Carbon dioxide capture in the presence of water vapour in InOF-1†
Ricardo A.
Peralta
a,
Brenda
Alcántar-Vázquez
a,
Mayra
Sánchez-Serratos
a,
Eduardo
González-Zamora
*b and
Ilich A.
Ibarra
*a
aInstituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior s/n, CU, Del. Coyoacán, 04510, México D. F., Mexico. E-mail: argel@unam.mx
bDepartamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, San Rafael Atlixco 186, Col. Vicentina, Iztapalapa, C. P. 09340, México D. F., Mexico
First published on 25th August 2015
Abstract
Kinetic uptake experiments on InOF-1 confirm a maximum of 5.42 wt% CO2 capture at 30 °C and a significant 2-fold increase (∼11 wt%) in CO2 capture under 20% relative humidity of water vapour. InOF-1 captures CO2 under humid conditions (10% and 20% RH) and at relatively high temperatures (40 and 50 °C) without any degradation of the crystalline structure which was corroborated by PXRD.
Introduction
Global warming is one of the biggest threats that our society has to solve since it causes extreme climate changes. The cumulative carbon dioxide (CO2) emissions in the atmosphere are continuously increasing due to anthropogenic activities and these, unwittingly, generate the undesirable green gas effect.1 The accelerating global energy demands and consumption of carbon-based fuels are the main triggers to the increasing CO2 levels,2 and these energy requirements are expanding promptly due to rapid world population growth, increases in the standard of living and the development of technologies, leading to a doubling in the energy demand over the last three decades.3 Therefore, CO2 separation and capture have motivated many governments to invest in the development of new methods for efficiently and effectively capturing CO2.4Typical CO2 absorption in aqueous alkanolamine solutions has been widely used and studied, but it has many major limitations as an adsorbent for industrial CO2 capture due to its heat instability and corrosion on vessels and pipelines.5 Thus, the use of porous solids for the adsorption of CO2 is a timely research area and the search for materials with a high adsorption capacity, structural stability, high tolerance against humidity, fast sorption kinetics and mild regeneration properties remains a major challenge for practical applications.
Porous coordination polymers (PCPs) or metal–organic frameworks (MOFs) are among the most interesting candidates for gas separation, because their sorption selectivity towards small molecule adsorbates is directly tunable as a function of the topology and chemical composition of the micropores.6 Porous metal–organic materials having high surface areas and high pore volumes normally show high CO2 storage capacities at room temperature and at relatively high pressures.7 Although the PCPs show high CO2 capacity and selectivity, many gas separation processes in industry involve the exposure to water vapour. However, water molecules can compete with any gas molecules for the active sites (within PCPs) or disrupt the ligand bonding between organic molecules and metals, resulting in the collapse of the structure.8 Capturing CO2 from real flue gas (high humidity and high temperature) is indeed a great challenge.
Recently, there is a considerable number of PCPs that have shown relatively good stability to water,9 and some interesting examples are: UiO-66,10 HKUST-1,11 MIL-100,12 MIL-101,13 and MIL-53.14 Doonan et al.15 reported a water stable MOF (Cu(bcppm)H2O, H2bcppm = bis(4-(4-carboxyphenyl)-1H-pyrazolyl)methane) that also showed exceptionally selective separation for CO2 over N2.
However, more than structural stability, the direct contact of PCPs to water can seriously reduce their gas storage capacity and water is most often unfavourable to gas separations.16 The effect of water on the CO2 capture has only recently been investigated on PCPs.17 Matzger and co-workers17b studied the effect of humidity on the performance of M/DOBDC (M = ZnII, NiII, CoII or MgII) by collecting N2/CO2/H2O breakthrough curves at different relative humidities. LeVan et al.18 found that a small amount of water did not decrease and may in fact increase the CO2 capacity of PCPs. Eddaoudi and co-workers19 demonstrated that a material entitled SIFSIX-3-Cu was a recyclable and moisture stable MOF that showed enhanced CO2 uptake and selectivity in highly diluted gas streams.
Llewellyn and co-workers20 investigated the CO2 adsorption in some PCPs under different relative humidities of water vapour. Certainly, HKUST-1, was shown to degrade in the presence of water vapour, and UiO-66 did not show any enhanced CO2 uptake.20 In the case of MIL-100(Fe), a remarkable 5-fold increase in CO2 uptake was observed with increasing relative humidity (RH), 105 mg g−1 at 40% RH. In addition, Yaghi et al.21 showed that the presence of hydroxyl functional groups increases the affinity of the framework for water. Thus, in the present work we have chosen a material entitled InOF-122 (Fig. 1), a water-stable PCP based on a binuclear [In2(μ2-OH)] building block (Fig. S1, ESI†), constructed from a flexible BPTC4− ligand (H4BPTC = biphenyl-3,3′,5,5′-tetracarboxylic acid) and possessing hydroxo functional groups (μ2-OH), to study the CO2 capture in the presence of water vapour.
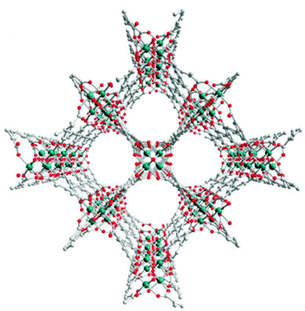 | ||
| Fig. 1 View of the crystal structure of InOF-1 along the c-axis (indium: green; oxygen: red; carbon: grey; hydrogen omitted for clarity).22 | ||
Experimental
Indium nitrate, In(NO3)3 (156 mg, 0.40 mmol) and biphenyl-3,3′,5,5′-tetracarboxylic acid, H4BPTC (33 mg, 0.10 mmol) were dispersed in CH3CN (5 ml), DMF (5 ml) and HNO3 (65%, 0.2 ml) and sealed in a pressure tube. The clear solution was heated at 85 °C in an oil bath for 72 h. The tube was cooled to room temperature over a period of 12 h and the colourless crystalline product was separated by filtration, washed with DMF (5 ml) and dried in air. Yield: 72% (based on ligand).The uncoordinated solvent molecules in the pores of the as-synthesized InOF-1 were exchanged for acetone and this promotes accessibility to the desolvated framework after activation by heating. Thus, thermogravimetric analysis (see Fig. S2, ESI†) and bulk powder X-ray diffraction patterns (see Fig. S3, ESI†) of the as-synthesised and desolvated InOF-1 confirmed that the material consistently retains its structural integrity upon solvent removal. N2 adsorption isotherms for activated InOF-1 at 77 K were used to calculate the BET surface area (0.01 < p/po < 0.04) of 1060 m2 g−1.
Kinetic uptake experiments were performed by using a thermobalance (Q500 HR, from TA) at different temperatures with a constant CO2 flow (60 mL min−1). Then, acetone-exchanged samples of InOF-1 were placed into the thermobalance and activated by heating from room temperature to 180 °C for 1 h and under a flow of N2 gas. After the activated sample was cooled down, the desired temperature and a constant CO2 flow (60 mL min−1) were set. With a humidity-controlled thermobalance (Q5000 SA, from TA), kinetic uptake experiments at 30, 40 and 50 °C with a constant CO2 flow (60 mL min−1) were carried out on activated samples (180 °C for 1 h and under a flow of N2 gas) of InOF-1.
Results and discussion
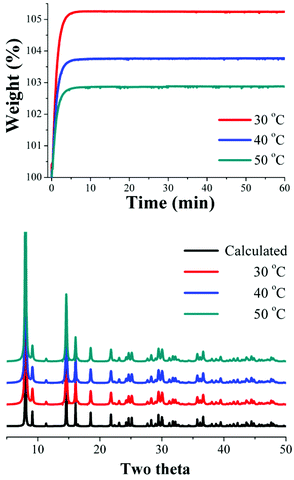
Dynamic and isothermal CO2 experiments were carried out on InOF-1. Fig. 1, left, shows the kinetic uptake experiments from 30 °C to 50 °C. At 30 °C the material exhibited the maximum weight% gain, which represents the maximum amount of CO2 captured. This amount corresponds to 5.24 wt%, which was rapidly reached after just 5 min and it was constant until the end of the experiment (60 min). At 40 °C the uptake was estimated to be 3.77 wt%, which was also reached after approximately 5 min (Fig. 2, top). Finally, at 50 °C the maximum uptake was 2.88 wt%.Clearly, while the temperature is increased (from 30 to 50 °C), the CO2 weight (%) gradually decreases (Fig. 2, top) from 5.24 to 2.88 wt%. In order to corroborate that this decrease is not due to sample degradation, we have carried out PXRD experiments on each sample after these CO2 capture experiments. Fig. 2 (bottom) confirms that the crystallinity of the samples after each CO2 capture experiment was retained.
Hong et al.22 showed, by PXRD experiments, that InOF-1 is a water stable PCP and this water-stability can be attributed to the presence of the hydroxo functional groups (inside the pores of InOF-1) as previously reported.17 Thus, kinetic isotherm experiments at 30, 40 and 50 °C, with a constant CO2 flow, and a relative humidity (RH) of 40% were carried out. It was decided to perform these experiments with a 40% RH based on the extraordinary results that Llewellyn et al.20 previously reported (a 5-fold increase in CO2 uptake for MIL-100(Fe)).
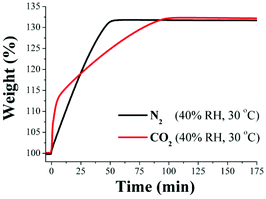
First, an activated InOF-1 sample (180 °C for 1 h and under a flow of N2 gas) was placed into a humidity-controlled thermobalance. After activation of the material, the equipment was stabilized at 40% RH (30 °C) and a constant CO2 flow (60 mL min−1) was started. Afterwards, we repeated this experimental procedure on a new activated InOF-1 sample and set a constant N2 flow (60 mL min−1). Fig. 3 exhibits the kinetic uptake experiments at 30 °C and 40% RH for CO2 and N2. For both isotherms, it is clear to observe that the material shows a constant increase in weight (while the experiment is continuous in time, see Fig. 3). This increase in weight is due to the contribution of H2O and CO2 or H2O and N2, respectively.
 | ||
| Fig. 3 Kinetic uptake experiments carried out at 30 °C and 40% RH with CO2 (red line) and N2 (black line) flow rates of 60 mL min−1, respectively. | ||
In order to find the maximum CO2 capture under 40% RH conditions, we need to differentiate the contribution of H2O to the weight increase. By taking the difference between the two isotherms (CO2 and N2) we could obtain the CO2 capture at 40% RH. This is valid if the material does not capture any N2 at 30 °C. Consequently, by performing a kinetic uptake experiment on a newly activated InOF-1 sample at 30 °C without the presence of H2O vapor (0% RH) with a constant N2 flow (60 mL min−1) we obtained a N2 capture of approximately 0.01 wt%. This result is consistent with the previous reports where the capture capacity of N2 capture in PCPs at room temperatures is basically negligible.23 In Fig. 3, the gradual weight increase for N2/H2O starts at 0 min and stabilises at ∼55 min. In the case of CO2/H2O the weight increase starts at 0 min and stabilises after approximately 110 min.
In contrast, under anhydrous conditions the CO2 uptake rapidly reached stability (5 min, see Fig. 2, top). This equilibrium discrepancy is due to the nature of the vapour adsorption process that in general takes considerably more time to stabilise than the gas adsorption process in microporous materials.24 Then, from 110 min until approximately 175 min both isotherms seem to reach a plateau where both uptake capacities are practically constant (Fig. 3). At 175 min, the maximum amounts of CO2/H2O and N2/H2O captured are 132 wt% and 131 wt%, respectively and by taking the difference between these two values (since there is no N2 uptake at 30 °C) the CO2 capture in the material is ∼1 wt%.
Therefore, the CO2 capture at 40% RH and 30 °C was considerably reduced in comparison with anhydrous conditions (from 5.24 to 1.00 wt%). At 40% RH and 40 °C (see Fig. S4, ESI†), as well as 40% RH and 50 °C (see Fig. S5, ESI†) the CO2 capture was approximately the same (∼1 wt%). This CO2 capture reduction is not due to the degradation of the material after each experiment, since the PXRD experiments carried out on the samples after the N2 and CO2 capture experiments (see Fig. 4) showed that the retention of the crystallinity was maintained (see Fig. 4).
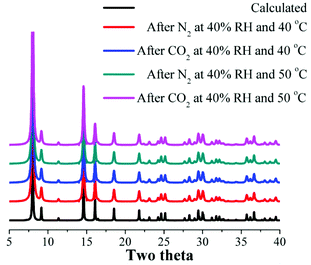 | ||
| Fig. 4 Calculated PXRD pattern of InOF-1 and PXRD patterns of each InOF-1 sample after the kinetic N2 and CO2 isotherms at different temperatures. | ||
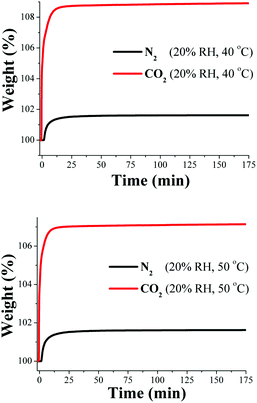
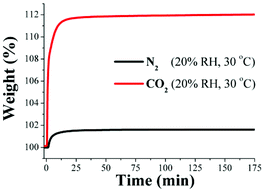
Then, in closer inspection of the porosity of InOF-1, this corresponds to the microporosity regime with a pore diameter of 7.6 Å.22 Interestingly, the remarkable result of a 5-fold increase in CO2 uptake was obtained in a mesoporous material at 40% RH and 30 °C (MIL-100(Fe))20 which comprises two types of mesoporous cages of free apertures of ca. 25 and 29 Å.20 We then rationalised that at 40% RH the saturation of the micropores in InOF-1, with H2O molecules, was completed and therefore, the inclusion of CO2 molecules, into the micropores, was unfeasible. In order to confirm this hypothesis, we reduced the relative humidity to half (20% RH) and more CO2 capture experiments were carried out. Then, an activated InOF-1 sample was stabilised, in a humidity-controlled thermobalance, at 20% RH (30 °C) and a constant CO2 flow (60 mL min−1) was started. Later, we replicated this experimental procedure on a new activated InOF-1 sample and set a constant N2 flow (60 mL min−1).
Fig. 5 exhibits the kinetic uptake experiments at 30 °C and 20% RH for CO2 and N2. Again, for both isotherms, the material clearly shows a constant increase in weight. However, this time both isotherms exhibited a much faster weight increase for N2/H2O and CO2/H2O than at 40% RH, starting at 0 min and stabilising at ∼15 min. Thus, from 15 min until approximately 175 min both isotherms reach a plateau where both uptake capacities are constant (Fig. 5). At 175 min, the maximum amounts of CO2/H2O and N2/H2O captured are 112 wt% and 101 wt%, respectively and by taking the difference between these two values (since there is no N2 uptake at 30 °C) the CO2 capture in the material is ∼11 wt%.
 | ||
| Fig. 5 Kinetic uptake experiments carried out at 30 °C and 20% RH with CO2 (red line) and N2 (black line) flow rates of 60 mL min−1, respectively. | ||
Therefore, the CO2 capture was approximately 2-fold increased with a 20% RH (from 5.24 wt% to 11 wt%) in comparison with anhydrous conditions. Clearly, when the relatively humidity was reduced to 20%, the micropores of InOF-1 were partially saturated with H2O molecules, allowing the CO2 molecules to enter into the micropores of InOf-1. This enhancement in CO2 uptake in the presence of water can be explained by CO2 confinement effects induced by bulky molecules (H2O).25 Walton et al.26 proposed that the functional groups (e.g. hydroxo functional groups inside the pores of InOF-1) act as a directing agent for water in the pores, which allows for more efficient packing. Additionally, we previously observed this enhanced-CO2 uptake phenomenon in a couple of Sc(III) water stable PCPs.27
The cycle stability of the material was investigated by running 11 kinetic uptake experiments on the same sample. Thus, an activated InOF-1 sample (180 °C for 1 h and under a flow of N2 gas) was placed into a humidity-controlled thermobalance and a constant CO2 flow (60 mL min−1) started at 20% RH. The experiment was stopped after 20 min (only to obtain the maximum CO2 uptake) and then, this protocol was repeated ten more times (with the same sample) to make a total of 10 cycles. The average CO2 uptake of the cycles was 11.02 wt% (see Fig. S8, ESI†). In addition, the PXRD experiments confirmed the retention of the overall framework crystallinity after 10 cycles (see Fig. S9, ESI†).
We also performed kinetic uptake experiments at 30 °C and 10% RH finding a maximum CO2 uptake of approximately 9 wt% (see Fig. S6, ESI†). Thus the highest amount of CO2 capture was obtained under a relative humidity of 20%.
Additionally, we decided to perform a CO2 experiment (60 mL min−1) at 20% RH and 30 °C on an activated PCM-1428 sample (150 °C for 2 h, under a flow of N2 gas). Since PCM-14 is a non-porous coordination polymer, when activated between 25–150 °C, it offered a direct CO2 capture in comparison with InOF-1 (a microporous material). Thus, from 0 min to ∼180 min the maximum CO2 uptake (under 20% RH) was 0.8 wt% (see Fig. S7, ESI†). This result corroborated that there is no CO2 sequestration in a non-porous material when the relative humidity is 20% at 30 °C.
Finally, kinetic uptake experiments were performed on InOF-1 at 20% RH and 40 °C (see Fig. 6, top) and 20% RH and 50 °C (see Fig. 6, bottom). The total CO2 capture values were 8 wt% and 6 wt% (Fig. 6), respectively. Thus, these values represent an approximately 2-fold CO2 increase (from anhydrous conditions to 20% RH) from 3.77 wt% to 8 wt% at 40 °C and from 2.88 wt% to 6 wt% at 50 °C. These results are indeed very promising for the application of PCPs in a more realistic CO2 capture situation like flue gas (high humidity and high temperature).
Conclusions
In conclusion, InOF-1, a In(III) porous coordination polymer, exhibited a total CO2 amount of 5.42 wt% at 30 °C shown by kinetic isotherm experiments, which was rapidly reached after approximately 5 min. While increasing the temperature of the kinetic isotherm experiments, the CO2 capture capacity of InOf-1 decreased to 2.88 wt% at 50 °C. Remarkably, InOF-1 exhibits high stability towards humidity, which was confirmed by PXRD. We attributed this water stability, as previously reported,21 to the presence of hydroxo functional groups within the pores of InOF-1.Due to this particularly high water stability, InOF-1 performs CO2 uptake under relative humidity conditions. Finding the best partial saturation of H2O molecules (percentage of relative humidity) into the micropores of InOF-1 is essential to increase the CO2 uptake. Thus, after testing different relative humidity conditions (40%, 20% and 10% RH) and temperatures (30, 40 and 50 °C), we found that the maximum CO2 capture was obtained at 20% RH and 30 °C with a total amount of ∼11 wt%. Significantly, this CO2 capture, under humid conditions, represents a 2-fold increase in comparison with anhydrous conditions.
It is also worth mentioning that this material captures CO2 under humid conditions and at relatively high temperatures (40 and 50 °C) which are desirable in a more realistic CO2 capturing scenario like flue gas. PCM-14 showed non-CO2 capture under RH conditions, suggesting that the microporosity provided by InOF-1 is fundamental for this capture process. Since PCM-14 is a non-porous coordination polymer, when activated between 25–150 °C, the CO2 confinement effects induced by H2O25 in porous materials cannot take place unlike in InOF-1, where these effects occur within the micropores as well as the directing effect of the hydroxo functional groups (inside the micropores of InOF-1) which can accommodate CO2 more efficiently.26
Acknowledgements
The authors thank Dr A. Tejeda-Cruz (X-ray; IIM-UNAM), CONACyT Mexico (212318), PAPIIT UNAM Mexico (IN100415) for financial support. E. G.-Z. thanks CONACyT (156801 and 236879), Mexico for financial support. Thanks to U. Winnberg (ITAM and ITESM) for scientific discussions.Notes and references
- J. T. Litynski, S.M. Klara, H. G. McIlvried and R. D. Srivastava, Environ. Int., 2006, 32, 128 CrossRef CAS PubMed.
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148 CAS.
- International Energy Agency (IEA), Key World Energy Statistics, OECD/IEA, France, 2013 Search PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, Z. T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- (a) G. T. Rochelle, Science, 2009, 325, 1652 CrossRef CAS PubMed; (b) F. Karadas, M. Atilhan and S. Aparicio, Energy Fuels, 2010, 24, 5817 CrossRef CAS.
- (a) S. Yang, G. S. B. Martin, G. J. J. Titman, A. J. Blake, D. R. Allan, N. R. Champness and M. Schröder, Inorg. Chem., 2011, 50, 9374 CrossRef CAS PubMed; (b) A. J. Nuñez, L. N. Shear, N. Dahal, I. A. Ibarra, J. W. Yoon, Y. K. Hwang, J.-S. Chang and S. M. Humphrey, Chem. Commun., 2011, 47, 11855 RSC.
- (a) H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424 CrossRef CAS PubMed; (b) A. M. Bohnsack, I. A. Ibarra, P. W. Hatfield, J. W. Yoon, Y. K. Hwang, J.-S. Chang and S. M. Humphrey, Chem. Commun., 2011, 47, 4899 RSC; (c) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed.
- (a) J. A. Greathouse and M. D. Allendorf, J. Am. Chem. Soc., 2006, 128, 1067 Search PubMed; (b) S. S. Han, S.-H. Choi and A. C. T. van Duin, Chem. Commun., 2010, 46, 5713 RSC.
- (a) P. Li, J. Chen, J. Zhang and X. Wang, Sep. Purif. Rev., 2015, 44, 19 CrossRef CAS PubMed; (b) J. Canivet, J. Bonnefoy, C. Daniel, A. Legrand, B. Coasne and D. Farrusseng, New J. Chem., 2014, 38, 3102 RSC; (c) P.-Q. Liao, H. Chen, D.-D. Zhou, S.-Y. Liu, C.-T. He, Z. Rui, H. Ji, J.-P. Zhang and X.-M. Chen, Energy Environ. Sci., 2015, 8, 1011 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148 CrossRef CAS.
- K. A. Cychosz and A. J. Matzger, Langmuir, 2010, 26, 17198 CrossRef CAS PubMed.
- D.-Y. Hong, Y. K. Hwang, C. Serre, G. Férey and J.-S. Chang, Adv. Funct. Mater., 2009, 19, 1537 CrossRef CAS PubMed.
- J. Liu, F. Zhang, X. Zou, G. Yu, N. Zhao, S. Fan and G. Zhu, Chem. Commun., 2013, 49, 7430 RSC.
- W. M. Bloch, R. Babaro, M. R. Hill, C. J. Doonan and C. J. Sumby, J. Am. Chem. Soc., 2013, 135, 10441 CrossRef CAS PubMed.
- (a) S. S. Nagarkar, A. K. Chaudhari and S. K. Ghosh, Inorg. Chem., 2012, 51, 572 CrossRef CAS PubMed; (b) H. J. Choi, M. Dincă, A. Daily and J. R. Long, Energy Environ. Sci., 2010, 3, 117 RSC.
- (a) J. Liu, A. I. Benin, A. M. B. Furtado, P. Jakubczak, R. R. Willis and M. D. LeVan, Langmuir, 2011, 27, 11451 CrossRef CAS PubMed; (b) A. C. Kizzie, A. G. Wong-Foy and A. J. Matzger, Langmuir, 2011, 27, 6368 CrossRef CAS PubMed; (c) H. Jasuja, Y.-g. Huang and K. S. Walton, Langmuir, 2012, 28, 16874 CrossRef CAS PubMed; (d) H. Jasuja, J. Zang, D. S. Sholl and K. S. Walton, J. Phys. Chem. C, 2012, 116, 23526 CrossRef CAS; (e) J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-g. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642 RSC.
- J. Liu, Y. Wang, A. I. Benin, P. Jakubczak, R. R. Willis and M. D. LeVan, Langmuir, 2010, 26, 14301 CrossRef CAS PubMed.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 1 Search PubMed.
- E. Soubeyrand-Lenoir, C. Vagner, J.W. Yoon, P. Bazin, F. Ragon, Y. K. Hwang, C. Serre, J.-S. Chang and P. L. Llewellyn, J. Am. Chem. Soc., 2012, 134, 10174 CrossRef CAS PubMed.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369 CrossRef CAS PubMed.
- J. Qian, F. Jiang, D. Yuan, M. Wu, S. Zhang, L. Zhang and M. Hong, Chem. Commun., 2012, 48, 9696 RSC.
- (a) E. Haldoupis, S. Nair and D. S. Sholl, J. Am. Chem. Soc., 2012, 134, 4313 CrossRef CAS PubMed; (b) Y.-S. Bae, O. K. Farha, J. T. Hupp and R. Q. Snurr, J. Mater. Chem., 2009, 19, 2131 RSC.
- I. P. O'koye, M. Benham and K. M. Thomas, Langmuir, 1997, 13, 4054 CrossRef.
- N. L. Ho, F. Porcheron and R. J.-M. Pellenq, Langmuir, 2010, 26, 13287 CrossRef CAS PubMed.
- G. E. Cmarik, M. Kim, S. M. Cohen and K. S. Walton, Langmuir, 2012, 28, 15613 CrossRef PubMed.
- (a) M. R. Gonzalez, J. H. González-Estefan, H. A. Lara-García, P. Sánchez-Camacho, E. I. Basaldella, H. Pfeiffer and I. A. Ibarra, New J. Chem., 2015, 39, 2400 RSC; (b) H. A. Lara-García, M. R. Gonzalez, J. H. González-Estefan, P. Sánchez-Camacho, E. Lima and I. A. Ibarra, Inorg. Chem. Front., 2015, 2, 442 RSC.
- I. A. Ibarra, K. E. Tan, V. M. Lynch and S. M. Humphrey, Dalton Trans., 2012, 41, 3920 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: GA data, PXRDP data and kinetic uptake experiments. See DOI: 10.1039/c5qi00077g |
| This journal is © the Partner Organisations 2015 |