CuSO4-H-phosphonate catalyzed highly stereo- and regioselective dimerization of terminal alkynes†
Xu Lia,
Xiao-Lan Chen*a,
Qing Zhanga,
Ling-Bo Quab,
Wen-Zhu Bia,
Kai Suna,
Jian-Yu Chena,
Xin Chena and
Yu-Fen Zhao*ac
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450052, China. E-mail: chenxl@zzu.edu.cn; Fax: +86 371 67767051; Tel: +86 371 67767051
bChemistry and Chemical Engineering School, Henan University of Technology, Zhengzhou, 450001, China
cDepartment of Chemistry, Xiamen University, Xiamen, 361005, China. E-mail: yfzhao@xmu.edu.cn
First published on 10th December 2014
Abstract
The readily available CuSO4-H-phosphonate catalytic system can catalyze the head-to-head dimerization of terminal alkynes to give the corresponding (E) conjugated enynes selectively in high yield. The present protocol provides an efficient and practical approach to various conjugated enynes with the advantages of cheap catalysts, operational simplicity and high stereo-and regioselectivity.
Conjugated enynes, as a versatile building block, have been widely used for the synthesis of active pharmaceutical ingredients (API) and organic electronic materials (OEM).1–3 For example, naturally occurring endiynes are a class of DNA-cleaving molecules with potent and selective anticancer activity.1a Calipeltoside A, an active enyne from a marine sponge, displays antitumor activity, as well as in vitro protection of HIV infected cells.1b Hou's group reported that π-conjugated aromatic enynes can serve as an excellent single-emitting component for white electroluminescence.4 Some powerful methodologies of using enynes to assemble the benzene or heterocyclic rings have also been developed over the past few years. For example, substituted pyrroles could be synthesized via spontaneous cycloisomerization of (Z)-(2-en-4-ynyl) amines.5 Pyridines could be synthesized via gold-catalyzed intermolecular cycloaddition of captodative dienynes with nitriles.6 Polysubstituted phenols could be assembled via the palladium catalyzed enyne-diyne cross-benzannulation.7
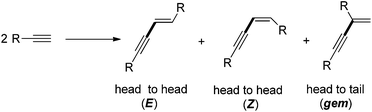
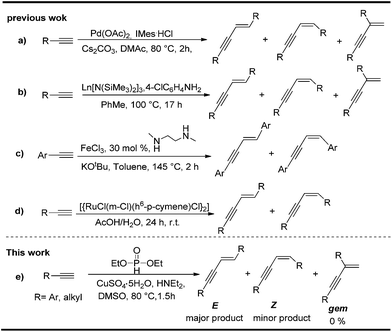
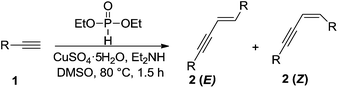
The metal-catalyzed dimerization of terminal alkynes provided a facile and atom economic entry into the chemistry of conjugated enynes.8 Three isomers of enynes, (E), (Z), and gem-enyne, would possibly be formed in the dimerization process (Scheme 1). High regio- and stereoselectivity has always been the emphasis in the area of alkyne dimerization.9–15 According to the literature, the dimerization of terminal alkynes was usually achieved by using expensive transition metals as catalysts or under harsh reaction conditions. For example, Nolan's group employed a palladium/imidazolium chloride/Cs2CO3 system to catalyze the dimerization of terminal alkynes (Scheme 2a).12b Komeyama's group carried out the reaction using a complicated catalyst system, Ln[N(SiMe3)2]3(Scheme 2b);12c A much more complicated catalyst system, [{RuCl(μ-Cl)(η6-C6Me6)}]2, was employed by Bassetti' group (Scheme 2d).13a Even though Dash' group has made progress in developing a FeCl3–DMEDA catalyst system for the E-selective head-to-head dimerization of terminal aryl alkynes, the reaction still needed to be performed in the presence of t-BuOK, a strong base, under relatively high temperature conditions (Scheme 2c).13b It is worth noticing that the previous dimerization reactions were generally carried out from aryl terminal alkynes. Aliphatic terminal alkynes were seldom mentioned for this purpose. Therefore, further developments for general and economical dimerization methodologies toward enynes are still strongly desired. Recently, we found that cheap and readily available CuSO4-H-phosphonate catalytic system can catalyzed head-to-head dimerization of terminal aryl and aliphatic alkynes to give the corresponding (E) selective conjugated enynes in high yield. Herein, we report this efficient E-selective head-to-head dimerization of terminal alkynes (Scheme 2e).
Initially, p-methylphenylacetylene (1a) was employed as model substrate to screen the reaction conditions (Table 1). The influence of variety of catalysts on the model reaction was first investigated in the presence of diethyl H-phosphonate and diethylamine in DMSO (entries 1–6). It is shown that the dimerization reaction did not work with FeCl3·3H2O, ZnBr2, MgCl2 and Ni(OAc)2·4H2O, and CuI brought about only trace product, however, CuSO4·5H2O comparatively offered the corresponding product 2a in high yield with the high stereo-and regioselectivity (ratio E/Z = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, yield: 98%). The amount of CuSO4·5H2O was also examined. Increase in the amount of CuSO4·5H2O from 20% to 50 % resulted in an increase in yield from 4% to 98%, however, the yield began to decrease when the amount of the catalyst reached the 60% (entries 7–10). The subsequent investigation on influence of the solvents indicates that DMSO was the best solvent among the solvents tested (entries 11–14). As indicated in entries 11–14, DMF gave a relatively low yield (64%), and the reaction even failed to occur in THF, CH3CN and ethanol (entries 12–14). The further investigation on influence of temperature revealed that the dimerization reaction worked best at a temperature of 80 °C (entries 6 and 15–18). It is worth emphasizing that the reaction did not happen in the absence of dialkyl H-phosphonate (entry 19). The influence of a variety of dialkyl H-phosphonates (entries 19–22) revealed that diethyl H-phosphonate still brought about the best yield among the dialkyl H-phosphonates tested. As indicated in entries 20–22, diisopropyl H-phosphonate (DIPPH) gave a low yield 17% (ratio E/Z = 72
24, yield: 98%). The amount of CuSO4·5H2O was also examined. Increase in the amount of CuSO4·5H2O from 20% to 50 % resulted in an increase in yield from 4% to 98%, however, the yield began to decrease when the amount of the catalyst reached the 60% (entries 7–10). The subsequent investigation on influence of the solvents indicates that DMSO was the best solvent among the solvents tested (entries 11–14). As indicated in entries 11–14, DMF gave a relatively low yield (64%), and the reaction even failed to occur in THF, CH3CN and ethanol (entries 12–14). The further investigation on influence of temperature revealed that the dimerization reaction worked best at a temperature of 80 °C (entries 6 and 15–18). It is worth emphasizing that the reaction did not happen in the absence of dialkyl H-phosphonate (entry 19). The influence of a variety of dialkyl H-phosphonates (entries 19–22) revealed that diethyl H-phosphonate still brought about the best yield among the dialkyl H-phosphonates tested. As indicated in entries 20–22, diisopropyl H-phosphonate (DIPPH) gave a low yield 17% (ratio E/Z = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28), while other dialkyl H-phosphonates even failed to afford the corresponding products. Finally, the influence of various bases upon the dimerization reaction was investigated (entries 23–27). Triethylamine appears to slightly less efficient than diethylamine (entries 24, 6), however it is obviously better than the other bases used such as K2CO3, Cs2CO3 and t-BuOK. Therefore, diethylamine was still proved to be the most efficient base among the bases tested. The optimum reaction conditions for this reaction were therefore found as follows: p-methyl phenylacetylene (2.5 mmol), diethyl phosphonate (1.1 equiv.), CuSO4·5H2O (50 mol%) and HNEt2 (30 mol%) in DMSO (5.0 mL) at 80 °C for 1.5 h.
28), while other dialkyl H-phosphonates even failed to afford the corresponding products. Finally, the influence of various bases upon the dimerization reaction was investigated (entries 23–27). Triethylamine appears to slightly less efficient than diethylamine (entries 24, 6), however it is obviously better than the other bases used such as K2CO3, Cs2CO3 and t-BuOK. Therefore, diethylamine was still proved to be the most efficient base among the bases tested. The optimum reaction conditions for this reaction were therefore found as follows: p-methyl phenylacetylene (2.5 mmol), diethyl phosphonate (1.1 equiv.), CuSO4·5H2O (50 mol%) and HNEt2 (30 mol%) in DMSO (5.0 mL) at 80 °C for 1.5 h.
| Entry | Cat(equiv.) | H-phosphonate (HPO(OR)2, R = ) | Solvent, T/°C | Base | Ratiob,c of 2a (E/Z), yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: p-methyl phenylacetylene (2.5 mmol), H-phosphonate (1.1 equiv.), cat (0.2–0.6 equiv.), diethylamine (0.3 equiv.) in DMSO (5 mL) for 1.5 h.b Determined by 1H NMR analysis of the crude reaction mixture.c No ratio was detected due to low yield. | |||||
| 1 | Cul (50%) | CH2CH3 | DMSO, 80 | Et2NH | Trace |
| 2 | FeCl3 (50%) | CH2CH3 | DMSO, 80 | Et2NH | NR |
| 3 | ZnBr2 (50%) | CH2CH3 | DMSO, 80 | Et2NH | NR |
| 4 | Ni(OAc)2·4H2O(50%) | CH2CH3 | DMSO, 80 | Et2NH | NR |
| 5 | MgCl2 (50%) | CH2CH3 | DMSO, 80 | Et2NH | NR |
| 6 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | Et2NH | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 98 24, 98 |
| 7 | CuSO4·5H2O(20%) | CH2CH3 | DMSO, 80 | Et2NH | 4c |
| 8 | CuSO4·5H2O(30%) | CH2CH3 | DMSO, 80 | Et2NH | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 56 25, 56 |
| 9 | CuSO4·5H2O(40%) | CH2CH3 | DMSO, 80 | Et2NH | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 86 25, 86 |
| 10 | CuSO4·5H2O(60%) | CH2CH3 | DMSO, 80 | Et2NH | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26, 91 26, 91 |
| 11 | CuSO4·5H2O(50%) | CH2CH3 | DMF, 80 | Et2NH | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 64 25, 64 |
| 12 | CuSO4·5H2O(50%) | CH2CH3 | THF, 80 | Et2NH | NR |
| 13 | CuSO4·5H2O(50%) | CH2CH3 | CH3CN, 80 | Et2NH | NR |
| 14 | CuSO4·5H2O(50%) | CH2CH3 | Ethanol, 80 | Et2NH | NR |
| 15 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 70 | Et2NH | NR |
| 16 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 90 | Et2NH | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23, 93 23, 93 |
| 17 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 100 | Et2NH | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27, 92 27, 92 |
| 18 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 110 | Et2NH | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 90 25, 90 |
| 19 | CuSO4·5H2O(50%) | — | DMSO, 80 | Et2NH | NR |
| 20 | CuSO4·5H2O(50%) | CH(CH3)2 | DMSO, 80 | Et2NH | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28, 17 28, 17 |
| 21 | CuSO4·5H2O(50%) | CH2CH2CH3 | DMSO, 80 | Et2NH | NR |
| 22 | CuSO4·5H2O(50%) | CH2Ph | DMSO, 80 | Et2NH | NR |
| 23 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | None | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23, 82 23, 82 |
| 24 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | Et2N | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 96 24, 96 |
| 25 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | K2CO3 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 86 24, 86 |
| 26 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | Cs2CO3 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 80 24, 80 |
| 27 | CuSO4·5H2O(50%) | CH2CH3 | DMSO, 80 | t-BuOK | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 80 24, 80 |
With the optimized conditions in hand, we next explored the scope of this dimerization of terminal alkynes. As we can see in Table 2, not only terminal aromatic alkynes, but also terminal aliphatic alkynes including primary (2q), secondary (2r) and tertiary aliphatic alkynes (entries 2s – t), proceeded with better stereo- and regioselectivity, affording the corresponding conjugated enynes in good to excellent yield from 62 % to 98%. It is worth mentioning that the electron-donating or electro-withdrawing substituents on the benzene ring (2a–p), did not make an obvious difference on the corresponding reaction yields. All functionalities such as methoxy, halogen, nitrile groups were well tolerated in this process, affording the corresponding products in good to excellent yield (2h–p) as well.
| Entry | Substrate 1 | Conv.b | Ratiob of 2(E/Z) | Yieldc (%) 2 |
|---|---|---|---|---|
| a Reaction condition: terminal alkynes (2.5 mmol), diethyl phosphonate 2.7 mmol (1.1 equiv.), CuSO4·5H2O 1.25 mmol (0.5 equiv.), HNEt2 0.75 mmol (0.3 equiv.) in DMSO (5.0 mL) at 80 °C for 1.5 h.b Determined by 1H NMR analysis of the crude reaction mixture.c Isolated yields by silica gel chromatography. | ||||
| 2a |  |
>99 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
92 |
| 2b |  |
>99 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
91 |
| 2c |  |
98 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
89 |
| 2d | 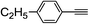 |
97 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
89 |
| 2e | 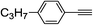 |
97 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
87 |
| 2f | 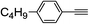 |
98 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
90 |
| 2g | 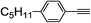 |
95 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
84 |
| 2h | 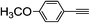 |
94 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
93 |
| 2i | 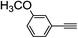 |
93 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
73 |
| 2j | 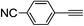 |
92 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
83 |
| 2k | 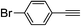 |
92 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
83 |
| 2l | 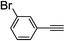 |
94 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
83 |
| 2m | 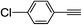 |
96 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
80 |
| 2n | 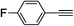 |
95 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
84 |
| 2o | 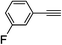 |
94 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
80 |
| 2p | 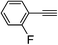 |
90 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
79 |
| 2q | 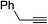 |
89 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44 |
70 |
| 2r | 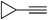 |
83 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
62 |
| 2s | 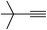 |
92 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
80 |
| 2t | 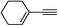 |
85 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
68 |
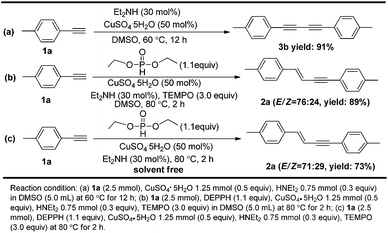
More synthetic experiments were subsequently carried out to deepen our understanding of the mechanism (Scheme 3). An experiment in the absence of diethyl H-phosphonate was firstly carried out (Scheme 3a). Diyne 3b was obtained instead in this case, indicating that dialkyl H-phosphonate was essential to synthesize the final enynes. TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy), as a widely used radical scavenger, is frequently employed to determine whether a free radical mechanism is the main path way involved in the corresponding reaction. The model reaction was carried out in the presence of TEMPO as shown in Scheme 3b. The reaction proceeded smoothly in good yield (89%), indicating that the reaction might take place via an ionic mechanism rather a free radical mechanism. Solvent are often crucial for chemical reactions. A neat reaction in the absence of DMSO was subsequently carried out as shown in Scheme 3c. In comparison with the model reaction, the net reaction proceeded smoothly with a slightly lower yield (73%), implying that DMSO didn't take part the actual reaction but slightly improve the efficiency of the reaction. The pH of the reaction was also monitored as the reaction progressed through time. We observed a quick decrease in pH from 11 to 4.5 (see details in ESI†) once the reaction started. This phenomenon implies that continuous generation of protons can occur once the reaction starts.
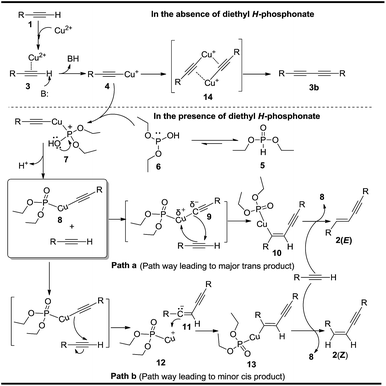
Two reaction mechanisms are accordingly depicted in Scheme 4. The possible mechanism of the dimerization of terminal alkynes catalyzed by CuSO4-H-phosphonate in the presence of diethyl H-phosphonate is shown in Scheme 4. The initial reaction of terminal alkyne 3 with copper(II) ion affords metal acetylide 4. We want to mention here that, a yellow precipitate, which might be the copper(II) acetylide 4 mentioned in Scheme 4, was observed transiently at the beginning of this dimerization reaction.16 It is well known that dialkyl H-phosphonates are especially important phosphorus compounds in the field of organophosphorus chemistry, and as very commonly used intermediates, they are frequently used to synthesize large varieties of biologically important phosphorus-containing compounds.17 The versatile synthetic applications of dialkyl H-phosphonates are closely related to the tautomeric equilibria between tricoordinated phosphorus form and tetracoordinated form.18 Here, cheap, easily prepared and preserved diethyl H-phosphonate 6 (tricoordinated form) exists with its tautomer diethyl phosphite 5 (tetracoordinated form). Diethyl phosphite 6 then acts as a ligand since it has one lone pair on phosphorus. Diethyl phosphite 6 subsequently coordinates with 4, giving a positively charged complex 7. Then deprotonation of 7 generates a neutral copper complex 8. The copper complex 8 subsequently reacts with terminal alkyne in two possible ways, path a leading to major trans-enzyne 2(E), and path b leading to minor cis-enzyne 2(Z). A regioselective head-to-head addition of partially cleaved 8 (9) to alkyne 1 (syn addition) afterwards leads to the formation of alkenyl intermediate 10 by path a. Acting as a template, intermediate 10 quickly reacts with another molecule of alkyne to yield conjugated trans-enyne 2(E), and at the same time regenerate the alkynide complex 8. In contrast, via path b, an alkynide anion, produced from 8 after the cleavage of its Cu–Csp3 bond, adds regioselectively to an alkyne molecule, resulting in the formation of vinyl anion 11 along with Cu(I) complex 12. Cu(I) complex 12 then has an opportunity to attack the vinyl anion 11 from the bottom of 11, affording alkenyl intermediate 13, which reacts alkyne 1 further, leading to the formation of conjugated trans-enyne 2(Z) along with the regeneration of another alkynide complex 8. It can be concluded that energetically favored syn-addition shown in path a is dominant over the two step trans-addition shown in path b. The experiment shown Scheme 3a, demonstrated that, in the absence of diethyl H-phosphonate, diyne 3b was obtained. The corresponding mechanism is shown in Scheme 4. Terminal alkyne 3 reacts with copper(II) ion, affording metal acetylide 4. In the absence of diethyl H-phosphonate, the two molecules of acetylide 4, via a four-membered ring transition state 14, couple together, affording the final cross-coupled diyne 3b. Similar Cu-catalyzed coupling reactions for the synthesis of the diynes were also reported.19,20 It can be concluded here that the presence of diethyl H-phosphonate played a key role in forming the alkenyl intermediate 10, a key intermediate leading to the formation of final conjugated enynes. The two mechanisms shown above might give a plausible reason that why a large variety of enynes especially E-enynes were synthesized via the dimerization of terminal alkynes in the presence diethyl H-phosphonate, otherwise diynes were synthesized instead.
Conclusion
In summary, easily available CuSO4-H-phosphonate catalytic system can catalyzed head-to-head dimerization of terminal alkynes to give the corresponding (E) selective conjugated enynes in high yield. This catalytic system represents an alternative to expensive transition metals. It may possess some advantages of cheap catalysts, operation simplicity and high stereo- and regioselectivity, opening a new door to construction of enynes under mild reaction conditions. Further mechanistic investigations and applications of this new methodology are currently underway.Experimental
General procedure
Terminal alkynes 1 (2.5 mmol), diethyl phosphonate 2.7 mmol (1.1 equiv.), CuSO4·5H2O 1.25 mmol (0.5 equiv.), HNEt2 0.75 mmol (0.3 equiv.) in DMSO (5.0 mL) at 80 °C for 1.5 h, the solvent was evaporated under vacuum, and the residue was quenched with water (5.0 mL), extracted with dichloromethane (3 × 5.0 mL). The combined organic layers were washed with brine (15.0 mL) and dried over anhydrous Na2SO4. After filtration, the solvent was evaporated in vacuo. The crude product was purified with pure petroleum ether by silica gel chromatography to give the desired product.Acknowledgements
This work was financially supported by NSFC (no. 21072178), Innovation Specialist Projects of Henan Province (no. 114200510023), Technology and Science talent Cultivation Project in Zhengzhou City (no. 112PLJRC359), The Special Fund for the Doctoral Program of Higher Education (no. 20124101110003), Foundational and Frontier Technology Research Foundation of Henan Province for their financial support (no. 132300410027).Notes and references
- (a) K. C. Nicolaou, W. M. Dai, S. C. Tsay, V. A. Estevez and W. Wrasidlo, Science, 1992, 256, 1172 CrossRef CAS; (b) D. A. Evans and J. D. Burch, Org. Lett., 2001, 3(4), 503 CrossRef CAS PubMed; (c) J. F. Dijoseph, M. E. Goad and M. M. Dougher, Clin. Cancer Res., 2004, 10, 8620 CrossRef CAS PubMed.
- (a) B. M. Trost, F. D. Toste and A. B. Pinkerton, Chem. Rev., 2001, 101, 2067 CrossRef CAS PubMed; (b) H. Kinoshita, T. Ishikawa and K. Miura, Org. Lett., 2011, 13, 122 CrossRef PubMed; (c) Y. Nishibayashi, M. Yamanashi, I. Wakiji and M. Hidai, Angew. Chem., Int. Ed., 2000, 39, 2909 CrossRef CAS.
- (a) L. M. Geary, S. K. Woo, J. C. Leung and M. J. Krische, Angew. Chem., Int. Ed., 2012, 51, 2972 CrossRef CAS PubMed; (b) V. Ritleng, C. Sirlin and M. Pfeffer, Chem. Rev., 2002, 102, 1731 CrossRef CAS PubMed; (c) H. Katayama and F. O. Coord, Chem. Rev., 2004, 248, 1703 CAS; (d) P. Wessig and G. Muller, Chem. Rev., 2008, 108, 2051 CrossRef CAS PubMed.
- Y. Liu, M. Nishiura, Y. Wang and Z. M. Hou, J. Am. Chem. Soc., 2006, 128, 5592 CrossRef CAS PubMed.
- (a) B. Gabriele, G. Salerno and A. Fazio, J. Org. Chem., 2003, 68, 7853 CrossRef CAS PubMed; (b) H. M. Peng, J. Zhao and X. W. Li, Adv. Synth. Catal., 2009, 351, 1371 CrossRef CAS.
- J. Barluenga, M. A. Fernandez-Rodriguez, P. Gatricia-Garcia and E. Aguilar, J. Am. Chem. Soc., 2008, 130, 2764 CrossRef CAS PubMed.
- V. Gevorgyan, L. G. Quan and Y. Yamamoto, J. Org. Chem., 1998, 63, 1244 CrossRef CAS.
- (a) B. M. Trost, Angew. Chem., 1995, 34, 285 (Angew. Chem., Int. Ed., 1995, 34, 259) CrossRef; (b) B. M. Trost, F. D. Toste and A. B. Pinkerton, Chem. Rev., 2002, 35, 695 CAS.
- (a) B. M. Trost, M. T. Sorum, C. Chan, A. E. Harms and G. Ruhter, J. Am. Chem. Soc., 1997, 119, 698 CrossRef CAS; (b) M. Rubina and V. Gevorgyan, J. Am. Chem. Soc., 2001, 123, 11107 CrossRef CAS; (c) C. Yang and S. P. Nolan, J. Org. Chem., 2002, 67, 591 CrossRef CAS PubMed; (d) S. Ogoshi, M. Ueta, M. A. Oka and H. Kurosawa, Chem. Commun., 2004, 2732 RSC; (e) W. Weng, C. Guo, R. Celenligil-Cetin, B. M. Foxman and O. V. Ozerov, Chem. Commun., 2006, 197 RSC; (f) M. Bassetti, C. Pasquini, A. Raneri and D. Rosato, J. Org. Chem., 2007, 72, 4558 CrossRef CAS PubMed.
- (a) M. Nishiura, Z. Hou, Y. Wakatsuki, T. Yamaki and T. Miyamoto, J. Am. Chem. Soc., 2003, 125, 1184 CrossRef CAS PubMed; (b) K. Komeyama, T. Kawabata, K. Takehira and K. Takaki, J. Org. Chem., 2005, 70, 7260 CrossRef CAS PubMed; (c) S. Ge, V. F. Q. Norambuena and B. Hessen, Organometallics, 2007, 26, 6508 CrossRef CAS.
- A. K. Dash and M. S. Eisen, Org. Lett., 2000, 2, 737 CrossRef CAS PubMed.
- (a) Y. Liu, M. Nishiura, Y. Wang and Z. Hou, J. Am. Chem. Soc., 2006, 128, 5592 CrossRef CAS PubMed; (b) C. Yang and S. P. Nolan, J. Org. Chem., 2002, 67, 591 CrossRef CAS PubMed; (c) K. Komeyama, T. Kawabata, K. Takehira and K. Takaki, J. Org. Chem., 2005, 70, 7260 CrossRef CAS PubMed.
- (a) A. Coniglio, M. Bassetti, S. E. Garcia-Garrido and J. Gimeno, Adv. Synth. Catal., 2012, 354, 148 CrossRef CAS; (b) G. C. Midya, S. Paladhi, K. Dhara and J. Dash, Chem. Commun., 2011, 47, 6698 RSC.
- (a) O. V. Zatolochnaya, E. G. Gordeev, C. Jahier, V. P. Ananikov and V. Gevorgyan, Chem.–Eur. J., 2014, 20, 9578 CrossRef CAS PubMed; (b) C. Jahier, O. V. ZAatolochnaya, N. V. Zvyagintsev, V. P. Ananikov and V. Gevorgyan, Org. Lett., 2012, 14, 2846 CrossRef CAS PubMed; (c) M. Schafer, J. Wolf and H. Werner, Dalton Trans., 2005, 1468 RSC; (d) M. A. Esteruelas, J. Herrero, A. M. Lopez and M. Olivan, Organometallics, 2001, 20, 3202 CrossRef CAS.
- (a) S. Ogoshi, M. Ueta, M. A. Oka and H. Kurosawa, Chem. Commun., 2004, 2732 RSC; (b) W. Weng, C. Y. Guo, R. Celenligil-Cetin, B. M. Foxman and O. V. Ozerov, Chem. Commun., 2006, 197 RSC; (c) S. Z. Ge, V. F. Q. Norambuena and B. Hessen, Organometallics, 2007, 26, 6508 CrossRef CAS; (d) A. Hijazi, K. Parkhomenko, J. P. Djukic, A. Chemmi and M. Pfeffer, Adv. Synth. Catal., 2008, 350, 1493 CrossRef CAS.
- (a) P. Siemsen, R. C. Livingston and F. Diederich, Angew. Chem., Int. Ed., 2000, 39, 2632 CrossRef CAS; (b) Y. Gao, G. Wang, L. Chen, P. Xu, Y. F. Zhao, Y. Zhou and L.-B. Han, J. Am. Chem. Soc., 2009, 131, 7956 CrossRef CAS PubMed; (c) Z. B. Qu, X. L. Chen, J. W. Yuan, L. B. Qu, X. Li, F. J. Wang, X. L. Ding and Y. F. Zhao, Can. J. Chem., 2012, 90, 747–752 CrossRef CAS.
- (a) J. Stawinski and A. Kraszewski, Acc. Chem. Res., 2002, 35, 952 CrossRef CAS PubMed; (b) J. Stawinski, in Handbook of Organophosphorus Chemistry, ed. R. Engel, MarcelDekker, New York, 1992, pp. 377–434 Search PubMed; (c) Y. X. Gao, G. Wang, L. Chen and Y. F. Zhao, J. Am. Chem. Soc., 2009, 131, 7956 CrossRef CAS PubMed; (d) K. D. Troev, Chemistry and application of H-phosphonates [M], Elsevier Science, 2006, vol. 84, pp. 438–442 Search PubMed; (e) Z. R. Wang, Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc., Hoboken, NJ, 2009, ISBN 978-0-471-70450-8 Search PubMed.
- (a) A. Kraszewski and J. Stawinski, Pure Appl. Chem., 2007, 79(12), 2217 CrossRef CAS; (b) W. J. Pietro and W. J. Hehre, J. Am. Chem. Soc., 1982, 104, 3594 CrossRef CAS; (c) X. L. Chen, X. Li, L. B. Qu, Y. C. Tang, W. P. Mai, D. H. Wei, W. Z. Bi, L. K. Duan, K. Sun, J. Y. Chen, D. D. Ke and Y. F. Zhao, J. Org. Chem., 2014, 79, 8407 CrossRef CAS PubMed.
- (a) N. A. Milas and O. L. Mageli, J. Am. Chem. Soc., 1953, 75, 5970 CrossRef; (b) C. Glaser, Ber. Dtsch. Chem. Ges., 1869, 2, 422 CrossRef; (c) A. S. Hay, J. Org. Chem., 1962, 27, 3320 CrossRef CAS; (d) L. Fomina, B. Vazquez, E. Tkatchouk and S. Fomine, Tetrahedron, 2002, 58, 6741 CrossRef CAS; (e) G. Eglinton and A. R. Galbraith, J. Am. Chem. Soc., 1959, 889 RSC.
- (a) S. I. Ghazala, F. Paul, L. Toupet, T. Roisnel, P. Hapiot and C. Lapinte, J. Am. Chem. Soc., 2006, 128, 2463 CrossRef CAS PubMed; (b) M. Akita, M. Terada and Y. Moroka, Chem. Commun., 1997, 265 RSC; (c) G. Argouarch, G. Grelaud and F. Paul, Organometallics, 2010, 29, 4414 CrossRef CAS; (d) W. Y. Yin, C. He, M. Chen, H. Zhang and A. W. Lei, Org. Lett., 2009, 2, 709 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c4ra10617b |
| This journal is © The Royal Society of Chemistry 2015 |