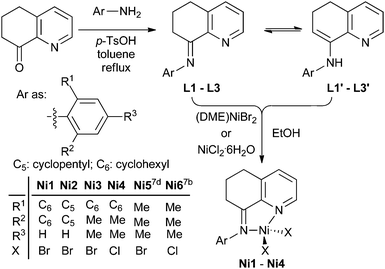
8-(2-Cycloalkylphenylimino)-5,6,7-trihydro-quinolylnickel halides: polymerizing ethylene to highly branched and lower molecular weight polyethylenes†
Zelin
Sun
ab,
Erlin
Yue
b,
Mengnan
Qu
*a,
Irina V.
Oleynik
c,
Ivan I.
Oleynik
c,
Kanshe
Li
a,
Tongling
Liang
b,
Wenjuan
Zhang
b and
Wen-Hua
Sun
*b
aCollege of Chemistry and Chemical Engineering, Xi'an University of Science and Technology, Xi'an 710054, China
bKey laboratory of Engineering Plastics and Beijing National Laboratory for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: whsun@iccas.ac.cn
cN.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Pr. Lavrentjeva 9, Novosibirsk 630090, Russia
First published on 12th January 2015
Abstract
Cycloalkyl-modified 8-arylimino-5,6,7-trihydroquinolylnickel pre-catalysts, activated with either MAO or Et2AlCl, are highly active for the polymerization of ethylene into branched polyethylene waxes with narrow polydispersity.
Late transition metal complexes have been the hot topic in ethylene polymerization in the past two decades1,2 since the discovery of α-diiminometal (nickel or palladium)3 and bis(imino)pyridylmetal (iron or cobalt) complexes4 as highly active pre-catalysts. The nickel complex pre-catalysts show the unique characteristic of producing highly branched polyethylenes.1,3 Therefore, modifications of existing α-diiminonickel complexes have been focused towards higher activities of the catalytic systems and better properties of the obtained polyethylenes,5 meanwhile alternative models of nickel pre-catalysts have also been developed using N,N-bidentate 2-iminopyridine6 or other sophisticated derivatives.7–11 Interestingly, 8-arylimino-5,6,7-trihydroquinolinylnickel halide pre-catalysts showed high activities towards ethylene reactivity,7 selectively catalyzing either the oligomerization7a or polymerization7b–e on the basis of the presence of 2-substituents within the 5,6,7-trihydroquinoline framework or not.
To verify the suitability of aniline derivatives, cycloalkyl-substituted anilines have been used to synthesize bis(iminoalkyl)pyridyliron complexes as pre-catalysts in ethylene polymerization.12 Subsequently, the cycloalkyl-substituted 8-arylimino-5,6,7-trihydroquinolylnickel halides were synthesized and are presented herein. Activated with MAO or Et2AlCl, all these nickel complexes show high activities towards ethylene polymerization, producing highly branched polyethylenes with low molecular weights and narrow polydispersity.
The 8-(2-cycloalkylphenylimino)-5,6,7-trihydroquinoline derivatives (L1–L3 and their enamine forms L1′–L3′, Scheme 1) were synthesized as yellowish oil compounds in moderate yields according to the literature.7b These compounds individually reacted with (DME)NiBr2 or NiCl2 to afford the corresponding nickel halide complexes (Scheme 1). The compositions of these nickel complexes were confirmed with elemental analysis and FT-IR spectroscopy. The structure of Ni1 was further confirmed through a single-crystal X-ray diffraction study and is shown in Fig. 1. The nickel atom is penta-coordinated with an N,N-bidentate ligand, two bromides and also an additional water molecule, and the complex adopts a distorted trigonal bipyramidal geometry, consistent with its analogs.13
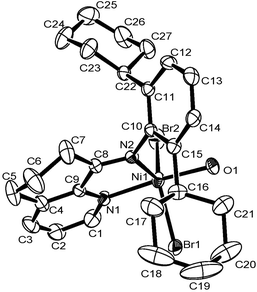 | ||
| Fig. 1 ORTEP drawing of the molecular structure of Ni1·H2O. Thermal ellipsoids are shown at the 30% probability level. Hydrogen atoms are omitted for clarity. | ||
Ethylene polymerization
Similar to determining the catalytic performance of analogous nickel pre-catalysts,7b–d complex Ni1 was used to select a suitable co-catalyst by employing MAO, MMAO, Et2AlCl, and Me2AlCl (Table 1). The promising co-catalysts turned out to be MAO, MMAO, and Et2AlCl.| Entry | Co-cat. | Al/Ni | Yield (g) | Act.b | M w (kg mol−1) | M w/Mnc | T m (°C) |
|---|---|---|---|---|---|---|---|
| a 3 μmol of Ni1; 30 min; 30 °C; 10 atm of ethylene; total volume 100 mL. b Values in units of 106 g(PE) mol(Ni)−1 h−1. c Determined using GPC. d Determined using DSC. | |||||||
| 1 | MAO | 1000 | 5.43 | 3.62 | 2.55 | 1.62 | 66.52 |
| 2 | MMAO | 1000 | 3.52 | 2.35 | 2.49 | 1.63 | 67.72 |
| 3 | Et2AlCl | 200 | 4.46 | 3.00 | 1.83 | 1.49 | 55.98 |
| 4 | Me2AlCl | 200 | Trace | Trace | — | — | — |
Ethylene polymerization in the presence of MAO
The molar ratio of MAO to Ni1 was changed from 1000 to 3500, and the highest activity of 4.70 × 106 g(PE) mol(Ni)−1 h−1 was observed at 2500 (entry 4, Table 2). Along with increasing the Al/Ni ratio (entries 1–6, Table 2), the polyethylenes obtained show slightly decreasing molecular weights due to higher chain transfer rates;14 an impressively narrow polydispersity between 1.57 to 1.60 reflected the well-defined single site catalysis.| Entry | Cat. | Al/Ni | T (°C) | t (min) | Yield (g) | Act.b | M w (kg mol−1) | M w/Mnc | T m (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a 3 μmol of Ni; 10 atm of ethylene; total volume 100 mL. b Values in units of 106 g(PE) mol(Ni)−1 h−1. c Determined using GPC. d Determined using DSC. | |||||||||
| 1 | Ni1 | 1000 | 30 | 30 | 5.43 | 3.62 | 2.55 | 1.62 | 66.52 |
| 2 | Ni1 | 1500 | 30 | 30 | 5.62 | 3.75 | 2.39 | 1.60 | 65.79 |
| 3 | Ni1 | 2000 | 30 | 30 | 5.84 | 3.89 | 2.32 | 1.60 | 62.65 |
| 4 | Ni1 | 2500 | 30 | 30 | 7.05 | 4.70 | 2.28 | 1.59 | 60.24 |
| 5 | Ni1 | 3000 | 30 | 30 | 6.17 | 4.11 | 2.15 | 1.59 | 59.41 |
| 6 | Ni1 | 3500 | 30 | 30 | 6.07 | 4.05 | 2.06 | 1.57 | 56.66 |
| 7 | Ni1 | 2500 | 20 | 30 | 6.70 | 4.47 | 2.92 | 1.72 | 75.08 |
| 8 | Ni1 | 2500 | 40 | 30 | 3.45 | 2.30 | 1.81 | 1.50 | 51.00 |
| 9 | Ni1 | 2500 | 50 | 30 | 1.55 | 1.03 | 1.53 | 1.43 | 10.12 |
| 10 | Ni1 | 2500 | 30 | 15 | 2.00 | 2.67 | 2.26 | 1.56 | 61.57 |
| 11 | Ni1 | 2500 | 30 | 45 | 8.04 | 3.57 | 2.32 | 1.59 | 61.66 |
| 12 | Ni1 | 2500 | 30 | 60 | 9.93 | 3.31 | 2.43 | 1.68 | 63.49 |
| 13 | Ni2 | 2500 | 30 | 30 | 3.80 | 2.53 | 3.33 | 1.51 | 67.94 |
| 14 | Ni3 | 2500 | 30 | 30 | 8.00 | 5.33 | 2.65 | 1.72 | 70.37 |
| 15 | Ni4 | 2500 | 30 | 30 | 5.96 | 3.97 | 3.09 | 1.70 | 78.84 |
| 16 | Ni5 | 2500 | 30 | 30 | 7.47 | 4.98 | 3.42 | 2.32 | 70.26 |
| 17 | Ni6 | 2500 | 30 | 30 | 5.54 | 3.69 | 2.83 | 1.72 | 77.90 |
The ethylene polymerization was conducted at temperatures from 20 to 50 °C (entries 4 and 7–9, Table 2), and the maximum activity was observed at 30 °C (entry 4, Table 2). In addition, the higher temperature resulted in a lower activity and produced the polyethylene with a lower molecular weight (entries 4, 8, and 9, Table 2). This means that both the deactivation of active species and fast chain transfer take place at elevated temperatures,4,14 which was consistent with the analogous pre-catalysts.7 The narrow polydispersity was observed for all polyethylenes obtained, as confirmed by their GPC curves (Fig. 2).
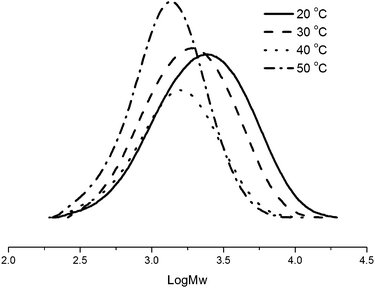 | ||
| Fig. 2 GPC curves of PEs using Ni1/MAO at different temperatures (entries 4, 7–9, Table 2). | ||
More interestingly, the lower melting points (Tm) of the resulting polyethylenes were confirmed using differential scanning calorimetry (DSC); a low melting point is commonly caused by the low molecular weight and/or high branching. It was surprising to observe a quite low Tm value of 10.12 °C for the polyethylene with a molecular weight of 1.53 kg mol−1 and a PDI of 1.43 (entry 9 in Table 2). It is worth mentioning the possibility of inaccurate data regarding measuring molecular weights of such polyethylene waxes because the measurement range of our equipment is set for 10 K to one million g mol−1. The 13C NMR measurement was conducted for the polymer at ambient temperature in deuterated 1,2-dichlorobenzene with TMS as an internal standard. Interpreted according to the literature,15 the spectrum (Fig. 3) indicated a branching number of 177 per 1000 carbons; the resonances of the carbons illustrate that the majority of the carbons are either tertiary or close to tertiary carbons, which are attributed to hydrogen migration happening on the active nickel species.16 The polyethylenes with lower molecular weights and high branching are in high demand as additives for lubricants and pour-point depressants in industry.
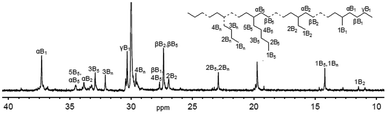 | ||
| Fig. 3 13C NMR spectrum of the polyethylene formed using Ni1/MAO at 50 °C (entry 9, Table 2). | ||
The polymerization tests were also conducted for different time periods from 15 to 60 min (entries 4 and 10–12, Table 2); the activities were well maintained along with prolonging the reaction time, indicating the long lifetime of the active species.17 Moreover, higher molecular weights and larger polydispersity indices of the polyethylenes were observed for longer reaction times.
Other nickel analogs (Ni2–Ni4) were extensively investigated at the Al/Ni ratio of 2500 and the temperature of 30 °C. For the purpose of comparison, the 8-(2,4,6-trimethylphenylimino)-5,6,7-trihydroquinolylnickel halides (Ni5 and Ni6),7b without cyclohexyl-substituents, were also investigated. The low molecular weight waxes were obtained with narrow polydispersity, indicating single-site active species. Better catalytic activities were observed for the nickel complexes bearing cyclohexyl-modified ligands (Ni1 and Ni3) (entries 4 and 13–17, Table 2); moreover, the bromo-nickel pre-catalysts showed higher activities than their chloro-nickel analogs (entries 14 to 15, and 16 to 17, Table 2), which was attributed to the better solubility of the bromide complexes.18
Ethylene polymerization in the presence of Et2AlCl
Similarly, complex Ni1 was extensively investigated with Et2AlCl to optimize the polymerization parameters (entry 1–11, Table 3). Within the Al/Ni ratios from 200 to 600 (entries 1–5, Table 3), the best activity of 4.85 × 106 g(PE) mol(Ni)−1 h−1 was observed at the Al/Ni ratio of 300 (entry 2, Table 3). Within the temperature range of 20 °C to 50 °C (entry 2 and 6–8, Table 3), the best activity was achieved at 30 °C (entry 2, Table 3). The obtained polyethylenes showed gradually lower molecular weights along with increasing the reaction temperature.| Entry | Cat. | Al/Ni | T (°C) | t (min) | Yield (g) | Act.b | M w (kg mol−1) | M w/Mnc | T m (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a 3 μmol of Ni; 10 atm of ethylene; total volume 100 mL. b Values in units of 106 g(PE) mol(Ni)−1 h−1. c Determined using GPC. d Determined using DSC. | |||||||||
| 1 | Ni1 | 200 | 30 | 30 | 4.46 | 3.00 | 1.83 | 1.49 | 55.98 |
| 2 | Ni1 | 300 | 30 | 30 | 7.27 | 4.85 | 1.84 | 1.56 | 55.15 |
| 3 | Ni1 | 400 | 30 | 30 | 6.83 | 4.55 | 1.86 | 1.58 | 55.80 |
| 4 | Ni1 | 500 | 30 | 30 | 6.38 | 4.25 | 1.90 | 1.59 | 56.49 |
| 5 | Ni1 | 600 | 30 | 30 | 5.89 | 3.93 | 2.02 | 1.59 | 59.18 |
| 6 | Ni1 | 300 | 20 | 30 | 4.94 | 3.29 | 2.13 | 1.62 | 65.05 |
| 7 | Ni1 | 300 | 40 | 30 | 3.70 | 2.47 | 1.51 | 1.46 | 16.42 |
| 8 | Ni1 | 300 | 50 | 30 | 0.73 | 0.49 | 1.43 | 1.38 | 9.11 |
| 9 | Ni1 | 300 | 30 | 15 | 2.12 | 2.92 | 1.81 | 1.54 | 55.19 |
| 10 | Ni1 | 300 | 30 | 45 | 7.88 | 3.50 | 1.88 | 1.56 | 56.07 |
| 11 | Ni1 | 300 | 30 | 60 | 8.27 | 2.76 | 1.88 | 1.92 | 56.21 |
| 12 | Ni2 | 300 | 30 | 30 | 3.60 | 2.40 | 3.21 | 1.79 | 63.77 |
| 13 | Ni3 | 300 | 30 | 30 | 6.05 | 4.03 | 1.72 | 1.54 | 57.02 |
| 14 | Ni4 | 300 | 30 | 30 | 5.08 | 3.39 | 2.19 | 1.87 | 60.11 |
| 15 | Ni5 | 300 | 30 | 30 | 6.10 | 4.10 | 2.24 | 1.42 | 68.25 |
| 16 | Ni6 | 300 | 30 | 30 | 4.47 | 2.98 | 1.98 | 1.71 | 60.61 |
Regarding the catalytic lifetime with Et2AlCl (entry 2 and 9–11, Table 3), the tendency observed is similar to that with MAO (entries 4 and 10–12, Table 2). With Et2AlCl, however, a significant change illustrated that the active species were rather formed with an initiating period because of the lower activity observed after 15 min than that after 30 min. Employing the Al/Ni ratio of 300 at 30 °C for 30 min (entry 2, Table 3), all other complexes also show high activities (entry 12–16, Table 3), producing polyethylenes with low molecular weights and narrow polydispersity. In general, compared with the systems using MAO, the resulting polyethylenes with Et2AlCl show somewhat lower molecular weights. Therefore, the current catalytic systems have a high potential for producing massive amounts of additives for lubricants and pour-point depressants. Again, the bromo-nickel pre-catalysts showed higher activities than their chloro-analogs (Table 3, entries 13 and 14 for complexes Ni3 and Ni4; entries 15 and 16 for complexes Ni5 and Ni6).
Conclusions
In conclusion, cycloalkyl-substituted 8-(2,4,6-trimethylphenylimino)-5,6,7-trihydroquinolylnickel halides (Ni1–Ni4) showed higher and adaptable activities than their analogous 8-(2,4,6-trimethylphenylimino)-5,6,7-trihydroquinolylnickel halides.7b Upon activation with either MAO or Et2AlCl, all nickel complexes exhibited high activities (up to 5.33 × 106 g(PE) mol(Ni)−1 h−1) with the single-site feature of the catalytic system. The resulting polyethylenes were found to be highly branched waxes with low molecular weights and narrow polydispersity, which indicates a high potential in their application as additives for lubricants and pour-point depressants.Acknowledgements
This work is supported by the NSFC (No. 21374123, 51411130208 and U1362204).Notes and references
- (a) W.-H. Sun, Adv. Polym. Sci., 2013, 258, 163–178 CrossRef CAS; (b) R. Gao, W.-H. Sun and C. Redshaw, Catal. Sci. Technol., 2013, 3, 1172–1179 RSC; (c) S. Wang, W.-H. Sun and C. Redshaw, J. Organomet. Chem., 2014, 751, 717–741 CrossRef CAS PubMed; (d) C. Bianchini, G. Giambastiani, L. Luconi and A. Meli, Coord. Chem. Rev., 2010, 254, 431–455 CrossRef CAS PubMed.
- (a) W. Zhang, W.-H. Sun and C. Redshaw, Dalton Trans., 2013, 42, 8988–8997 RSC; (b) J. Ma, C. Feng, S. Wang, K.-Q. Zhao, W.-H. Sun, C. Redshaw and G. A. Solan, Inorg. Chem. Front., 2014, 1, 14–34 RSC.
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414–6415 CrossRef CAS.
- (a) B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049–4050 CrossRef CAS; (b) G. J. P. Britovsek, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. J. McTavish, G. A. Solan, A. J. P. White and D. J. Williams, Chem. Commun., 1998, 849–850 RSC.
- (a) H. Liu, W. Zhao, X. Hao, C. Redshaw, W. Huang and W.-H. Sun, Organometallics, 2011, 30, 2418–2424 CrossRef CAS; (b) H. Liu, W. Zhao, J. Yu, W. Yang, X. Hao, C. Redshaw, L. Chen and W.-H. Sun, Catal. Sci. Technol., 2012, 2, 415–422 RSC; (c) S. Kong, C.-Y. Guo, W. Yang, L. Wang, W.-H. Sun and R. Glaser, J. Organomet. Chem., 2013, 725, 37–45 CrossRef CAS PubMed.
- (a) T. V. Laine, M. Klinga and M. Leskelä, Eur. J. Inorg. Chem., 1999, 959–964 CrossRef CAS; (b) T. V. Laine, K. Lappalainen, J. Liimatta, E. Aitola, B. Löfgren and M. Leskelä, Macromol. Rapid Commun., 1999, 20, 487–491 CrossRef CAS; (c) T. V. Laine, U. Piironen, K. Lappalainen, M. Klinga, E. Aitola and M. Leskel, J. Organomet. Chem., 2000, 606, 112–124 CrossRef CAS; (d) B. Y. Lee, X. Bu and G. C. Bazan, Organometallics, 2001, 20, 5425–5431 CrossRef CAS; (e) J. M. Benito, E. De Jesús, F. J. de la Mata, J. C. Flores, R. Gómez and P. Gómez-Sal, Organometallics, 2006, 25, 3876–3887 CrossRef CAS; (f) S. Jie, D. Zhang, T. Zhang, W.-H. Sun, J. Chen, Q. Ren, D. Liu, G. Zheng and W. Chen, J. Organomet. Chem., 2005, 690, 1739–1749 CrossRef CAS PubMed; (g) S. Zai, F. Liu, H. Gao, C. Li, G. Zhou, S. Cheng, L. Guo, L. Zhang, F. Zhu and Q. Wu, Chem. Commun., 2010, 46, 4321–4323 RSC; (h) H. Gao, H. Hu, F. Zhu and Q. Wu, Chem. Commun., 2012, 48, 3312–3314 RSC; (i) H. Hu, L. Zhang, H. Gao, F. Zhu and Q. Wu, Chem. – Eur. J., 2014, 20, 3225–3233 CrossRef CAS PubMed; (j) S. Zai, H. Gao, Z. Huang, H. Hu, H. Wu and Q. Wu, ACS Catal., 2012, 2, 433–440 CrossRef CAS.
- (a) J. Yu, X. Hu, Y. Zeng, L. Zhang, C. Ni, X. Hao and W.-H. Sun, New J. Chem., 2011, 35, 178–183 RSC; (b) J. Yu, Y. Zeng, W. Huang, X. Hao and W.-H. Sun, Dalton Trans., 2011, 40, 8436–8443 RSC; (c) L. Zhang, X. Hao, W.-H. Sun and C. Redshaw, ACS Catal., 2011, 1, 1213–1220 CrossRef CAS; (d) X. Hou, Z. Cai, X. Chen, L. Wang, C. Redshaw and W.-H. Sun, Dalton Trans., 2012, 41, 1617–1623 RSC; (e) W. Chai, J. Yu, L. Wang, X. Hu, C. Redshaw and W.-H. Sun, Inorg. Chim. Acta, 2012, 385, 21–26 CrossRef CAS PubMed.
- C. Shao, W.-H. Sun, Z. Li, Y. Hu and L. Han, Catal. Commun., 2002, 3, 405–410 CrossRef CAS.
- (a) Z. Guan and W. J. Marshall, Organometallics, 2002, 21, 3580–3586 CrossRef CAS; (b) F. Speiser, P. Braunstein, L. Saussine and R. Welter, Organometallics, 2004, 23, 2613–2624 CrossRef CAS; (c) Z. Weng, S. Teo and T. S. A. Hor, Organometallics, 2006, 25, 4878–4882 CrossRef CAS.
- (a) F. Speiser, P. Braunstein, L. Saussine and R. Welter, Inorg. Chem., 2004, 43, 1649–1658 CrossRef CAS PubMed; (b) X. Tang, D. Zhang, S. Jie, W.-H. Sun and J. Chen, J. Organomet. Chem., 2005, 690, 3918–3928 CrossRef CAS PubMed; (c) W.-H. Sun, Z. Li, H. Hu, B. Wu, H. Yang, N. Zhu, X. Leng and H. Wang, New J. Chem., 2002, 26, 1474–1478 RSC.
- (a) C. J. Stephenson, J. P. McInnis, C. Chen, M. P. Weberski, A. Motta, M. Delferro and T. J. Marks, ACS Catal., 2014, 4, 999–1003 CrossRef CAS; (b) T. Wiedemann, G. Voit, A. Tchernook, P. Roesle, I. Göttker-Schnetmann and S. Mecking, J. Am. Chem. Soc., 2014, 136, 2078–2085 CrossRef CAS PubMed.
- (a) N. I. Ivancheva, V. K. Badaev, I. I. Oleinik, S. S. Ivanchev and G. A. Tolstikov, Dokl. Phys. Chem., 2000, 374, 203–205 Search PubMed; (b) S. S. Ivanchev, G. A. Tolstikov, V. K. Badaev, N. I. Ivancheva, I. I. Oleinik, M. I. Serushkin and L. V. Oleinik, Polym. Sci., Ser. A, 2001, 43, 1189–1192 Search PubMed; (c) I. I. Oleinik, I. V. Oleinik, I. B. Abdrakhmanov, S. S. Ivanchev and G. A. Tolstikov, Russ. J. Gen. Chem., 2004, 74, 1575–1578 CrossRef CAS PubMed; (d) S. S. Ivanchev, G. A. Tolstikov, V. K. Badaev, I. I. Oleinik, N. I. Ivancheva, D. G. Rogozin, I. V. Oleinik and S. V. Myakin, Kinet. Catal., 2004, 45, 176–182 CrossRef CAS; (e) I. Oleinik, I. V. Oleinik, I. B. Abdrakhmanov, S. S. Ivanchev and G. A. Tolstikov, Russ. J. Gen. Chem., 2004, 74, 1423–1427 CrossRef CAS PubMed.
- X. Hou, T. Liang, W.-H. Sun, C. Redshaw and X. Chen, J. Organomet. Chem., 2012, 708–709, 98–105 CrossRef CAS PubMed.
- (a) G. J. P. Britovsek, M. Bruce, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. Mastroianni, S. J. McTavish, C. Redshaw, G. A. Solan, S. Strolmberg, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 1999, 121, 8728–8740 CrossRef CAS; (b) G. J. P. Britovsek, S. A. Cohen, V. C. Gibson and M. V. Meurs, J. Am. Chem. Soc., 2004, 126, 10701–10702 CrossRef CAS PubMed; (c) M. V. Meurs, G. J. P. Britovsek, V. C. Gibson and S. A. Cohen, J. Am. Chem. Soc., 2005, 127, 9913–9923 CrossRef PubMed.
- G. B. Galland, R. F. de Souza, R. S. Mauler and F. F. Nunes, Macromolecules, 1999, 32, 1620–1625 CrossRef CAS.
- M. M. Wegner, A. K. Ott and B. Rieger, Macromolecules, 2010, 43, 3624–3633 CrossRef CAS.
- Q. Xing, K. Song, T. Liang, Q. Liu, W.-H. Sun and C. Redshaw, Dalton Trans., 2014, 43, 7830–7837 RSC.
- E. Yue, L. Zhang, Q. Xing, X.-P. Cao, X. Hao, C. Redshaw and W.-H. Sun, Dalton Trans., 2014, 43, 423–431 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of all organic compounds and nickel complexes. The ethylene polymerization using nickel complex pre-catalysts as well as the characteristics of the obtained polyethylenes. CCDC 1029135 for Ni1. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00162a |
| This journal is © the Partner Organisations 2015 |