Cleavage of the C–C triple bond of ketoalkynes: synthesis of 4(3H)-quinazolinones†
Xifa
Yang
,
Guolin
Cheng
*,
Jinhai
Shen
,
Changsheng
Kuai
and
Xiuling
Cui
*
Key Laboratory of Xiamen Marine and Gene Drugs, Institutes of Molecular Medicine and School of Biomedical Sciences, Huaqiao University & Engineering Research Center of Molecular Medicine, Ministry of Education, Xiamen 361021, China. E-mail: cuixl@hqu.edu.cn; glcheng@hqu.edu.cn
First published on 11th February 2015
Abstract
A novel protocol for the synthesis of 4(3H)-quinazolinones via selective cleavage of the triple bond of ketoalkynes under oxidant-, metal-, and ligand-free conditions has been developed. Various 4(3H)-quinazolinones were obtained through fragmentation of the C–C triple bond and formation of two C–N bonds.
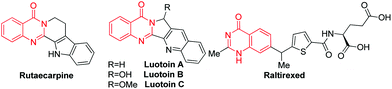
Quinazolinones are widely present in natural products. For example, Rutaecarpine from a Chinese herbal drug named Wu–Chu–Yu was used to treat headache and cholera;1 Luotonin A from a Chinese plant (Peganumn nigellastrum) showed cytotoxicity to the murine leukemia P388 cell line;2 and Raltitrexed (brand name: Tomudex) have played an effective role in clinical therapy (Scheme 1).3 They also exhibit a wide range of bioactivities, such as anticonvulsant,4 antiviral,5 anti-inflammatory,6 antifungal,7 antimicrobial,8 and antimalarial.9 As a result, there have been tremendous synthetic efforts towards building such a core. The condensation of aldehydes with o-aminobenzamides followed by oxidation has been used in industry.10 Recently, the transition metal-catalyzed synthesis of quinazolines has made great progress. Zhou and Fang built such a structure via iridium-catalyzed hydrogen transfer.11 Yokoyama and Hidemasa described a Pd-catalyzed benzylic C–H amidation with benzyl alcohols.12 Zhu and other groups developed a Pd-catalyzed CO insertion reaction to construct quinazolin-4(3H)-ones.13 Very recently, copper or iron-catalyzed C–N coupling of 2-halobenzoic acids or 2-halobenzamides with amidines to form quinazolines has been developed by Shafir, Buchwald and other groups.14 These procedures rely on metal catalysts and excessive oxidant or base. Suitable ligands or microwave irradiation were also necessary in some cases. The reaction of β-diketones with o-aminobenzamide to produce 4(3H)-quinazolinones has been mentioned in the literature.15 However, the generality of the β-diketones was limited and low efficiency was observed when 1,3-diarylpropane-1,3-diones were used as the starting materials. We reasoned that 1,3-diarylpropane-1,3-dione generate enaminone intermediate inefficiently. Recently, we easily synthesized a series of heterocycles, such as pyrroles, chromones and triazoles,16 from ketoalkynes through an enaminone intermediate, which could be generated in situ from the reaction of ketoalkyne with o-aminobenzamide in the presence of suitable additives. Herein, we report a new strategy for the synthesis of 2-arylquinazolin-4(3H)-one from ketoalkynes via C–C triple bond cleavage.
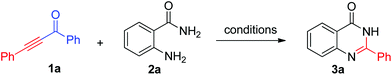
In our initial studies, o-aminobenzamide 1a and 1,3-diphenylprop-2-yn-1-one 2a were chosen as model substrates to optimize the reaction parameters. The desired product 2-phenylquinazolin-4(3H)-one 3a was obtained, with acetophenone as a byproduct, by using TFA as an additive in THF. No product was detected in the absence of TFA. Different solvents were tested with 5 equiv. of TFA at 90 °C (Table 1, entries 1–9). The highest yield was obtained in toluene (83%). Ethanol and DMSO led to 3a in 79% yield (Table 1 entries 2 and 3). The yield decreased significantly when NMP, DMF, and H2O were used as the reaction media. Then the additive loading was investigated. This transformation was not affected when the acid additive was decreased to 3 equiv. (Table 1, entry 11). However, further decreasing the loading of TFA resulted in relatively lower yield of 3a (Table 1, entry 12). 1 Equiv. of 1a made the yield of 3a decrease to 62% (entry 13). When the reaction temperature was decreased to room temperature, only 30% yield of 2-aryl-4(3H)-quinazolinone was obtained (Table 1, entry 14). The base, such as E3N and LiOtBu, did not favour this transformation (Table 1, entries 15 and 16). Therefore, the optimal reaction conditions were identified as follows: 3 equiv. of TFA at 90 °C in toluene, overnight.
| Entry | Additive (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1a (0.65 mmol), 2a (0.5 mmol), dry solvent (2 mL), 90 °C, overnight, N2.
b Isolated yields based on 2a.
c 60 °C, 16 h.
d RT.
e
1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a = 1 2a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||
| 1 | TFA (5) | THF | 78 |
| 2 | TFA (5) | DMSO | 79 |
| 3 | TFA (5) | Ethanol | 79 |
| 4 | TFA (5) | DCM | 71 |
| 5 | TFA (5) | DMF | 40 |
| 6 | TFA (5) | H2O | 38 |
| 7 | TFA (5) | Toluene | 83 |
| 8 | TFA (5) | NMP | 55 |
| 9c | TFA (5) | DCM | 67 |
| 10 | TFA (4) | Toluene | 82 |
| 11 | TFA (3) | Toluene | 83 |
| 12 | TFA (2) | Toluene | 78 |
| 13e | TFA (3) | Toluene | 62 |
| 14d | TFA (5) | Toluene | 30 |
| 15 | E3N (5) | Toluene | 0 |
| 16 | LiOtBu (5) | Toluene | 0 |
Having the optimal reaction conditions in hand, the scope and generality of the substrates were investigated. As shown in Scheme 2 (3a–o), good to excellent yields were obtained for most of the substrates examined. 1,3-Diphenylprop-2-yn-1-one and diarylprop-2-yn-1-one bearing electron-donating groups on the 3-aryl ring, such as methyl and methoxyl, proceeded well, affording 2-arylquinazolin-4(3H)-ones 3a–d in good yields (Scheme 2). Meanwhile, o-methyl-substituted substrates reacted smoothly and resulted in the desired 4(3H)-quinazolinones in 81% yield (Scheme 2, 3bvs. 3c), implying that influence of steric hindrance of ketoalkynes was not significant. In addition, ketoalkynes with electron-withdrawing groups (fluoro- and chloro-) also reacted well and furnished the desired products 3e, 3f in 70% and 79% yield, respectively. Heteroaryl- and aliphatic ketoalkyne were also applicable under the standard reaction conditions. For instance, 1-phenyl-3-(thiophen-2-yl)prop-2-yn-1-one, 1-phenylnon-2-yn-1-one and 1-phenylprop-2-yn-1-one gave the corresponding quinazolin-4(3H)-ones 3g, 3h, 3i in 96%, 98%, and 98% yield, respectively. We then examined reactions of N-substituted 2-aminobenzamides, such as 2-amino-N-methylbenzamide and 2-amino-N-phenylbenzamide. The corresponding products 3j, 3k could be obtained in 91% and 65% yields, respectively. Next, the scope of the coupling reaction of substituted o-aminobenzamides 2l–p was further examined. We were pleased to find that the reaction of o-aminobenzamide with 3-chloro, 4-methyl, 5-methyl, 6-chloro, and 6-fluoro groups also worked well and provided quinazolin-4(3H)-one derivatives 3l–p in 79%–84% yields. To our delight, 2-aminothiophene-3-carboxamide could be a suitable substrate and resulted in the desired product in 45% yield (Scheme 2, 3o).
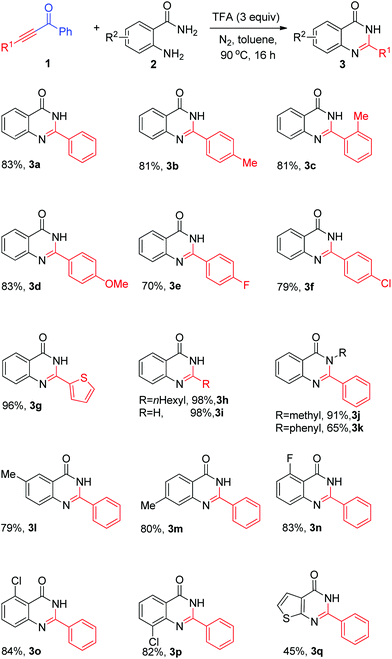 | ||
| Scheme 2 Substrate scope. Reaction conditions: 1a (0.65 mmol), 2a (0.5 mmol), TFA (1.5 mmol), dry toluene (2 mL), 90 °C, 16 h. Isolated yields based on 2a. | ||
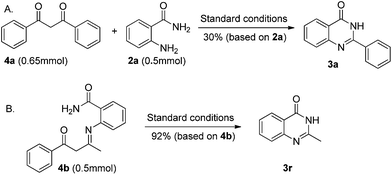
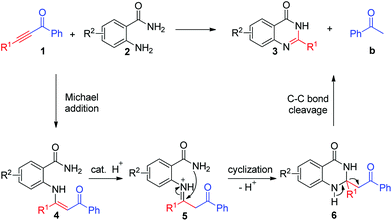
To clarify the reaction mechanism, 1.5 equiv. of 1,3-diphenylpropane-1,3-dione (Scheme 3, 4a) was treated with o-aminobenzamide under the standard reaction conditions. 4(3H)-Quinazolinone 3a was isolated in only 30% yield. These results indicated that β-diketones were not likely to have been intermediates in this metal-free C–C triple bond cleavage reaction. Meanwhile, a relatively accessible key intermediate (E)-2-(4-oxo-4-phenylbutan-2-ylideneamino) benzamide was successfully synthesized and resulted in the desired 4(3H)-quinazolinones in 92% yield under the standard reaction conditions. Based on the results obtained above and the literature,17 a plausible reaction mechanism was proposed and shown in Scheme 4. With the help of TFA, the condensation reaction of 1 with 2 would generate the enaminone intermediate 4. Then, intramolecular nucleophilic addition of 4 produced the intermediate 6. Then the C–C bond cleavage reaction occurred to generate the product 3.
In summary, we have demonstrated that the novel metal-free C–C triple bond cleavage reaction proceeded efficiently with TFA, affording 2-aryl(alkyl)-quinazolin-4(3H)-ones in moderate to excellent yields. In addition, the scope of this reaction was successfully expanded to heteroaryl ketoalkyne and 2-aminothiophene-3-carboxamide. Currently, we are exploring the application of the proposed cleavage of the C–C triple bond with TFA to the synthesis of the pharmaceutical molecules.
Acknowledgements
This work was supported by Program for Minjiang Scholar program (10BS216), Xiamen Southern Oceanographic Center (13GYY003NF16), NSF of China (21202048), and Promotion Program for Young and Middle-aged Teachers in Science and Technology Research of Huaqiao University (ZQN-PY120).Notes and references
- A. L. Chen and K. K. Chen, J. Am. Pharm. Assoc., 1933, 22, 716 CrossRef CAS.
- Z.-Z. Ma, Y. Hano, T. Nomura and Y.-J. Chen, Heterocycles, 1997, 46, 541 CrossRef CAS PubMed.
- Y. Nozoe, Y. Ogata, Y. Araki, T. Sasatomi, H. Fukumori and K. Shirouzu, Anticancer Res., 2002, 23, 4663 Search PubMed.
- M. M. Aly, Y. A. Mohamed, K. A. El-Bayouki, W. M. Basyouni and S. Y. Abbas, Eur. J. Med. Chem., 2010, 45, 3365 CrossRef CAS PubMed.
- Z. W. Wang, M. X. Wang, X. Yao, Y. Li, J. Tan, L. Z. Wang, W. T. Qiao, Y. Q. Geng, Y. X. Liu and Q. M. Wang, Eur. J. Med. Chem., 2012, 53, 275 CrossRef CAS PubMed.
- (a) J. A. Lowe III, R. L. Archer, D. S. Chapin, J. B. Cheng, D. Helweg, J. L. Johnson, B. K. Koe, L. A. Lebel, P. F. Moore, J. A. Nielsen, L. L. Russo and J. T. Shirley, J. Med. Chem., 1991, 34, 624 CrossRef; (b) S. E. de Laszlo, C. S. Quagliato, W. J. Greenlee, A. A. Patchett, R. S. L. Chang, V. J. Lotti, T.-B. Chen, S. A. Scheck, K. A. Faust, S. S. Kivlighn, T. S. Schorn, G. J. Zingaro and P. K. S. Siegl, J. Med. Chem., 1993, 36, 3207 CrossRef CAS.
- N. J. Liverton, D. J. Armstrong, D. A. Claremon, D. C. Remy, J. J. Baldwin, R. J. Lynch, G. X. Zhang and R. J. Gould, Bioorg. Med. Chem. Lett., 1998, 8, 483 CrossRef CAS.
- (a) O. M. Habib, E. B. Moawad, M. M. Girges and A. M. El-Shafei, Boll. Chim. Farm., 1995, 134, 503 CAS; (b) S. S. Ibrahim, A. M. Abdel-Halim, Y. Gabr, S. El-Edfawy and R. M. Abdel-Rahman, J. Chem. Res., Synop., 1997, 5, 154 RSC.
- (a) C. S. Jang, F. Y. Fu, C. Y. Wang, K. C. Huang, G. Lu and T. C. Thou, Science, 1946, 103, 59 CAS; (b) T.-Q. Chou, F. Y. Fu and Y. S. Kao, J. Am. Chem. Soc., 1948, 70, 1765 CrossRef CAS; (c) K. Murata, F. Takano, S. Fushiya and Y. Oshima, J. Nat. Prod., 1998, 61, 729 CrossRef CAS PubMed.
- (a) D. Zhan, T. B. Li, X. P. Zhang, C. Dai, H. D. Wei, Y. Y. Zhang and Q. L. Zeng, Synth. Commun., 2013, 43, 2493 CrossRef CAS; (b) A. Davoodnia, S. Allameh, A. R. Fakhari and N. Tavakoli-Hoseini, Chin. Chem. Lett., 2010, 21, 550 CrossRef CAS PubMed; (c) J. J. Naleway, C. M. Fox, D. Robinhold, E. Terpetschnig, N. A. Olson and R. P. Haugland, Tetrahedron Lett., 1994, 35, 8569 CrossRef CAS; (d) M. Bakavoli, O. Sabzevari and M. Rahimizadeh, Chin. Chem. Lett., 2007, 18, 1466 CrossRef CAS PubMed.
- J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730 CrossRef CAS PubMed.
- H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046 CrossRef CAS PubMed.
- (a) B. Ma, Y. Wang, J. L. Peng and Q. Zhu, J. Org. Chem., 2011, 76, 6362 CrossRef CAS PubMed; (b) X. F. Wu, L. He, H. Neumann and M. Beller, Chem. – Eur. J., 2013, 19, 12635 CrossRef CAS PubMed; (c) H. Q. Li, L. He, H. Neumann, M. Beller and X.-F. Wu, Green Chem., 2014, 16, 1336 RSC.
- (a) X. W. Liu, H. Fu, Y. Y. Jiang and Y. F. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348 CrossRef CAS PubMed; (b) X. D. Zhang, D. J. Ye, H. F. Sun, D. L. Guo, J. Wang, H. Huang, X. Zhang, H. L. Jiang and H. Liu, Green Chem., 2009, 11, 1881 RSC; (c) D. S. Yang, H. Fu, L. M. Hu, Y. Y. Jiang and Y. F. Zhao, J. Comb. Chem., 2009, 11, 653 CrossRef CAS PubMed; (d) W. Xu, Y. B. Jin, H. X. Liu, Y. Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274 CrossRef CAS PubMed; (e) L. T. Xu, Y. W. Jiang and D. W. Ma, Org. Lett., 2012, 14, 1150 CrossRef CAS PubMed.
- (a) L. Lu, M. M. Zhang, H. Jiang and X. S. Wang, Tetrahedron Lett., 2013, 54, 757 CrossRef CAS PubMed; (b) J. Lessel, Arch. Pharm., 1994, 327, 571 CrossRef CAS.
- (a) J. H. Shen, G. L. Cheng and X. L. Cui, Chem. Commun., 2013, 49, 10641 RSC; (b) X. S. Wang, G. L. Cheng and X. L. Cui, Chem. Commun., 2014, 50, 652 RSC; (c) G. L. Cheng, X. B. Zeng, J. H. Shen, X. S. Wang and X. L. Cui, Angew. Chem., Int. Ed., 2013, 52, 13265 CrossRef CAS PubMed.
- S. Roy, M. P. Davydova, R. Pal, K. Gilmore, G. A. Tolstikov, S. F. Vasilevsky and I. V. Alabugin, J. Org. Chem., 2011, 76, 7482 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c4qo00260a |
| This journal is © the Partner Organisations 2015 |