Enantioselective Friedel–Crafts reaction of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes†
Hao
Wu
,
Ren-Rong
Liu
,
Chong
Shen
,
Ming-Di
Zhang
,
Jianrong
Gao
and
Yi-Xia
Jia
*
College of Chemical Engineering, Zhejiang University of Technology, Chaowang Road 18#, Hangzhou 310014, China. E-mail: yxjia@zjut.edu.cn
First published on 16th December 2014
Abstract
Using a Ni(ClO4)2–bisoxazoline complex as a catalyst, Friedel–Crafts alkylations of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes were carried out with high enantioselectivities (up to 91%) to give alkylated dihydroindoles bearing trifluoromethylated all-carbon quaternary stereocenters in good yields. The corresponding chiral C2 alkylated indoles were obtained with complete preservation of enantiomeric purity by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).
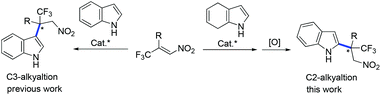
Indole is a pivotal structural motif existing in many biologically active compounds and natural products.1 Therefore, extensive efforts have been devoted to the synthesis of optically active indolic compounds. The catalytic asymmetric Friedel–Crafts alkylation reaction is an attractive approach for this purpose through the direct functionalization of the indole ring.2 Most of the examples that have been documented have been C3 alkylations of the indole ring.2 In contrast, Friedel–Crafts alkylation of indole at its C2 position has been difficult due to the higher nucleophilicity of its C3 position.3 Therefore, an indirect strategy was developed for the synthesis of chiral 2-alkylated indoles via enantioselective Friedel–Crafts alkylation of 4,7-dihydroindoles at the pyrrolic C2 position, followed by an oxidation reaction. A range of electrophiles have been successfully applied in Friedel–Crafts alkylations of 4,7-dihydroindoles.4–7 However, the construction of an all-carbon quaternary stereocenter at the C2 position of the indole ring has remained unexploited. Development of an efficient approach for constructing such stereocenters would be highly valuable.
Recently, the enantioselective construction of an all-carbon quaternary stereogenic center via an asymmetric Friedel–Crafts C3 alkylation of indole has been achieved.8 Kwiatkowski and co-workers reported a high-pressure accelerated Friedel–Crafts reaction of indole with enone, with modest enantioselectivity.9 Excellent results were then presented by Liu and Zhang for the reactions with isatin-derived α,β-unsaturated aldehydes as alkylating reagents.10 Both of these research groups applied chiral amine as a catalyst. At the same time, Arai's group and our group independently reported the chiral Lewis-acid-catalyzed Friedel–Crafts reactions of indoles with β,β-disubstituted nitroalkenes (isatin-derived nitroalkenes and β-CF3-β-disubstituted nitroalkenes), leading to the desired products with excellent enantioselectivities.11 Our group, as well as the Meggers and Akiyama groups, have reported successful Friedel–Crafts reactions of indoles with α-substituted-β-nitroacrylates.12 Inspired by the above described progress, we envisioned that the Friedel–Crafts alkylation of 4,7-dihydroindole with β,β-disubstituted unsaturated substrates followed by oxidation would provide a good opportunity to prepare chiral 2-alkylated indoles bearing all-carbon quaternary stereocenters (Scheme 1).13 Herein, we present our primary results on the Friedel–Crafts alkylation of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes.
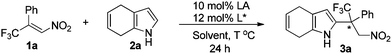
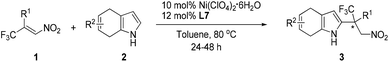
Initially, (E)-1-phenyl-1-trifluoromethyl-2-nitroethene (1a) and 4,7-dihydroindole (2a) were selected as the model substrates to study the Friedel–Crafts reaction. To our satisfaction, the reaction proceeded smoothly to give the desired product with 85% yield and 59% ee in the presence of 10 mol% Ni(ClO4)2·6H2O and 12 mol% ligand L1 in toluene at 80 °C (entry 1, Table 1). Different chiral bisoxazoline ligands were then investigated. Poor to modest enantioselectivities were detected when modifying the chiral substituents and the linker groups of ligand L1 (entries 2–6). Gratifyingly, the reaction with ligand L7, which bears trans-diphenyl groups, gave the highest ee value (entry 7). The Lewis acid Zn(ClO4)2·6H2O could also promote this reaction with slightly lower enantioselectivity (entry 8), while Ni(OTf)2 gave an inferior result (entry 9). Finally, changing the solvent and lowering the temperature did not improve the enantioselectivity (entries 10–12).
| Entry | LA | L* | Solvent | T (°C) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), Lewis acid (LA) (10 mol%), and chiral ligand (L*) (12 mol%) in toluene (2.0 mL) at 80 °C for 24 h.
b Isolated yield.
c Determined by chiral HPLC.
d DCE = 1,2-dichloroethane.
e 48 h. 
|
||||||
| 1 | Ni(ClO4)2·6H2O | L1 | Toluene | 80 | 85 | 59 |
| 2 | Ni(ClO4)2·6H2O | L2 | Toluene | 80 | 78 | 23 |
| 3 | Ni(ClO4)2·6H2O | L3 | Toluene | 80 | 83 | 55 |
| 4 | Ni(ClO4)2·6H2O | L4 | Toluene | 80 | 80 | 69 |
| 5 | Ni(ClO4)2·6H2O | L5 | Toluene | 80 | 77 | 14 |
| 6 | Ni(ClO4)2·6H2O | L6 | Toluene | 80 | 89 | 43 |
| 7 | Ni(ClO4)2·6H2O | L7 | Toluene | 80 | 94 | 91 |
| 8 | Zn(ClO4)2·6H2O | L7 | Toluene | 80 | 80 | 84 |
| 9 | Ni(OTf)2 | L7 | Toluene | 80 | 82 | 67 |
| 10 | Ni(ClO4)2·6H2O | L7 | DCEd | 80 | 65 | 89 |
| 11 | Ni(ClO4)2·6H2O | L7 | Ether | 80 | 78 | 87 |
| 12e | Ni(ClO4)2·6H2O | L7 | Toluene | 50 | 75 | 88 |
With the optimal conditions established, we then checked the substrate scope. A range of substituted nitroalkenes and 4,7-dihydroindoles were investigated. As shown in Table 2, either electron-donating or electron-withdrawing substituents in the para- or meta-position on the phenyl ring of nitroalkenes were well tolerated, and their reactions with 4,7-dihydroindole smoothly afforded the corresponding products with excellent yields and good to excellent enantioselectivities (entries 2–8, Table 2). However, the reactivity was sharply influenced by the steric effect of nitroalkene, and no reaction was observed for the substrate 1m, indicating the limitation of the present method. In addition, the 3-thienyl and 2-naphthyl products 3ia and 3ja were isolated in good yields, but the enantioselectivity of 3ia was lower (entries 9 and 10). Modest enantioselectivities were also observed in the reactions of alkylated nitroalkenes 1k and 1l, though good yields were obtained (entries 11 and 12). The reaction was successfully extended to 4,7-dihydroindoles bearing 5-Me, 5-F, and 6-F substituents, achieving good yields and enantioselectivities in their reactions with nitroalkene 1a (entries 13–15).
| Entry | R1 | R2 | Yieldb (%) | eec (%) | |
|---|---|---|---|---|---|
a Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), 10 mol% Ni(ClO4)2·6H2O and 12 mol% L7 in 2.0 mL toluene at 80 °C for 24–48 h.
b Isolated yield.
c Determined by chiral HPLC. 
|
|||||
| 1 | Ph (1a) | H (2a) | 3aa | 94 | 91 |
| 2 | 3-Me-Ph (1b) | H (2a) | 3ba | 95 | 85 |
| 3 | 4-Me-Ph (1c) | H (2a) | 3ca | 92 | 88 |
| 4 | 4-MeO-Ph (1d) | H (2a) | 3da | 90 | 88 |
| 5 | 3,5-Me2-Ph (1e) | H (2a) | 3ea | 90 | 84 |
| 6 | 4-Cl-Ph (1f) | H (2a) | 3fa | 89 | 81 |
| 7 | 3-F-Ph (1g) | H (2a) | 3ga | 92 | 85 |
| 8 | 4-CF3-Ph (1h) | H (2a) | 3ha | 88 | 86 |
| 9 | 3-Thienyl (1i) | H (2a) | 3ia | 86 | 68 |
| 10 | 2-Naphthyl (1j) | H (2a) | 3ja | 85 | 82 |
| 11 | 2-Phenylethyl (1k) | H (2a) | 3ka | 84 | 71 |
| 12 | 1-Octyl (1l) | H (2a) | 3la | 88 | 62 |
| 13 | Ph (1a) | 5-Me (2b) | 3ab | 90 | 88 |
| 14 | Ph (1a) | 5-F (2c) | 3ac | 87 | 88 |
| 15 | Ph (1a) | 6-F (2d) | 3ad | 89 | 88 |
To determine the practicality of the present method for synthesizing chiral 2-substituted indole derivatives, a one-pot process combining the Friedel–Crafts alkylation and the subsequent oxidation was developed. As shown in Scheme 2, the direct addition of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to the reaction mixture after completion of the Friedel–Crafts alkylation led to the corresponding 2-alkylated indoles in good yields. The enantioselectivities of the original Friedel–Crafts adducts were maintained in the 2-alkylated indoles, indicating the perfect preservation of the stereochemistry during the oxidation step.
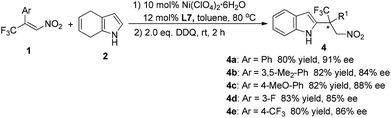 | ||
| Scheme 2 One-pot synthesis of 2-alkylated indoles 4.14 | ||
Conclusions
In summary, we have developed a highly enantioselective Friedel–Crafts reaction of 4,7-dihydroindoles with β-CF3-β-disubstituted nitroalkenes by employing a Ni(ClO4)2–bisoxazoline complex as a catalyst. The reaction produced 2-substituted 4,7-dihydroindoles bearing trifluoromethylated all-carbon quaternary stereocenters, with good yields and modest to excellent enantioselectivities. Moreover, a one-pot process combining alkylation and subsequent oxidation was developed to prepare the optically active 2-alkylated indoles in good yields, and no loss of enantioselectivity was observed in the oxidation reaction. Further extension of this methodology for organic synthesis is currently underway in our laboratory.Acknowledgements
The project was supported by the National Natural Science Foundation of China (grant nos. 21002089; 21372202), New Century Excellent Talents in University (NCET-12-1086), and Zhejiang Natural Science Fund for Distinguished Young Scholars (R14B020005).Notes and references
- (a) M. d'Ischia, A. Napolitano and A. Pezzella, Compr. Heterocycl. Chem. III, 2008, 3, 353 CrossRef; (b) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed; (c) S. E. O'Connor and J. J. Maresh, Nat. Prod. Rep., 2006, 23, 532 RSC.
- For a book on the asymmetric Friedel–Crafts reaction, see: (a) Catalytic Asymmetric Friedel-Crafts Alkylations, ed. M. Bandini and A. Umani-Ronchi, Wiley-VCH, Weinheim, 2009 Search PubMed. For reviews, see: (b) T. B. Poulsen and K. A. Jørgensen, Chem. Rev., 2008, 108, 2903 CrossRef CAS PubMed; (c) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (e) V. Terrasson, R. M. Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635 CrossRef CAS.
- For the reactions of 3-substituted indole, see: (a) H.-G. Cheng, L.-Q. Lu, T. Wang, Q.-Q. Yang, X.-P. Liu, Y. Li, Q.-H. Deng, J.-R. Chen and W.-J. Xiao, Angew, Chem., Int. Ed., 2013, 52, 3250 CrossRef CAS PubMed; (b) Y.-L. Zhang, X.-H. Liu, X.-H. Zhao, J.-L. Zhang, L. Zhou, L.-L. Lin and X.-M. Feng, Chem. Commun., 2013, 49, 11311 RSC.
- For α,β-unsaturated carbonyl compounds: (a) D. A. Evans, K. R. Fandrick, H. J. Song, K. A. Scheidt and R. Xu, J. Am. Chem. Soc., 2007, 129, 10029 CrossRef CAS PubMed; (b) G. Blay, I. FernSndez, J. R. Pedro and C. Vila, Tetrahedron Lett., 2007, 48, 6731 CrossRef CAS PubMed; (c) M. Zeng, Q. Kang, Q.-L. He and S.-L. You, Adv. Synth. Catal., 2008, 350, 2169 CrossRef CAS; (d) L. Hong, C. Liu, W. Sun, L. Wang, K. Wong and R. Wang, Org. Lett., 2009, 11, 2177 CrossRef CAS PubMed; (e) L. Hong, W. Sun, C. Liu, L. Wang, K. Wong and R. Wang, Chem. – Eur. J., 2009, 15, 11105 CrossRef CAS PubMed; (f) T. Sakamoto, J. Itoh, K. Mori and T. Akiyama, Org. Biomol. Chem., 2010, 8, 5448 RSC.
- For β-monosubstituted nitroalkenes: (a) Y.-F. Sheng, G.-Q. Li, Q. Kang, A.-J. Zhang and S.-L. You, Chem. – Eur. J., 2009, 15, 3351 CrossRef CAS PubMed; (b) N. Takenaka, J. Chen, B. Captain, R. S. Sarangthem and A. Chandrakuma, J. Am. Chem. Soc., 2010, 132, 4536 CrossRef CAS PubMed.
- For active ketones: (a) T. Wang, G. W. Zhang, Y. Teng, J. Nie, Y. Zheng and J.-A. Ma, Adv. Synth. Catal., 2010, 352, 2773 CrossRef CAS; (b) G. Blay, I. Fernández, M. C. Muñoz, J. R. Pedro, A. Recuenco and C. Vila, J. Org. Chem., 2011, 76, 6286 CrossRef CAS PubMed.
- For imines: (a) Q. Kang, X.-J. Zheng and S.-L. You, Chem. – Eur. J., 2008, 14, 3539 CrossRef CAS PubMed; (b) J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC.
- For reviews of catalytic asymmetric construction of all-carbon quaternary stereocenters, see: (a) E. J. Corey and A. Guzman-Perez, Angew. Chem., Int. Ed., 1998, 37, 388 CrossRef; (b) C. J. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363 CrossRef CAS PubMed; (c) J. Christoffers and A. Baro, Adv. Synth. Catal., 2005, 347, 1473 CrossRef CAS; (d) B. M. Trost and C. Jiang, Synthesis, 2006, 369 CrossRef CAS PubMed; (e) C. Hawner and A. Alexakis, Chem. Commun., 2010, 46, 7295 RSC.
- D. Lyzwa, K. Dudzinski and P. Kwiatkowski, Org. Lett., 2012, 14, 1540 CrossRef CAS PubMed.
- R. Liu and J. Zhang, Org. Lett., 2013, 15, 2266 CrossRef CAS PubMed.
- (a) J.-R. Gao, H. Wu, B. Xiang, W.-B. Yu, L. Han and Y.-X. Jia, J. Am. Chem. Soc., 2013, 135, 2983 CrossRef CAS PubMed; (b) T. Arai, Y. Yamamoto, A. Awata, K. Kamiya, M. Ishibashi and A. Arai, Angew. Chem., Int. Ed., 2013, 52, 2486 CrossRef CAS PubMed.
- (a) K. Mori, M. Wakazawa and T. Akiyama, Chem. Sci., 2014, 5, 1799 RSC; (b) L.-A. Chen, X. Tang, J. Xi, W. Xu, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2013, 52, 14021 CrossRef CAS PubMed; (c) J.-Q. Weng, Q.-M. Deng, L. Wu, K. Xu, H. Wu, R.-R. Liu, J.-R. Gao and Y.-X. Jia, Org. Lett., 2014, 16, 776 CrossRef CAS PubMed.
- M. G. Banwell, D. A. S. Beck and A. C. Willis, ARKIVOC, 2006, iii, 163 Search PubMed.
- The absolute configuration of product 4a was assigned to be S, see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of substrates, characterization data, 1H, 13C NMR, HRMS. See DOI: 10.1039/c4qo00265b |
| This journal is © the Partner Organisations 2015 |