Metal free access to quinolines via C–C bond cleavage of styrenes†
Ramesh
Deshidi
ab,
Shekaraiah
Devari
ab and
Bhahwal Ali
Shah
*ab
aAcademy of Scientific and Innovative Research, India
bNatural Product Microbes, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu-Tawi, 180001, India. E-mail: bashah@iiim.ac.in; Fax: +91(191)-2569333/2569017; Tel: +91-(191)-2569000-010
First published on 3rd March 2015
Abstract
A new metal free self-sorting tandem reaction between styrenes and anilines to access 2,4-disubstituted quinolines has been developed. The reaction relies on simultaneous C–C and C–N bond formation along with a C–C bond cleavage, under metal free conditions. The reaction proceeds efficiently over a broad range of substrates with excellent functional group tolerance. The utility of the method was also demonstrated in a first synthesis of bis-indoles from styrenes.
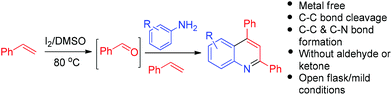
The quinoline nucleus is a characteristic underlying element of myriad bioactive scaffolds and synthetic therapeutic agents.1 Also, quinolines serve as crucial ligands in the synthesis of OLED materials2 and asymmetric catalysts.3 This have given impetus to the development of innumerable methods for their synthesis.4,5 Recently, reactions mediated by iodine have attracted great interest because of its low toxicity, the ability to operate under mild reaction conditions and its low cost compared to transition metal catalysts.6 In this direction, as part of our continuing research interests,7 we were intrigued to explore the possibility of an oxidative cross-coupling between terminal alkenes and amines using I2 as an activator and DMSO as an oxidizing agent. During the course of our attempted reaction of styrene with p-methyl aniline at 80 °C using I2 in DMSO, we discovered the rather surprising formation of 6-methyl-2,4-diphenyl quinolines, involving a fascinating C–C bond cleavage of styrene. The reaction features the formation of two new C–C bonds and one C–N bond, along with a metal free C–C bond cleavage. To the best of our knowledge, very few methods are known that involve metal free C–C bond cleavage of styrenes.8 Notably, for the cleavage of alkenes, rather harsh reaction conditions are required, such as the use of ozone (which is explosive),9 permanganate/periodic acid,10 ruthenium tetraoxide with hypochlorite or periodate,11 or hydrogen peroxide with peroxotungstate catalysts under acidic conditions.12 Moreover, general methods that convert commercially available or readily accessible materials by simultaneous C–C bond formation and cleavage in one pot are of great interest.13 An advantage of direct cleavage of a C–C bond with concomitant formation of new C–C and C–N bonds is that it avoids the traditional requirement of preinstalled functional handles.
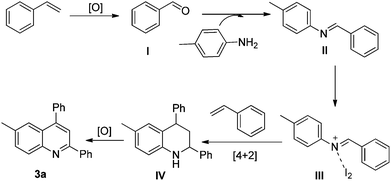
Thus, in the current study, we have developed a one pot, two component method for the generation of quinolines, wherein styrene has a dual role. One corresponds to its tendency to produce imines, and the second is to act as a dienophile. The method so developed has shown the capability of I2/DMSO to facilitate the formation of quinolines, which is achieved via C–C bond cleavage followed by C–N bond formation and then [4 + 2] cycloaddition-aromatization (Fig. 1). This work presents the first two component synthesis of quinolines, as well as the first synthesis of bis-indoles from styrenes.
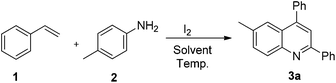
To efficiently synthesize quinolines and translate this into a general method for their synthesis, it was imperative to optimize the reaction conditions. The identification of the optimum loading was an important aspect of the reaction strategy. An increase in the loading of iodine to 1 equiv. resulted in an improvement of the product yield to 81% (Table 1, entry 2). Increasing the iodine loading to 2 equiv. did not significantly alter the reaction yield (Table 1, entry 3). Thus, an iodine loading of 1 equiv. was found to be the condition of choice. Further, to establish the role of iodine, we performed the reaction with NIS and TBAI. While the use of NIS resulted in the formation of product in 48% yield, TBAI failed to promote the reaction (Table 1, entries 4 and 5). The effect of temperature on the reaction was also examined; decreasing the temperature to rt resulted in no product formation (Table 1, entry 6). We also examined the feasibility of the reaction in other solvents, such as THF, DMF, DCM and toluene. The reaction in THF gave the corresponding product in 46% yield, while in DMF and DCM no product was formed, and toluene gave the product in 22% yield (Table 1, entries 7–10). Thus, the optimization of the reaction conditions revealed that 1 equiv. of iodine at 80 °C in DMSO is ideal to mediate the reaction with good yields. It is worth mentioning that the reaction under inert conditions resulted in no product formation whatsoever, which unambiguously establishes the role of aerial oxidation.
| Entry | Reagent | Equiv. | Solvent | Temp (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reactants: 1 (1 mmol), 2 (0.5 mmol). b Isolated yields. | ||||||
| 1 | I2 | 0.5 | DMSO | 80 | 18 | 52 |
| 2 | I2 | 1 | DMSO | 80 | 18 | 81 |
| 3 | I2 | 2 | DMSO | 80 | 18 | 83 |
| 4 | NIS | 1 | DMSO | 80 | 18 | 48 |
| 5 | TBAI | 1 | DMSO | 80 | 18 | — |
| 6 | I2 | 1 | DMSO | rt | 24 | — |
| 7 | I2 | 1 | THF | 80 | 18 | 46 |
| 8 | I2 | 1 | DMF | 80 | 18 | — |
| 9 | I2 | 1 | DCM | 40 | 18 | Trace |
| 10 | I2 | 1 | Toluene | 80 | 18 | 22 |
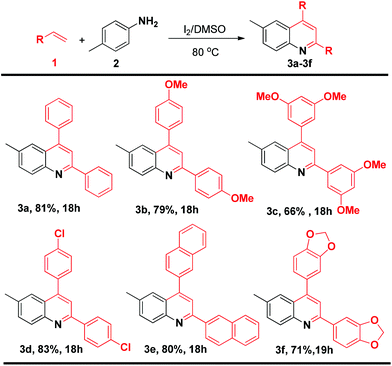
To determine whether terminal alkenes could engage in the efficient synthesis of quinolines, the scope of different styrenes in the reaction with p-methyl aniline under the optimized conditions was investigated. The reaction with a range of styrenes gave the corresponding products in good yields (Scheme 1). Styrenes with electron donating groups, i.e., p-methoxy and 3,4-dimethoxy styrene, gave the corresponding products (3b–c) in 79% and 66% yields, respectively. A halogen-substituted styrene, p-chloro styrene, also gave the corresponding product (3d) in 83% yield, offering the possibility of further functionalization. 2-naphthyl and 3,4-methylenedioxy styrene could also be efficiently transformed into the corresponding products (3e–f) in 80% and 71% yields, respectively. Notably, the reaction with o-chloro and p-nitro styrene did not give the corresponding products.
The study of the two component reaction leading to quinolines was further expanded to a range of substituted anilines (Scheme 2). Both electron-rich and electron-deficient anilines could be smoothly transformed into the desired products. The reaction of 4-methoxy, 3-methoxy and 3,5-dimethoxy styrene gave the corresponding products (3h–j) in 81%, 76% and 79% yields. Of particular note, the reaction with halogenated anilines such as 4-bromo, 4-chloro, 4-fluoro and 3,4-difluoro aniline gave the corresponding products (3k–n) in 74%, 69%, 71% and 75% yields, respectively. These molecules with halogen functionalities provide the possibility for further functionalization. The reaction of 4-OCF3-substituted aniline also gave the corresponding product 3o in 64% yield. The reaction of p-nitro and o-methyl aniline did not yield the corresponding products.
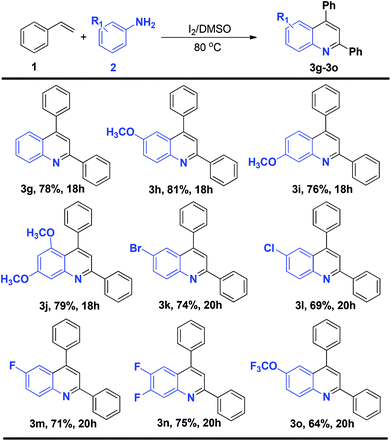 | ||
| Scheme 2 Generality of the reaction in terms of amines for the construction of substituted quinolines. Reaction conditions: 1 (1 mmol), 2 (0.5 mmol), I2 (1 mmol), DMSO (2 mL), 80 °C; isolated yields. | ||
Having established the scope of the reaction, we attempted to find out the probable mechanism by carrying out a controlled set of experiments. As we know, in the presence of iodine, styrenes can also lead to the formation of acetophenones, 2-oxoaldehydes and α-iodoacetophenones.14 Thus, to eliminate the role of these intermediates, we investigated the reaction of p-bromo substituted acetophenone, phenylglyoxal and α-iodoacetophenone with p-methyl aniline and styrene under the optimized reaction conditions. All the reactions led to the formation of product 4 instead of the desired product 3a, implying that the reaction does not proceed through these intermediates. It is worth mentioning here that, since the reaction of styrenes and anilines also takes place in THF, it is implicit that acetophenones, 2-oxoaldehydes and α-iodoacetophenones are not possible intermediates. We also attempted the reaction with phenylacetaldehyde, only to observe no product formation. In view of the fact that the reaction proceeds via oxidative cleavage of styrenes, we carried out the reaction of p-methoxybenzaldehyde with p-methyl aniline to give an iminium ion, which undergoes [4 + 2] cycloaddition in the presence of styrene, leading to the formation of 5 (Scheme 3).15 This result demonstrates that the reaction involves the formation of an iminium ion intermediate. Furthermore, the iminium ion formation was unequivocally established by GC-MS analysis of the crude reaction mixture, which showed a mass peak corresponding to N-benzylideneaniline and also matched with the GC-MS library database (see ESI†).
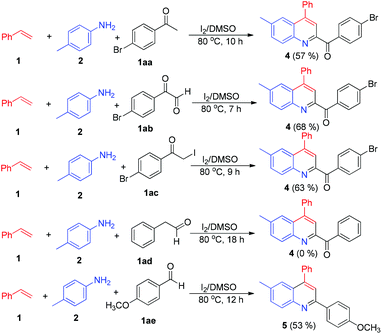 | ||
| Scheme 3 Controlled experiments. Reactants: 1 (1 mmol), 2 (1 mmol), 1aa–1ae (1 mmol), DMSO (2 mL), 80 °C; isolated yields. | ||
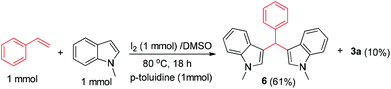
Believing that benzaldehyde is the possible intermediate from styrene, we were intrigued to see if the reaction could also be used for the synthesis of bis-indoles via C–C bond cleavage of styrenes, which is hitherto unknown. The synthesis of structurally diverse bis-indole skeletons represents an important domain in organic synthesis16 as they form part of myriad bioactive scaffolds17 and naturally occurring compounds such as topsentins,18 isoborreverine,19 and indirubin.20 Thus, we carried out the reaction of styrene and N-methyl indole, which was anticipated to lead to the formation of bis-indole 6 (Scheme 4). Notably, the reaction does not take place in the absence of toluidine or any other aniline. This may be because of the probable formation of imine as a more favourable intermediate for the reaction. Not only could this be an attractive method for the synthesis of bis-indoles, but it also confirms benzaldehyde as the intermediate involved in the synthesis of 2,4-disubstituted quinolines.
Thus, the reaction possibly proceeds via benzaldehyde formation from styrenes. Therefore, a plausible explanation of the reaction mechanism involves the formation of the corresponding aromatic aldehyde (I) via C–C bond cleavage of styrene. The aldehyde thus formed reacts with the amine to give the corresponding imine (II), as in the case of the Povarov reaction.15 The imine subsequently reacts with iodine to give an activated iminium ion (III), which then undergoes the key step of the Povarov reaction and reacts with styrene to give intermediate IV in the presence of an excess of regenerated iodine.21 The intermediate IV subsequently undergoes oxidation and aromatization to yield quinoline 3a (Fig. 2).
Conclusions
In summary, we have developed an efficient two component strategy for the synthesis of substituted quinolines. The reaction features an intriguing metal free C–C bond cleavage of styrenes to generate imines, along with C–C and C–N bond formation. The simple experimental procedure, absence of metal/oxidizing agent, stoichiometric quantities of reactants and wide substrate scope are some of the advantages of the process. The reaction may also be used for the synthesis of diverse bis-indoles. Further studies to expand the utility of this methodology to other cycloaddition and nucleophilic reactions are currently underway in our laboratory.Acknowledgements
We thank DST, New Delhi (SB/S1/OC-07/2013) for financial assistance. R. D. and S. D. thank UGC and CSIR for the award of Research Fellowships (IIIM/1772/2015).Notes and references
- (a) T. Eicher and S. Hauptmann, The Chemistry of Heterocycles, Wiley-VCH, Weinheim, 2nd edn, 2003, p. 316 CrossRef; (b) K. Andries, P. Verhasselt, J. Guillemont, W. H. Gohlmann, J. M. Neefs, H. Winkler, J. Van Gestel, P. Timmerman, M. Zhu, E. Lee, P. Williams, D. de Chaffoy, E. Huitric, S. Hoffner, E. Cambau, C. Truffot-Pernot, N. Lounis and V. Jarlier, Science, 2005, 307, 223 CrossRef CAS PubMed; (c) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (d) D. C. Behenna, J. L. Stockdill and B. M. Stoltz, Angew. Chem., Int. Ed., 2008, 47, 2365 CrossRef CAS PubMed; (e) J. K. Natarajan, J. N. Alumasa, K. Yearick, K. A. Ekoue-Kovi, L. B. Casabianca, A. C. de Dios, C. Wolf and P. D. Roepe, J. Med. Chem., 2008, 51, 3466 CrossRef CAS PubMed; (f) K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 245 Search PubMed; (g) R. Musiol, M. Serda, S. Hensel-Bielowka and J. Polanski, Curr. Med. Chem., 2010, 17, 1960 CrossRef CAS; (h) B. D. Bax, P. F. Chan, D. S. Eggleston, A. Fosberry, D. R. Gentry, F. Gorrec, I. Giordano, M. M. Hann, A. Hennessy, M. Hibbs, J. Huang, E. Jones, J. Jones, K. K. Brown, C. J. Lewis, E. W. May, M. R. Saunders, O. Singh, C. E. Spitzfaden, C. Shen, A. Shillings, A. J. Theobald, A. Wohlkonig, N. D. Pearson and M. N. Gwynn, Nature, 2010, 466, 935 CrossRef PubMed; (i) M. Rouffet, C. A. de Oliveira, Y. Udi, A. Agrawal, I. Sagi, J. A. McCammon and S. M. Cohen, J. Am. Chem. Soc., 2010, 132, 8232 CrossRef CAS PubMed; (j) S. Andrews, S. J. Burgess, D. Skaalrud, J. X. Kelly and D. H. Peyton, J. Med. Chem., 2010, 53, 916 CrossRef CAS PubMed; (k) V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488 CrossRef CAS and references cited therein.
- (a) J. I. Kim, I. S. Shin, H. Kim and J. K. Lee, J. Am. Chem. Soc., 2005, 127, 1614 CrossRef CAS PubMed; (b) H. Li and F. Jakle, Macromolecules, 2009, 42, 3448 CrossRef CAS; (c) S. Tao, L. Li, J. Yu, Y. Jiang, Y. Zhou, C.-S. Lee, S.-T. Lee, X. Zhang and O. Kwon, Chem. Mater., 2009, 21, 1284 CrossRef CAS; (d) M. Velusamy, C.-H. Chen, Y. S. Wen, J. T. Lin, C.-C. Lin, C.-H. Lai and P.-T. Chou, Organometallics, 2010, 29, 3912 CrossRef CAS; (e) V. Bhalla, V. Vij, M. Kumar, P. R. Sharma and T. Kaur, Org. Lett., 2012, 14, 1012 CrossRef CAS PubMed.
- (a) I. Abrunhosa, L. Delain-Bioton, A. C. Gaumont, M. Gulea and S. Masson, Tetrahedron, 2004, 60, 9263 CrossRef CAS PubMed; (b) M. M. Biddle, M. Lin and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 3830 CrossRef CAS PubMed; (c) Y. Zhang and M. S. Sigman, J. Am. Chem. Soc., 2007, 129, 3076 CrossRef CAS PubMed; (d) B. Tan, Z. Shi, P. J. Chua and G. Zhong, Org. Lett., 2008, 10, 3425 CrossRef CAS PubMed.
- For representative reviews, see: (a) F. W. Bergstrom, Chem. Rev., 1944, 35, 153 CrossRef; (b) F. W. Bergstrom, Chem. Rev., 1944, 35, 156 CrossRef; (c) R. H. Reitsema, Chem. Rev., 1948, 43, 47 CrossRef; (d) R. H. F. Manske and M. Kukla, Org. React., 1953, 7, 59 Search PubMed; (e) G. Jones, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky and C. W. Rees, Pergamon Press, New York, 1996, vol. 5, p. 167 Search PubMed; (f) V. V. Kouznetsov, L. Y. V. Mendez and C. M. M. Gomez, Curr. Org. Chem., 2005, 9, 141 CrossRef CAS; (g) S. Madapa, Z. Tusi and S. Batra, Curr. Org. Chem., 2008, 12, 1116 CrossRef CAS; (h) J. Marco-Contelles, E. Perez-Mayoral, A. Samadi, M. D. Carreiras and E. Soriano, Chem. Rev., 2009, 109, 2652 CrossRef CAS PubMed.
- (a) H. Skraup, Chem. Ber., 1880, 13, 2086 Search PubMed; (b) O. Doebner and W. von Miller, Chem. Ber., 1881, 14, 2812 CrossRef; (c) P. Friedlander, Chem. Ber., 1882, 15, 2572 CrossRef; (d) W. J. Pfitzinger, Prakt. Chem., 1886, 33, 100 CrossRef; (e) A. Combes, Bull. Chim. Soc. France, 1888, 49, 89 Search PubMed; (f) S. A. Yamashkin and E. A. Oreshkina, Chem. Heterocycl. Compd., 2006, 42, 701 CrossRef CAS.
- (a) Y.-P. Zhu, M.-C. Liu, F.-C. Jia, J.-J. Yuan, Q.-H. Gao, M. Lian and A.-X. Wu, Org. Lett., 2012, 14, 3392 CrossRef CAS PubMed; (b) Q. Gao, X. Wu, Y. Li, S. Liu, X. Meng and A. Wu, Adv. Synth. Catal., 2014, 356, 2924 CrossRef CAS; (c) S. Tang, Y. Wu, W. Liao, R. Bai, C. Liu and A. Lei, Chem. Commun., 2014, 50, 4496 RSC; (d) A. Ilangovan and G. Satish, J. Org. Chem., 2014, 79, 4984 CrossRef CAS PubMed; (e) Y. Yuan and H. Zhu, Eur. J. Org. Chem., 2012, 329 CrossRef CAS; (f) W. J. Xue, Q. Li, Y. P. Zhu, J. G. Wang and A. X. Wu, Chem. Commun., 2012, 48, 3485 RSC; (g) Y. P. Zhu, M. Lian, F. C. Jia, M. C. Liu, J. J. Yuan, Q. H. Gao and A. X. Wu, Chem. Commun., 2012, 48, 9086 RSC; (h) Y. P. Zhu, M. C. Liu, F. C. Jia, J. J. Yuan, Q. H. Gao, M. Lian and A. X. Wu, Org. Lett., 2012, 14, 3392 CrossRef CAS PubMed; (i) Y. P. Zhu, F. C. Jia, M. C. Liu and A. X. Wu, Org. Lett., 2012, 14, 4414 CrossRef CAS PubMed.
- (a) R. Deshidi, M. Kumar, S. Devari and B. A. Shah, Chem. Commun., 2014, 50, 9533 RSC; (b) R. Deshidi, S. Devari and B. A. Shah, Eur. J. Org. Chem., 2015, 1428 CrossRef CAS; (c) S. Devari, A. Kumar, R. Deshidi and B. A. Shah, Chem. Commun., 2015 10.1039/C4CC10438B; (d) M. Kumar, S. Devari, A. Kumar, S. Sultan, Q. N. Ahmed and B. A. Shah, Asian J. Org. Chem., 2015 DOI:10.1002/ajoc.201500022.
- (a) J.-H. Xu, Q. Jiang and C.-C. Guo, J. Org. Chem., 2013, 78, 11881 CrossRef CAS PubMed; (b) E. L. Eliel, R. T. McBride and S. Kaufmann, J. Am. Chem. Soc., 1953, 75, 4291 CrossRef CAS.
- (a) Ozonation in Organic Chemistry, Vol. 1 Olefinic Compounds, ed. P. S. Bailey, Academic Press, New York, 1978 Search PubMed; (b) U. Biermann, U. Bornscheuer, M. A. R. Meier, J. O. Metzger and H. J. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 3854 CrossRef CAS PubMed.
- (a) S. Dash, S. Patel and B. K. Mishra, Tetrahedron, 2009, 65, 707–739 CrossRef CAS PubMed; (b) P. H. J. Carlsen, T. Katsuki, V. S. Martin and K. B. Sharpless, J. Org. Chem., 1981, 46, 3936 CrossRef CAS.
- J. L. Courtney, in Organic Synthesis by Oxidation with Metal Compounds, ed. W. J. Mijs and C. R. H. I. De Jonge, Plenum, New York, 1986, p. 445 Search PubMed.
- (a) E. Antonelli, R. D'Aloisio, M. Gambaro, T. Fiorani and C. Venturello, J. Org. Chem., 1998, 63, 7190 CrossRef CAS PubMed; (b) Y. Ishii, K. Yamawaki, T. Ura, H. Yamada, T. Yoshida and M. Ogawa, J. Org. Chem., 1988, 53, 3587 CrossRef CAS.
- (a) F. Chen, T. Wang and N. Jiao, Chem. Rev., 2014, 114, 8613 CrossRef CAS PubMed; (b) Y. Yang, F. Ni, W.-M. Shu and A.-X. Wu, Chem. – Eur. J., 2014, 20, 11776 CrossRef CAS PubMed; (c) C. Zhang, P. Feng and N. Jiao, J. Am. Chem. Soc., 2013, 135, 15257 CrossRef CAS PubMed; (d) H. Liu, C. Dong, Z. Zhang, P. Wu and X. Jiang, Angew. Chem., Int. Ed., 2012, 51, 12570 CrossRef CAS PubMed; (e) Q. Gao, Y. Zhu, M. Lian, M. Liu, J. Yuan, G. Yin and A. Wu, J. Org. Chem., 2012, 77, 9865 CrossRef CAS PubMed; (f) R. Lin, F. Chen and N. Jiao, Org. Lett., 2012, 14, 4158 CrossRef CAS PubMed; (g) C. Han, E. H. Kim and D. A. Colby, J. Am. Chem. Soc., 2011, 133, 5802 CrossRef CAS PubMed; (h) H. Li, Y. Li, X.-S. Zhang, K. Chen, X. Wang and Z.-J. Shi, J. Am. Chem. Soc., 2011, 133, 15244 CrossRef CAS PubMed; (i) A. Sattler and G. Parkin, Nature, 2010, 463, 523 CrossRef CAS PubMed; (j) N. Z. Burns and P. S. Baran, Angew. Chem., Int. Ed., 2008, 47, 205 CrossRef CAS PubMed; (k) N. Z. Burns, M. Jessing and P. S. Baran, Tetrahedron, 2009, 65, 6600 CrossRef CAS PubMed; (l) F. Dagorn, L.-H. Yan, E. Gravel, K. Leblanc, A. Maciuk and E. Poupon, Tetrahedron Lett., 2011, 52, 3523 CrossRef CAS PubMed.
- (a) H. Nakayama and A. Itoh, Tetrahedron Lett., 2007, 48, 1131 CrossRef CAS PubMed; (b) R. D. Evans and J. H. Schauble, Synthesis, 1986, 727 CrossRef CAS.
- (a) L. S. Povarov, Ser. Khim., 1963, 953 Search PubMed; (b) L. S. Povarov, Ser. Khim., 1963, 2039 CAS; (c) L. S. Povarov, Russ. Chem. Rev., 1967, 36, 656 CrossRef PubMed; (d) V. V. Kouznetsov, Tetrahedron, 2009, 65, 2721 CrossRef CAS PubMed; (e) V. Sridharan, P. A. Suryavanshi and J. C. Menendez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed; (f) G. Masson, C. Lalli, M. Benohoud and G. Dagousset, Chem. Soc. Rev., 2013, 42, 902 RSC; (g) X. X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS PubMed; (h) M. Fochi, L. Caruana and L. Bernardi, Synthesis, 2014, 135 Search PubMed; (i) M. S. Xie, X. H. Chen, Y. Zhu, B. Gao, L. L. Lin, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2010, 49, 3799 CrossRef CAS PubMed; (j) G. Dagousset, J. P. Zhu and G. Masson, J. Am. Chem. Soc., 2011, 133, 14804 CrossRef CAS PubMed; (k) S. Ali, H.-T. Zhu, X.-F. Xia, K.-G. Ji, Y.-F. Yang, X.-R. Song and Y.-M. Liang, Org. Lett., 2011, 13, 2598 CrossRef CAS PubMed; (l) Z. L. Chen, B. L. Wang, Z. B. Wang, G. Y. Zhu and J. W. Sun, Angew. Chem., Int. Ed., 2013, 52, 2027 CrossRef CAS PubMed.
- (a) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (b) S. Hibino and T. Choshi, Nat. Prod. Rep., 2001, 18, 66 RSC; (c) Y. Nishizawa, Nature, 1984, 308, 693 CrossRef; (d) Y. Nishizawa, Nature, 1988, 334, 661 CrossRef PubMed; (e) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (f) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (g) M. Platon, R. Amardeil, L. Djakovitch and J.-C. Hierso, Chem. Soc. Rev., 2012, 41, 3929 RSC; (h) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed; (i) R. Vicente, Org. Biomol. Chem., 2011, 9, 6469–6480 RSC; (j) K. Sapeta, T. P. Lebold and M. A. Kerr, Synlett, 2011, 1495 CAS; (k) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC.
- (a) N. K. Garg and B. M. Stoltz, Tetrahedron Lett., 2005, 46, 2423 CrossRef CAS PubMed; (b) L.-P. Fu, Q.-Q. Shi, Y. Shi, B. Jiang and S.-J. Tu, ACS Comb. Sci., 2013, 15, 135 CrossRef CAS PubMed; (c) J. Chen, A. J. Ferreira and C. M. Beaudry, Angew. Chem., Int. Ed., 2014, 53, 11931 CrossRef CAS PubMed.
- (a) S. Tsuji, K. L. Rinehart, S. P. Gunasekera, Y. Kashman, S. S. Cross, M. S. Lui, S. A. Pomponi and M. C. Diaz, J. Org. Chem., 1988, 53, 5446 CrossRef; (b) S. A. Morris and R. J. Andersen, Can. J. Chem., 1989, 67, 677 CrossRef CAS; (c) L. M. Murray, T. K. Lim, J. N. A. Hooper and R. J. Capon, Aust. J. Chem., 1995, 48, 2053 CrossRef CAS; (d) J. Shin, Y. Seo, K. W. Cho, J.-R. Rho and C. J. Sim, J. Nat. Prod., 1999, 62, 647 CrossRef CAS PubMed.
- F. Tillequin, M. Koch, M. Bert and T. Sevenet, J. Nat. Prod., 1979, 42, 92 CrossRef CAS.
- (a) R. Hoessel, S. Leclerc, J. Endicott, M. Noble, A. Lawrie, P. Tunnah, M. Leost, E. Damiens, D. Marie, D. Marko, E. Niederberger, W. Tang, G. Eisenbrand and L. Meijer, Nat. Cell Biol., 1999, 1, 60 CrossRef CAS PubMed; (b) M. Kritsanida, P. Magiatis, A.-L. Skaltsounis, Y. Peng, P. Li and L. P. Wennogle, J. Nat. Prod., 2009, 72, 2199 CrossRef CAS PubMed.
- Q. Gao, S. Liu, X. Wu and A. Wu, Org. Lett., 2014, 16, 4582 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00010f |
| This journal is © the Partner Organisations 2015 |