein. The affinity of ouabain with its target protein, Na,K-ATPase, is enhanced as much as 26-fold by the glycosidation of the corresponding aglycone.17 The strong hydrogen bond network between the glycone moiety of ouabain and the ligand binding pocket of Na,K-ATPase strongly enhanced their binding. We examined this third possibility as shown below.
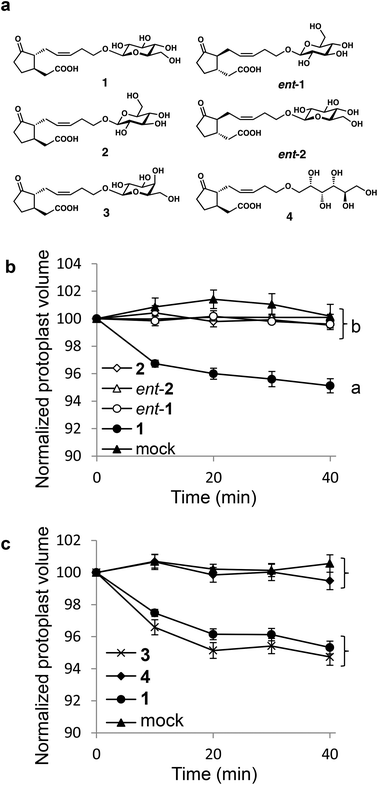
We first carried out structure activity relationship (SAR) studies on 1 to estimate the importance of the D-glucopyranosyl moiety. We designed ent-1,18 diastereomeric 12-O-β-L-glucopyranosyljasmonic acid (2), diastereomeric 12-O-β-D-glucopyranosyl-ent-jasmonic acid (ent-2), epimeric 12-O-β-D-galactopyranosyljasmonic acid (3),18 and open-chain type 12-O-D-sorbitoyljasmonic acid (4) (Fig. 1). The hydrophilicity of 1 was maintained in ent-1, 2, ent-2, 3, and 4, whereas the structure recognition of the glycone moiety may be affected by structural modifications in ent-1, ent-2, 3, and 4. We synthesized 1, ent-1, 2 (Scheme S1†), ent-2 (Scheme S2†), and 3 according to the procedure in ref. 19. The open-chain type 4 was synthesized as shown in Scheme S3.† In the previous study,15 we demonstrated that 1 induced shrinking of the extensor motor cell protoplasts isolated from S. saman, whereas ent-1 cannot induce the shrinking. As the shrinking of motor cells directly causes leaf-folding movement,10 the biological evaluations of 1–4, ent-1, and ent-2 were carried out based on this cell-shrinking assay (Fig. 1).
Interestingly, clear differences were observed among them. Only naturally occurring 1 induced cell-shrinking, whereas no cell-shrinking was observed with the enantiomeric and diastereomeric isomers (ent-1, 2 and ent-2) (Fig. 1b). It should be noted that the stereochemistry of the glycone moiety was proven to be important for the bioactivity of 1. Additionally, the substitution of glucopyranosyl into galactopyranosyl (4′-epimer) in 3 did not affect the cell-shrinking activity or the previously reported leaf-folding activity (Fig. 1c).18 Subtle structural modifications, such as epimerization of one hydroxy group in the sugar moiety, did not affect the biological activities of 1. In contrast, the glycopyranosyl structure is important because no cell-shrinking was observed in open-chain type 4. These results strongly suggested that the structure of the D-glycopyranosyl moiety is indispensable for cell-shrinking activity.
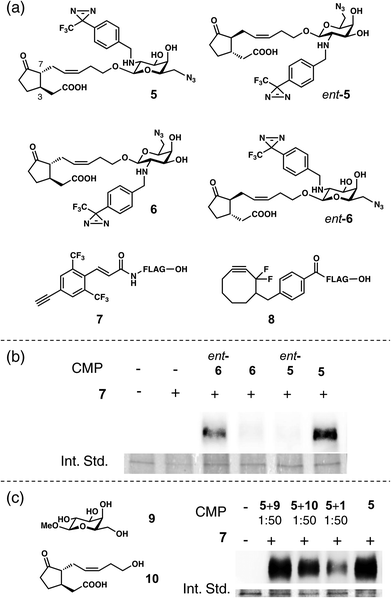
Next, we considered that the photoaffinity labeling experiments could provide direct proof that the stereostructure of the glycone moiety in 1 affected its affinity with its target protein, MTJG. We previously prepared four stereochemical hybrids of compact molecular probes19 (CMPs: 5, ent-5, 6, and ent-6, Fig. 2a) based on 1.20 The CMPs contained D-galactopyranosyl due to its stability against hydrolysis by glucosidases in living cells. Among them, only the naturally occurring form (5) could induce the shrinking of Samanea motor cells.20 The target protein in living cells was labeled by a sterically minimal azide handle and subsequent introduction of a tag was achieved by Hüsgen [3 + 2] cycloaddition employing Cu-catalyzed azide alkyne cycloaddition (CuAAC)21,22 or copper-free click chemistry (CFCC).23 Thus, the living motor cells were photo-crosslinked using CMP 5 (1 × 10−4 M) followed by either CuAAC with 4-ethynyl-2,6-bis(trifluoromethyl)-cinnamamide (7; 1 × 10−4 M)24,25 or CFCC with the strained difluorinated cyclooctyne DIFO23 derivative (8; 1 × 10−4 M). In both the alkyne units, the FLAG peptide was conjugated as a molecular tag.19,26 As shown in Fig. S1,† CuAAC using 7 gave the better result. CFCC using 8 gave many non-specific bindings (Fig. S1†). These nonspecific bindings were also observed when DIFO 8 was used alone without the addition of CMP 5 (Fig. S1†), and could be due to the nonspecific thiol adducts because the strained DIFO unit can react with thiol groups in proteins to give nonspecific adducts.27,28 In contrast, no nonspecific binding was observed in CuAAC, and the result was very clear because only one target band was observed (Fig. S1†).
The photo-crosslinking against a living Samanea motor cell was examined using these four CMPs (5, ent-5, 6, and ent-6) and subsequent cycloaddition was carried out with alkyne 7. Results of SDS-PAGE analysis and subsequent chemiluminescence detection are shown in Fig. 2b and S2.† The order of target affinity was 5 > ent-6 > 6 = ent-5. The difference between the results by probe 5 or ent-5 clearly demonstrated the stereospecific recognition of the probe by MTJG. Binding of probe 5 with this protein was competitively inhibited by the co-addition of excess 1 (50-fold), whereas it could not be inhibited by the excess co-addition of methyl-D-galactopyranoside (9) or 12-hydroxy jasmonic acid (10) (Fig. 2c and S3†). Interestingly, CMP ent-6 consisting of the enantiomeric aglycone and the D-galactosaminopyranosyl moiety showed a weak affinity with MTJG. The band was weakly observed using ent-6, whereas it could not be seen using 6, suggesting the importance of the D-glycopyranosyl moiety for binding with MTJG. Considering that no cell-shrinking activity was observed in ent-6,20 the affinity of ent-6 with MTJG may not be strong enough to induce cell-shrinking. However, these results provided direct proof that MTJG recognizes the glycone as well as the aglycone.
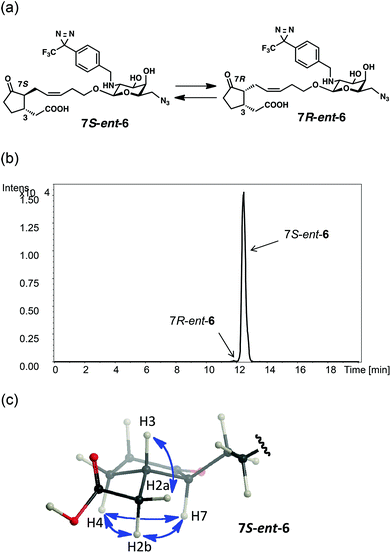
The weak affinity of ent-6 with MTJG can be attributed to thermodynamic equilibrium. JAG 1 is known to exist as an equilibrium mixture of two isomers at the α-position of the ketone (7R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7S = 95
7S = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5),18 and the bioactive form was revealed to be 7R-1.18 UPLC-MS analysis revealed that CMP ent-6 also exists as an equilibrium mixture of 7S- and 7R-forms (Fig. 3a,b and S4,† 7S
5),18 and the bioactive form was revealed to be 7R-1.18 UPLC-MS analysis revealed that CMP ent-6 also exists as an equilibrium mixture of 7S- and 7R-forms (Fig. 3a,b and S4,† 7S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7R = 95
7R = 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). The isomer of ent-6 predominantly observed in solution was confirmed to be the 7S-form from NOE correlations29 in the NMR spectrum (Fig. 3c and S5†). The 7R-ent-6 is an epimer of 5 at the 3-position, and is expected to be biologically active and have some affinity with MTJG. Thus, the presence of 7R-ent-6 (5%) can explain the weak band of MTJG in Fig. 2b observed when using ent-6.
5). The isomer of ent-6 predominantly observed in solution was confirmed to be the 7S-form from NOE correlations29 in the NMR spectrum (Fig. 3c and S5†). The 7R-ent-6 is an epimer of 5 at the 3-position, and is expected to be biologically active and have some affinity with MTJG. Thus, the presence of 7R-ent-6 (5%) can explain the weak band of MTJG in Fig. 2b observed when using ent-6.
The results of the photoaffinity labeling experiments demonstrated that the affinity between a CMP and MTJG can be affected by a structural modification in the D-galactopyranosyl moiety. Both the aglycone moiety and the D-galactopyranosyl moiety participate in the molecular recognition of 5 by MTJG.
Conclusions
Our previous SAR studies suggested that leaf-folding activity of 1 is strongly affected by the stereochemistry of the aglycone moiety.18 In this study it was also revealed that the structure and stereochemistry of the D-glucopyranosyl moiety of 1 also severely affected the cell-shrinking activity as well as its target affinity. Our results of the SAR studies of 1 revealed the unique role of its glycone moiety. Cell-shrinking activity and target affinity of 1 were severely affected by the stereochemistry of both the aglycone and glycone moieties. The stereochemical hybrid 6 with naturally occurring aglycone and the L-galactopyranosyl moiety showed no target affinity in photoaffinity experiments. In contrast, hybrid ent-6 with enantiomeric aglycone and the D-galactopyranosyl moiety containing 5% of 7R-ent-6 isomer, a 3-epimer of 5, showed a weaker target affinity than 5 with the naturally occurring stereochemistry. These results suggested that stereochemistries of both the aglycone and glycone in 1 contribute to the target affinity.JAG belongs to a unique class of ligands in which the glycone moiety plays an important role in the biological activity as well as the affinity with the target protein.30
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Chemical Biology of Natural Products” from MEXT, Japan, and a Grant-in-Aid for Scientific Research (no. 26282207) from JSPS, Japan.
Notes and references
- A. Devoto and J. G. Turner, Ann. Bot., 2003, 92, 329–337 CrossRef CAS PubMed.
- C. Wasternack, Ann. Bot., 2007, 100, 681–697 CrossRef CAS PubMed.
- J. Browse, Phytochemistry, 2009, 70, 1539–1546 CrossRef CAS PubMed.
- C. Wasternack and E. Kombrink, ACS Chem. Biol., 2009, 5, 63–77 CrossRef PubMed.
- M. Ueda and S. Yamamura, Angew. Chem., Int. Ed., 2000, 39, 1400–1414 CrossRef CAS.
- M. Ueda and Y. Nakamura, Nat. Prod. Rep., 2006, 23, 548–557 RSC.
- M. Ueda and Y. Nakamura, Plant Cell Physiol., 2007, 48, 900–907 CrossRef CAS PubMed.
- M. Ueda, M. Okazaki, K. Ueda and S. Yamamura, Tetrahedron, 2000, 56, 8101–8105 CrossRef CAS.
- C. Darwin and F. Darwin, The power of movement in plants, third thousand, John Murray, London, 1882 Search PubMed.
- R. L. Satter and A. W. Galston, Annu. Rev. Plant Physiol., 1981, 32, 83–110 CrossRef CAS.
- N. Moran, FEBS Lett., 2007, 581, 2337–2347 CrossRef CAS PubMed.
- A. Chini, S. Fonseca, G. Fernandez, B. Adie, J. M. Chico, O. Lorenzo, G. Garcia-Casado, I. Lopez-Vidriero, F. M. Lozano, M. R. Ponce, J. L. Micol and R. Solano, Nature, 2007, 448, 666–671 CrossRef CAS PubMed.
- B. Thines, L. Katsir, M. Melotto, Y. Niu, A. Mandaokar, G. Liu, K. Nomura, S. Y. He, G. A. Howe and J. Browse, Nature, 2007, 448, 661–665 CrossRef CAS PubMed.
- L. B. Sheard, X. Tan, H. Mao, J. Withers, G. Ben-Nissan, T. R. Hinds, Y. Kobayashi, F. F. Hsu, M. Sharon, J. Browse, S. Y. He, J. Rizo, G. A. Howe and N. Zheng, Nature, 2010, 468, 400–405 CrossRef CAS PubMed.
- Y. Nakamura, A. Mithöfer, E. Kombrink, W. Boland, S. Hamamoto, N. Uozumi, K. Tohma and M. Ueda, Plant Physiol., 2011, 155, 1226–1236 CrossRef CAS PubMed.
- Y. Nakamura, R. Miyatake and M. Ueda, Angew. Chem., Int. Ed., 2008, 47, 7289–7292 CrossRef CAS PubMed.
- F. Cornelius, R. Kanai and C. Toyoshima, J. Biol. Chem., 2013, 288, 6602–6616 CrossRef CAS PubMed.
- Y. Nakamura, R. Miyatake, S. Inomata and M. Ueda, Biosci., Biotechnol., Biochem., 2008, 72, 2867–2876 CrossRef CAS PubMed.
- M. Ueda, Y. Manabe, Y. Otsuka and N. Kanzawa, Chem. – Asian J., 2011, 6, 3286–3297 CrossRef CAS PubMed.
- M. Ueda, G. Yang, Y. Ishimaru, T. Itabashi, S. Tamura, H. Kiyota, S. Kuwahara, S. Inomata, M. Shoji and T. Sugai, Bioorg. Med. Chem., 2012, 20, 5832–5843 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- J. A. Codelli, J. M. Baskin, N. J. Agard and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 11486–11493 CrossRef CAS PubMed.
- Y. Manabe, M. Mukai, S. Ito, N. Kato and M. Ueda, Chem. Commun., 2010, 46, 469–471 RSC.
- Y. Nakamura, S. Inomata, M. Ebine, Y. Manabe, I. Iwakura and M. Ueda, Org. Biomol. Chem., 2011, 9, 83–85 CAS.
- M. Ueda, Y. Manabe and M. Mukai, Bioorg. Med. Chem. Lett., 2011, 21, 1359–1362 CrossRef CAS PubMed.
- K. E. Beatty, J. D. Fisk, B. P. Smart, Y. Y. Lu, J. Szychowski, M. J. Hangauer, J. M. Baskin, C. R. Bertozzi and D. A. Tirrell, ChemBioChem, 2010, 11, 2092–2095 CrossRef CAS PubMed.
- P. V. Chang, J. A. Prescher, E. M. Sletten, J. M. Baskin, I. A. Miller, N. J. Agard, A. Lo and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1821–1826 CrossRef CAS PubMed.
- H. Seto, E. Nomura, S. Fujioka, H. Koshino, T. Suenaga and S. Yoshida, Biosci., Biotechnol., Biochem., 1999, 63, 361–367 CrossRef CAS.
- M. Ueda, Chem. Lett., 2012, 41, 658–666 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ob02106a |
| This journal is © The Royal Society of Chemistry 2015 |