Graphene and graphene oxide: advanced membranes for gas separation and water purification
Quan
Xu
*a,
Hong
Xu
a,
Jiarui
Chen
b,
Yunzu
Lv
a,
Chenbo
Dong
c and
Theruvakkattil Sreenivasan
Sreeprasad
c
aState Key Laboratory of Heavy Oil Processing, Institute of New Energy, China University of Petroleum (Beijing), Beijing, 102249, China. E-mail: xuquan@cup.edu.cn
bDepartment of Chemical Engineering, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, China
cDepartment of Civil and Environmental Engineering, Rice University, Houston, Texas 77005, USA
First published on 4th March 2015
Abstract
Advanced membrane systems with excellent permeance are important for controllable separation processes, such as gas separation and water purification. The ideal candidate materials should be very thin to provide high permeance, be stiff enough to withstand working under high applied pressure, with a large surface area and micro- or nano-pore structure for excellent selectivity. Graphene oxide (GO) nanosheets are graphene with oxygen-containing functional groups, obtained by treating graphite with strong oxidizers. Graphene-based materials, by virtue of their high mechanical strength, large surface area, single-atom-thick unique two-dimensional honeycomb lattice structure, and narrow pore distribution, provide exciting opportunities to assemble novel types of advanced, ultra-thin, high-efficiency membrane devices. In this contribution, we discuss the progress made in the direction of using graphene oxide as high-efficiency membranes for gas separation and water purification. The primary focus will be on introducing the fabrication processes, exceptional properties, and innovative membrane applications of two-dimensional graphene oxide materials for controllable separation processes. This state-of-the-art review will provide a platform for understanding the intricate details of gas and water molecular transport through laminar graphene oxide membranes, as well as a summary of the latest process in the field.
 Jiarui Chen | Jiarui Chen is currently a senior student at the Department of Chemical Engineering, Xi'an Jiaotong University. His research interest is nanomaterial manufacture and characteristics. |
Introduction
Separation is a critical industrial process that finds a broad range of applications in natural gas purification, hydrogen production, and sea-water desalination.1,2 The low efficacy of the prevailing technologies necessitates the formulation of advanced separation techniques to solve urgent environmental and energy issues. In comparison to traditional distillation,3 absorption,4 adsorption5,6 or sublimation,7 membrane-based technology exhibits considerable promise. The key advantages of membrane-based strategies for small-to-medium scale gas separation and water filtration applications are (i) overall economics, (ii) safety, (iii) comparatively environmentally friendly nature, and (iv) easy recycling.8,9 Currently, membranes made from polymers, zeolite and silicon are being successfully applied in industry.10 However, permeability and selectivity are two inherent trade-offs for conventional membrane materials that limit their usage.Recently, considerable interest has been aroused by carbon-based nanomaterials because of their easy accessibility, excellent mechanical properties, biocompatibility, and environmental friendliness.11,12 One-dimensional carbon nanomaterials (e.g. carbon nanotubes, CNTs) were initially believed to be promising candidate materials for membrane assembly due to their unique hollow structure with open ends and extremely strong mechanical properties. However, there is a technical barrier in the assembly of vertically-aligned nanotube arrays with high density on a large scale,13 and the fabrication of CNT-based separation membranes remains a theoretical possibility. Another carbon-based nanomaterial with great mechanical properties, carbon diamond composed of amorphous sp3 hybridized carbon atoms, was also investigated for filtration applications. However, the large energy consumption, due to the low utilization efficiency of carbon diamond material-based membranes devices, inhibited their large scale industrial application.14 Thus, the development of more applicable and economical carbon nanomaterial-based separation membranes is urgently needed.
Graphene, a single-atom-thick sheet of sp2 hybridized carbon atoms arrayed in a honeycomb pattern, has opened the door for assembling separation membranes with improved separation capabilities.15 The concept and the preparation of graphene-based materials have been explored for over 100 years. As early as in 1859, Brodie prepared graphitic oxide with a strong oxidizing mixture.16 In 1958, Hummers reported a redox method to prepare graphitic oxide.17 Since its first isolation in 2004, graphene has drawn increasing attention in diverse branches of science and technology owing to its exceptional physicochemical properties, including mono-atomic thickness, high mechanical strength, and significant chemical inertness.18 With its ubiquitous properties, graphene has displayed great potential for atomic permeation, water transportation, and gas separation.19–21 For mass production, user-controllable pore distribution, and application under high-pressure conditions, graphene's oxygen-containing analogue, graphene oxide (GO), is being considered for filtration/membrane fabrication and utilization. The possibility of facile and large scale production of GO and its unique properties has opened up new opportunities for the production of advanced membrane separation technology. GO-based membranes have been used in water treatment and liquid organic molecular separation.22,23 It should be noted that the physicochemical status of the oxygen-containing groups in GO sheets plays a significant role in determining their separation performance. This offers several straightforward strategies to control pore size in the tiny channels of GO via tuning the types and forms of oxygen-containing groups.
In this mini-review paper, the application of graphene and GO under consideration for separation membrane applications is discussed. The discussion is divided into three parts. The first part describes the application of graphene and its derivatives in gas separation, while the second part focuses on their unique applications in ion sieving, as well as their role in water purification with different mechanisms. Lastly, the final part of the review gives a summary and outlook of graphene-based separation membranes, the existing challenges and new directions in this emerging research field. This critical review offers a comprehensive discussion of graphene-based membranes from the perspective of broad separation applications, as well as the advanced progress of such membranes with improved separation performance.
Graphene and GO for gas and water molecule separation
Compared to polymer, zeolite, and silica-based membranes, layered graphene and GO membranes display distinguishing characteristics including excellent stiffness, capacity for large-scale production, and hydrophobic properties. Recent studies have demonstrated that graphene-based membranes exhibit significant potential for gas24–26 and water molecule separation.27Graphene and GO for gaseous selectivity
A mono-layer of graphene is well known to resist the permeation of small gaseous molecules (e.g. He),28 as the electron density of its hexagonal rings will repel the atoms and molecules trying to pass through them. Based on this, an atomic layer of pristine graphene can be considered as a perfect gas barrier material. Although graphene is impermeable to gases, nanoporous graphene (NPG), due to its high surface porosity, can efficiently select gases. Hence, graphene, with its monatomic thickness, can potentially surpass the permeability and selectivity limits of conventional membranes and is considered as the ideal membrane material. Theoretical computational studies revealed that NPG had higher selectivity and flow rates compared to traditional polymer and silica-based membranes. For example, Jiang et al.29 (Fig. 1(a)) investigated the permeability and selectivity of nitrogen-doped porous single-layer graphene and found that the graphene layer exhibited high selectivity (up to 108) toward H2–CH4 gas flow after designing and adjusting the subnanometer-sized pores. In addition, Schrier30 simulated NPG with different functionalized pores for separating He, Ne and CH4 and found the possibility of high helium selectivity using a porous graphene membrane. Also, Hauser et al.31 pointed out that NPG with different functionalized graphene pores can efficiently select 3He over 4He. Du et al.,32via a simulated separation process for H2 and N2, reported that the H2 flow rate through NPG was linear in relation to the pore size of graphene, while N2 flow rate was independent of it. Trinh et al.,33 with the help of the calculated bonding energy between the molecule and graphene, analysed the selectivity and self-diffusion of CO2 and H2 on few-layer graphene (Fig. 1(b)) at different temperatures. It was suggested that the selectivity of CO2 over H2 is five times greater at lower temperatures compared to that at higher temperatures. The thickness of the graphene also was found to regulate the self-diffusion and flow rate of gases. The pore size, the nature of the functional groups at the edges of the pores, thickness, temperature, local defects and wrinkling (Fig. 1(c))34 are considered as the key factors affecting the selectivity of pristine graphene. Other mechanisms, such as the pore translocation-limited mechanism, surface adsorption mechanism and rearrangement reaction mechanism, are also intensively evaluated and discussed in the literature.35–38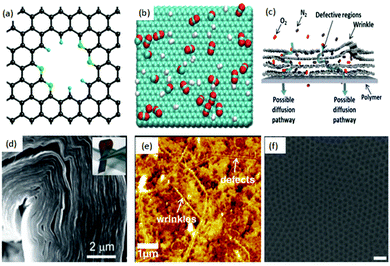 | ||
| Fig. 1 (a) Creation of a nitrogen-functionalized pore within a graphene sheet. Color code: C, black; N, green; H, cyan.29 (b) Typical snapshot of a gas mixture of CO2 and H2 in equilibrium with a graphite surface. The temperature is T = 300 K and the number of particles is N = 700. The green, red and white colors represent carbon, oxygen, and hydrogen atoms, respectively.33 (c) Schematic illustration of possible gas transport through a few-layered, defective graphene membrane.34 (d) Large area GO can be bent over 90 degrees.47 (e) Atomic force microscopy (AFM) image of a graphene/PTMSP membrane surface; wrinkles and defects are clearly seen in the surface.34 (f) Manufactured graphene nanomesh;40 the pore size of the graphene sheet can be controlled.52 | ||
Since theoretical results illustrated the tremendous promise of graphene and GO as next generation membranes (Fig. 2(b)),39 several strategies have been developed to synthesize NPG to achieve a functional separation membrane. The most important methods include electron beam40,41 and ultraviolet-induced oxidative42 etching of holes on graphene surfaces, creation of porous two-dimensional sheets from molecular building blocks,43 and sandwiching/tethering of graphene with carbon nanotubes to create 3D structures.44 The progress in experimental processes allowed the successful transfer of large area GO sheets onto different substrates under suitable conditions.45 Further, the ability to precisely control the thickness and chemical functionalization of GO46 resulted in the development of facile approaches for fabricating GO-based membranes. Compared to graphite foils, the unique interlocking-tile arrangement of the nanoscale GO showed a higher fracture energy (350 kJ m−3) and high tensile strength (42 ± 2 GPa).47 Although GO has high tensile strength and bending strength, it can still be folded over 90 degrees (Fig. 1(d)), and this stiff-yet-flexible property of GO makes it a promising membrane. Moreover, various routes have been successfully reported for the production of large area graphene48,49 and GO50 with accurate evaluation of its mechanical properties.51 For example, Kim et al.34 successfully transferred a large area of graphene and GO onto a polymer substrate and measured the transport and selectivity of O2–N2 and CO2–N2 on graphene and its derivative-based membrane (Fig. 1(e)). Selective gas diffusion can be achieved by controlling the gas flow channels and pores via different stacking processes. Heating the system and applying pressure could also result in a better selectivity of the system. During the flux process, gases interact with both pores and interlayers. Thus, functional groups inserted at the pore edges or between layers can further enhance the selectivity of the system. Other effective methods are also reported for the formation of structured pores in graphene membranes. Russo et al.41 successfully constructed NPG with pore radii as small as 3 Å with atomic precision using ion beam exposure. Bai et al.52 employed block copolymer lithography to prepare NPG with neck widths as low as 5 nm. Li et al.53 created GO with a thickness approaching 1.8 nm, supported on porous anodic aluminium oxide (AAO). These membranes showed mixture separation selectivities (as high as 3400 and 900) for H2–CO2 and H2–N2 mixtures. Bai et al.52 made a graphene nanomesh and could precisely control the pore size and pattern after a series of processes (Fig. 1(f)).
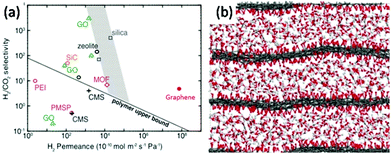 | ||
| Fig. 2 (a) Comparison of H2–CO2 separation performances of porous graphene membranes (7.6 nm pore diameter, with 4.0% porosity) and other membranes: graphene oxide (GO),34–53 poly(1-trimethylsilyl-1-propyne) (PTMSP), polyetherimide (PEI), carbon molecular sieve (CMS), zeolite, silica, metal–organic framework (MOF), and SiC.54 (b) Simulation result of CO2 flow between GO layers.39 | ||
Traditional membranes, such as silica, polymer and zeolite, follow the rule that selectivity is a product of diffusivity and solubility.10 Numerical attempts have been developed for increasing those two parameters to obtain the desired performance. However, this law is no longer valid for graphene and GO. For single-layer graphene, the selectivity is closely affected by the pore size. The traditional concept of thickness is no longer linearly related to the diffusion speed and needs to be reconsidered. Although graphene is one of the strongest materials in the world, due to its unique 2D ultrathin structure it has excellent flexibility and a high diffusion coefficient. For example, Celebi et al.54 deposited pre-synthesized bi-layers of graphene on a SiNx frame, punctured with 49 pores each of 4 um in diameter, forming graphene with a calculated thickness of 1 nm. The H2 separation permeance of the porous graphene membrane is much higher than those of membranes made of polymer, silicon or composite materials (Fig. 2(a)). Defects and pore size are considered to be two critical parameters which determine the selectivity. Pressure and temperature, which can control the effusion rate, will also affect the permeation.33
Graphene and GO as membranes for water purification
Water flux and the rejection of dissolved impurities are two main aspects governing the productivity and separation performance of graphene and GO-based membranes in water purification processes. When the pore diameter is larger than 0.8 nm, graphene membranes show higher water flux than CNT membranes due to higher velocity in the centre region.55 An interesting study indicated that graphene nanopores are able to block salt ions with 2–3 orders of magnitude greater water permeability than commercial reverse osmosis membranes.56 However, the helium-impermeable GO membrane allows unimpeded water permeation.57 Since unusual molecular behaviour occurs on nanometer-length scales, various mechanisms of water permeation through the layer-by-layer and porous microstructure of the GO membrane are proposed using molecular dynamics (MD) simulations. Nair et al.57 attributed the anomalous water permeation to low friction between the water monolayer and pristine graphene regions and proposed a capillary-driven flow mechanism. Based on the work of Nair et al., Boukhvalov et al.58 suggested that interlayer spaces determine the formation of a hexagonal ice bilayer between flakes in pristine regions and the melting transition of ice at the edges, and are responsible for perfect water permeation and the water flux. Since monolayer graphene sheets are hydrophobic with a water contact angle of 95–100°,59 Wei et al.60 investigated the wetting properties of GO and found that the patterns of the oxidized region and the morphological corrugation of the sheet critically influence the spreading of water droplets (Fig. 3(a)). Huang et al.61 recently developed an advanced filter membrane with a superior separation performance by constructing nanostrand-channelled GO ultrafiltration membranes with a narrow pore size distribution (3–5 nm). The enhanced viscous water flow through the channels was attributed to the intricate porous structure and significantly reduced channel length in the device. Wei and co-authors also attributed the enhanced water flow in graphenic membranes to their unique porous microstructures and pointed out that the side-pinning effect caused by H-bonds between water molecules and oxidized regions could inhibit fast water transport in graphene channels, and enhanced water flow was prominently attributed to the porous microstructure.62 Therefore, in order to improve water permeability, a small sheet size, high density of inter-edge spaces, and wide nanochannels are suggested in GO membrane fabrication.62 The interlayer channel size can be tuned by intercalating different-sized cross-linkers.63 Furthermore, Xu et al.64 quantified the nature of interlayer flow and concluded that the chemical functionalization and relaxation of nano-confinement in GO can cause the breakdown of flow enhancement. On the other hand, the effect of membrane structure and operation conditions on water flux in graphene and GO-based membranes have been explored both via simulation and experiments. By using computational methods, David et al.65 proposed that water flux through monolayer graphene has a linear relationship with pore area. Moreover, it was understood that hydrophilic pores increased water permeation due to H-bonding between water molecules and pore edges, and quantitatively the hydrophilic edges of the pores contribute more to high water flux compared to the pore area and size of graphenic membranes. For traditional polymeric membranes, flux is inversely proportional to the thickness. However, the same argument seems invalid for GO membranes. Meng Hu et al.66 synthesized GO membranes with different numbers of layers and tested the water separation performance and found no obvious correlation between water flux and the number of layers. Yi Han et al.,67via an investigation on base-reflux-induced reduced GO, concluded that water flux increased linearly with pressure under 7 bar and decreased exponentially with GO loading. The above two studies showed contradictory conclusions on the relationship between GO thickness and water flux, and reiterated that there is a need to comprehensively investigate this aspect.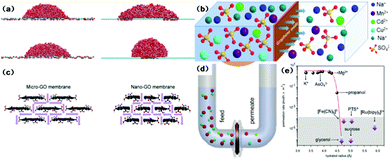 | ||
| Fig. 3 (a) (left) Simulation snapshots of a water droplet on wrinkled graphene with different corrugation amplitudes; (right) simulation snapshots of a droplet on GO patterned with separated oxidized regions (top) and a single oxidized region covered by the droplet (bottom).60 (b) Schematic diagram of the penetration processes of different ions through GO membranes.69 (c) Schematic diagrams of the structure of capillary networks in nano- and micro-GO membranes.72 (d) Schematic of experimental setup. A U-shaped tube 2.5 cm in diameter is divided by the GO membrane into two compartments referred to as feed and permeate. Each is filled to a typical level of ∼20 cm. Magnetic stirring is used so as to ensure no concentration gradients.71 (e) Sieving through atomic-scale mesh. The shown permeation rates are normalized per 1 M feed solution and are measured by using 5 mm-thick membranes.71 | ||
The rejection of ions and organic dye molecules by graphene and GO membranes is also intensively studied. The selectivity of this process is primarily attributed to size exclusion and interactions (including chemical and electrostatic ones) with functional groups. Grossman et al.65 proposed the high performance of monolayer graphene to filter out NaCl from water via their computational investigation. Small pores, low pressure, and hydrophobic pores reject salts more efficiently due to direct size exclusion, while the larger effective volume of ions and the lack of H-bonding can lead to a higher energy barrier to ionic passage. Hu et al.66 reported that GO membranes display significant rejection of monovalent and divalent salts and moderate or high rejection of organic dyes. Similar results are also observed in the work of Han et al.67 The high rejection for organic dyes is ascribed to size exclusion and electrostatic interaction, and the rejection of ions to Donnan's exclusion. Huang et al.68 investigated the effects of salt concentration, pH, and pressure on the controlled separation of small molecules through GO membranes. All these factors modulated the separation performance due to their role in changing the interlayer spacing. Specially, low pH (<6) and high pressure could result in low water flux and high rejection of organic dyes. Xu et al.69 demonstrated GO's selective permeation of different metal salts and reported fast permeation of sodium salts, slow permeation of heavy-metal salts and no permeation of copper ions and organic impurities (Fig. 3(b)). The simulation of the trans-membrane transport of alkali and alkaline earth cations indicated that the coordinative interactions are responsible for selectivity.70 Joshi et al.71 tested the permeation characteristics of micrometer-thick GO laminates for permeation ability (Fig. 3(d)). It was observed that ultrafast permeation is possible only for ions smaller than a specific size. In this work, the permeation rate was first quantified from the slope of the concentration curve (Fig. 3(e)). The permeation selectivity was attributed to size exclusion and the anomalous fast transportation is due to a capillary force.
Graphene and GO can be used as other kinds of water treatment membranes as well. Sun et al.72 used micron-sized and nano-sized GO (Fig. 3(c)) as cation exchange membranes for acid recovery from FeCl3 solution. The nano-GO membrane exhibited enhanced penetration of both H+ and Fe3+. While H+ transport through the nano-GO membrane was two orders of magnitude greater, no Fe3+ transportation occurred at very low concentrations (e.g. 0.01 mol L−1). Huang et al.73 employed a GO membrane to separate a dimethyl carbonate–water mixture through a pervaporation process. The separation process was believed to follow a sorption–diffusion mechanism. Tang et al.74 also used a pervaporation process for dehydration of ethanol using GO thin films. The selectivity obtained from the binary-component test was much lower than the ideal value calculated from single-component feed analysis. This can be attributed to the increased ethanol flux through the thin films caused by the enlarged interlayer space due to the H-bonding between water molecules and the functional groups on the GO surface.
Due to their excellent intrinsic mechanical strength,75 high chemical stability,76 high antibacterial activity,77 and exquisite antifouling properties,47 GO membranes demonstrated significant promise for applications in water purification in various studies. By adjusting the pore size and nanochannels via induction and modification of surface oxygen-containing groups, GO membranes can be oriented towards a narrow pore size distribution, which is advantageous for precise sieving based on the size exclusion mechanism.
Conclusions and outlook
In summary, membranes based on two-dimensional monolayer graphene and its functionalized analogue, GO, with their ultrafast permeance, outstanding mechanical properties as well as exceptional energy-efficiency, are increasingly coming to the fore as promising candidates for precise and selective gas separation and water purification processes (Fig. 4).Although graphene and GO demonstrate excellent selectivity and superior performance against multiple gas mixtures, several challenges still exist in their path to industrial application. Firstly, the grain boundary, defects, impurities, polymer, or molecular residues can block the pores on graphene or GO sheets. This will result in the dramatic reduction of the flow rate. Hence, the mixture needs to be carefully purified before entering graphene membrane systems. The humidity is also reported to affect the selectivity. In addition, for graphene or GO-based gas separation membranes, the selectivity for H2–H2S, CO2–CH4, or mixtures of three or more gases is still not well characterized. Thus a comprehensive analysis of the parameters that influence the process is necessary before predicting the mechanism of selectivity. These studies are expected to attract a great deal of interest from industry, especially for natural gas and shale gas industries, due to the importance of the separation process in their sustainability. Secondly, the possibility of finding a large number of inherent defects in the products hurts the chance for large scale industrial application of graphene and GO. Although a single layer of graphene has a Young's modulus of up to 1 TPa, local bond breaking and bond rotation at the crack tip of the graphene50 could lead to dramatic loss of mechanical properties in products. This can render the membranes less robust and more fragile, and even create unexpected large pores during use, and result in the failure of the separation system. Since pore size is the most important parameter in controlling the performance of graphenic membrane systems, GO can selectively separate gas mixtures though modulation of its layer-to-layer distance and the chemical groups on the surfaces and layer interfaces as well. The third challenge is the cost. Previously, the high cost of production of GO was retarding its large scale applications. However, the advent of newer technologies has decreased the cost of graphene and GO and more and more methods are being developed to fabricate defect-free, larger area graphene with controllable pore structure/size and interlayer distance. The authors envisage that this particular field will attract increased interest from academia and industry in the coming years.
As for water purification, although considerable progress has been achieved in the fundamental understanding and specialized industrial applications of graphene and GO-based membranes, there are still several promising topics that are worth exploring. For example, the precise design of user-controlled GO membranes, including the pattern of oxidized regions, the interlayer space, the pore size, modification of pore chemistry, and the number of graphene layers, is still not entirely successful. The lessons learned about the parameters that determine the small molecule permeation and separation performance can be transplanted to fabricate solid-state ionic devices or microfluidic devices. Likewise, detailed investigations on the effect of the interlayer space or surface defect on water flux, and whether size exclusion or interaction contributes more to the selectivity, are also essential. A compromise between water flux and selectivity may be made to determine the optimal membrane fabrication parameters. In order to achieve higher water flux without sacrificing selectivity, modifying the membrane with different atoms and functional groups to adjust water affinity could be employed. In addition, the swelling of GO in aqueous solutions needs to be regulated to achieve the expected selectivity. Moreover, for industrial processes, the operating pressure is an important factor which needs to be considered. Operating without applying a differential pressure will be highly beneficial. Furthermore, if high purity of the product (e.g. to produce high purity acid from a high-concentration FeCl3 solution) is required, pre-treatment or post-treatment will be needed, which increases energy consumption and total cost. Therefore, additional efforts are needed for minimizing the difference between theoretical and experimental results on water/ion transport mechanisms across GO nanosheets in water. There is also a need to consider and solve the contamination issue for graphene or GO-based membranes. The effects of adsorption of metals and organic dyes, ionic clogging inside the capillaries, and coordination occupation on the surface of oxidized regions needs further investigation and optimization. To further improve the outstanding antifouling capability of GO-based membranes, it is crucial to tune the balance between the fouling rejection and flux decline. Improving the antimicrobial efficiency and mechanical strength of the freestanding membranes can be the key to the successful balancing act.
Compared to GO, graphene is simpler to design, more toxic to microbacteria,78 and more resistant to swelling in water, ionic clogging inside the capillaries and coordination occupation on the surface of oxidized regions. Thus, it is more competitive compared to GO. For large scale productivity, and industrial use, novel reduction methods need to be developed. A suitable substrate is imperative to avoid dispersion in the liquid phase and to ensure water transportation. Experiments illustrated that the water flux is approximately linear in relation to the applied pressure, and so graphene membranes cannot be used without energy input. Study also showed that even at low pressure (<100 bar), graphene still allows ultra-high water flux,79 indicating that it is a promising membrane for water desalination. David et al.80 examined the mechanical strength of nanoporous graphene as a reverse osmosis membrane and GO showed robustness and suitability for reverse membranes. To further improve the productivity, it is crucial to achieve high pore density without compromising the mechanical strength.
Currently, MD simulation is used to explore the mechanism of molecular permeation, and various theories have been put forward. Experimental studies have analysed the effect of pressure, pH, pore chemistry, pore area, and number of layers on water flux and the permeation selectivity of various ions and molecules. However, discrepancies exist between theory and experiment. To solve this problem, dedicated MD simulation focusing on the permeation of different ions and molecules through graphene and GO membranes should be performed to correct the existing theoretical models and bring them close to experimental results. On the other hand, further simulation and experimental studies are needed to determine whether size exclusion or interaction contributes more to the permeation selectivity of ions and molecules. This could provide insights for designing next-generation membrane technologies and evaluating their potential for application in various fields.
Acknowledgements
We thank the Science Foundation of China University of Petroleum Beijing (no. 2462014YJRC011) for support.Notes and references
- R. W. Baker, J. Membr. Sci., 2010, 362, 134–136 CrossRef CAS PubMed.
- R. W. Baker and K. Lokhandwala, Ind. Eng. Chem. Res., 2008, 47, 2109–2121 CrossRef CAS.
- X. Gao, J. Chen, Z. Ma and L. Yang, Ind. Eng. Chem. Res., 2014, 53, 14440–14445 CrossRef CAS.
- Y. S. Sistla and A. Khanna, J. Ind. Eng. Chem., 2014, 20, 2497–2509 CrossRef CAS PubMed.
- H. Bi, Z. Yin, X. Cao, X. Xie, C. Tan, X. Huang, B. Chen, F. Chen, Q. Yang, X. Bu, X. Lu, L. Sun and H. Zhang, Adv. Mater., 2013, 25, 5916–5921 CrossRef CAS PubMed.
- H. Bi, X. Huang, X. Wu, X. Cao, C. Tan, Z. Yin, X. Lu, L. Sun and H. Zhang, Small, 2014, 10, 3544–3550 CrossRef CAS PubMed.
- J. L. Pi, C. F. You and C. H. Chuang, J. Anal. At. Spectrom., 2014, 29, 861–867 RSC.
- C. A. Scholes, G. W. Stevens and S. E. Kentish, Fuel, 2012, 96, 15–28 CrossRef CAS PubMed.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- C. Dong, A. S. Campell, R. Eldawud, G. Perhinschi, Y. Rojanasakul and C. Z. Dinu, Appl. Surf. Sci., 2013, 264, 261–268 CrossRef CAS PubMed.
- A. S. Campbell, C. Dong, J. S. Dordick and C. Z. Dinu, Process Biochem., 2013, 48, 1355–1360 CrossRef CAS PubMed.
- M. Yu, H. Funke, J. L. Falconer and D. Richard, Nano Lett., 2008, 9, 225–229 CrossRef PubMed.
- S. Karan, S. Samitsu, X. Peng, K. Kurashima and I. Ixhinose, Science, 2012, 335, 444–447 CrossRef CAS PubMed.
- H. Huang, Y. Ying and X. Peng, J. Mater. Chem. A, 2014, 2, 13772–13782 CAS.
- B. C. Brodie, Philos. Trans. R. Soc. London, 1859, 149, 249–259 CrossRef.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- L. Tsetseris and S. T. Pantelides, Carbon, 2014, 67, 58–63 CrossRef CAS PubMed.
- J. G. Gai, X. L. Gong, W. W. Wang, X. Zhang and W. L. Kang, J. Mater. Chem. A, 2014, 2, 4023–4028 CAS.
- H. Liu, S. Dai and D. Jiang, Solid State Commun., 2013, 175, 101–105 CrossRef PubMed.
- K. Goh, L. Setiawan, L. Wei, R. Si, A. G. Fane, R. Wang and Y. Chen, J. Membr. Sci., 2015, 474, 244–253 CrossRef CAS PubMed.
- R. Liu, G. Arabale, J. Kim, K. Sun, Y. Lee, C. Ryu and C. Lee, Carbon, 2014, 77, 933–938 CrossRef CAS PubMed.
- Y. Tao, Q. Xue, Z. Liu, M. Shan, C. Ling, T. Wu and X. Li, ACS Appl. Mater. Interfaces, 2014, 6, 8048–8058 CAS.
- G. Lei, C. Liu, H. Xie and F. Song, Chem. Phys. Lett., 2014, 599, 127–132 CrossRef CAS PubMed.
- A. Ambrosetti and P. L. Silvestrelli, J. Phys. Chem. C, 2014, 118, 19172–19179 CAS.
- K. A. Mahmoud, B. Mansoor, A. Mansour and M. Khraisheh, Desalination, 2014, 356, 208–225 CrossRef PubMed.
- J. S. Bunch, S. S. Verbridge, J. S. Alden, A. M. van der Zande, J. M. Parpia, H. G. Craighead and P. L. McEuen, Nano Lett., 2008, 8, 2458–2462 CrossRef CAS PubMed.
- D. Jiang, V. R. Cooper and S. Dai, Nano Lett., 2009, 9, 4019–4024 CrossRef CAS PubMed.
- J. Schrier, J. Phys. Chem. Lett., 2010, 1, 2284–2287 CrossRef CAS.
- A. W. Hauser and P. Schwerdtfeger, J. Phys. Chem. Lett., 2011, 3, 209–213 CrossRef.
- H. Du, J. Li, J. Zhang, G. Su, X. Li and Y. Zhao, J. Phys. Chem. C, 2011, 115, 23261–23266 CAS.
- T. T. Trinh, T. J. Vlugt, M. B. Hagg, D. Bedeaux and S. Kjelstrup, Front. Chem., 2013, 1, 1–9 Search PubMed.
- H. W. Kim, H. W. Yoon, S. M. Yoon, B. M. Yoo, B. K. Ahn, Y. H. Cho, H. J. Shin, H. Yang, U. Paik, S. Kwon, J. Y. Choi and H. B. Park, Science, 2013, 342, 91–95 CrossRef CAS PubMed.
- L. W. Drahushuk and M. S. Strano, Langmuir, 2012, 28, 16671–16678 CrossRef CAS PubMed.
- S. Eigler, C. Dotzer, A. Hirsch, M. Enzelberger and P. Muller, Chem. Mater., 2012, 24, 1276–1282 CrossRef CAS.
- C. Sun, M. S. Boutilier, H. Au, P. Poesio, B. Bai, R. Karnik and N. G. Hadjiconstantinou, Langmuir, 2014, 30, 675–682 CrossRef CAS PubMed.
- S. Zhou and A. Bongiorno, Acc. Chem. Res., 2014, 47, 3331–3339 CrossRef CAS PubMed.
- D. Kim, D. W. Kim, H. K. Lim, J. Jeon, H. Kim, H. T. Jung and H. Lee, J. Phys. Chem. C, 2014, 118, 11142–11148 CAS.
- M. D. Fischbein and M. Drndić, Appl. Phys. Lett., 2008, 93, 113107–113110 CrossRef PubMed.
- C. J. Russo and J. A. Golovchenko, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 5953–5957 CrossRef CAS PubMed.
- S. P. Koenig, L. Wang, J. Pellegrino and J. S. Bunch, Nat. Nanotechnol., 2012, 7, 728–732 CrossRef CAS PubMed.
- P. Kuhn, A. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333–13337 CrossRef CAS PubMed.
- J. Niu, M. Li, W. Choi, L. Dai and Z. Xia, Carbon, 2014, 67, 627–634 CrossRef CAS PubMed.
- S. C. O'Hern, C. A. Stewart, M. S. H. Boutilier, J. C. Idrobo, S. Bhaviripudi, S. K. Das, J. Kong, T. Laoui, M. Atieh and R. Karnik, ACS Nano, 2012, 6, 10130–10138 CrossRef PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed.
- X. Li, W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- S. S. Terdalkar, S. Huang, H. Yuan, J. J. Rencis, T. Zhu and S. Zhang, Chem. Phys. Lett., 2010, 494, 218–222 CrossRef CAS PubMed.
- J. Bai, X. Zhong, S. Jiang, Y. Huang and X. Duan, Nat. Nanotechnol., 2010, 5, 190–194 CrossRef CAS PubMed.
- H. Li, Z. Song, X. Zhang, Y. Huang, S. Li, Y. Mao, H. J. Ploehn, Y. Bao and M. Yu, Science, 2013, 342, 95–98 CrossRef CAS PubMed.
- K. Celebi, J. Buchheim, R. M. Wyss, A. Droudian, P. Gasser, I. Shorubalko, J. I. Kye, C. Lee and H. G. Park, Science, 2014, 344, 289–292 CrossRef CAS PubMed.
- M. E. Suk and N. Aluru, J. Phys. Chem. Lett., 2010, 1, 1590–1594 CrossRef CAS.
- T. M. Pendergast, T. Mary and M. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 Search PubMed.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed.
- D. W. Boukhvalov, M. I. Katsnelson and Y. W. Son, Nano Lett., 2013, 13, 3930–3935 CrossRef CAS PubMed.
- F. Taherian, V. Marcon, N. F. A. van der Vegt and F. Leroy, Langmuir, 2013, 29, 1457–1465 CrossRef CAS PubMed.
- N. Wei, C. Lv and Z. Xu, Langmuir, 2014, 30, 3572–3578 CrossRef CAS PubMed.
- H. Huang, Z. Song, N. Wei, L. Shi, Y. Mao, Y. Ying, L. Sun, Z. Xu and X. Peng, Nat. Commun., 2013, 4, 2979–2988 Search PubMed.
- N. Wei, X. Peng and Z. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 5877–5883 CAS.
- B. Mi, Science, 2014, 343, 740–742 CrossRef CAS PubMed.
- N. Wei, X. Peng and Z. Xu, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2014, 89, 012113–012118 CrossRef.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed.
- M. Hu and B. Mi, Environ. Sci. Technol., 2013, 47, 3715–3723 CrossRef CAS PubMed.
- Y. Han, Z. Xu and C. Gao, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- H. Huang, Y. Mao, Y. Ying, Y. Liu, L. Sun and X. Peng, Chem. Commun., 2013, 49, 5963–5965 RSC.
- P. Sun, M. Zhu, K. Wang, M. Zhong, J. Wei, D. Wu, Z. Xu and H. Zhu, ACS Nano, 2012, 7, 428–437 CrossRef PubMed.
- P. Sun, F. Zheng, M. Zhu, Z. Song, K. Wang, M. Zhong, D. Wu, R. B. Little, Z. Xu and H. Zhu, ACS Nano, 2014, 8, 850–859 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- P. Sun, K. Wang, J. Wei, M. Zhong, D. Wu and H. Zhu, J. Mater. Chem. A, 2014, 2, 7734–7737 CAS.
- K. Huang, G. Liu, Y. Lou, Z. Dong, J. Shen and W. Jin, Angew. Chem., Int. Ed., 2014, 53, 6929–6932 CrossRef CAS PubMed.
- Y. P. Tang, D. R. Paul and T. S. Chung, J. Membr. Sci., 2014, 458, 199–208 CrossRef CAS PubMed.
- Y. Liu, B. Xie, Z. Zhang, Q. Zheng and Z. Xu, J. Mech. Phys. Solids, 2012, 60, 591–605 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS PubMed.
- O. Akhavan and E. Ghaderi, ACS Nano, 2010, 4, 5731–5736 CrossRef CAS PubMed.
- D. Cohen-Tanugi and J. C. Grossman, J. Chem. Phys., 2014, 141, 0747041–0747044 Search PubMed.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2014, 14, 6171–6178 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |