Effect of methoxy group instead of polar group in the nematic phase of four-ring bent-core liquid crystals†
Amina Nafeesa,
Gayatri Kalitab,
Manoj Kumar Paulb,
Aloka Sinha*a and
Nandiraju V. S. Raob
aDepartment of Physics, Indian Institute of Technology Delhi, Hauz Khas, New Delhi-110016, India. E-mail: aloka@physics.iitd.ac.in; Tel: +91-11-26596003
bChemistry Department, Assam University, Silchar-788011, Assam, India. E-mail: drnvsrao@gmail.com; Tel: +91-9435522541
First published on 15th December 2014
Abstract
Two achiral four-ring bent-core liquid crystal possessing an alkoxy chain at one end of the molecules and methoxy group at the other end are investigated by using the optical polarizing microscope, electro-optical studies, and dielectric spectroscopy. Both the compounds exhibit enantiotropic nematic phase over a wide range of temperatures. In the dielectric spectroscopic studies of the compounds, only one sharp peak in the loss curve is observed in the entire nematic range. In addition, the dielectric permittivity of the sample is very large. The molecular structures of these two compounds are similar to the compounds studied by Ghosh et al., which are reported to exhibit the ferroelectric-like switching and electro-convection pattern. However, the differences are (a) replacement of the polar halogen substituent at one end of the molecule by a methoxy group and (b) an increased length of the alkyl chain at the other end. These studies confirm the requirement of a polar moiety for the ferro-nematic phase to exist in these four-ring bent-core compounds.
1 Introduction
The investigation of liquid crystals composed of bent-core molecules provides us with new and unexpected electro-optical properties. The most attractive aspects of this new class of liquid crystals are in their polarity1 and chirality2,3 despite being formed from achiral molecules. Some of the smectic phases exhibited by these bent-core liquid crystals are the first ferroelectric and anti-ferroelectric liquid crystalline phases realized without introducing chirality. In this class of materials, the nematic phase is uncommon due to a strong tendency for smectic layering generated by close packing of the kinked molecules. However, very recently a number of new bent-core compounds with nematic phases (BCNs) have been synthesized.4–6 Simultaneously, there has been a surge in the theoretical studies7–10 of these compounds predicting intriguing new thermotropic nematic and isotropic structures. These include biaxial phases, orientationally ordered but optically isotropic phases, and even spontaneously chiral and polar liquid phases; these are all made theoretically possible by the bent shape of the molecules. A nematic phase exhibited by these bent-core compounds is rare and less abundant than the nematic phase exhibited by calamitic counterparts, because of the kink shape and aromatic interactions, which prohibit translational freedom. As a result, bent-core nematic (BCN) phases exhibit some unique physical properties such as biaxiality,11 giant flexo-electricity,12 unprecedented scenarios in electro-convection,13 magnetic field induced birefringence,14 non-Newtonian fluid rheology15 and ferro-nematic phases or polar response in electro-optical switching.16–18Of the above-defined properties of the bent-core liquid crystal, one of the LC phase is considered to be very important in LC research because it exhibits the illusive biaxial nematic phase and ferro-nematic phases. Thermotropic biaxial nematic phases would not only be of fundamental scientific interest for soft matter physics, but also these phases are considered as possible candidates for next generation LC displays with enhanced switching performance.19 Moreover, the fluid ferroelectric nematic phase is expected to exhibit a much faster and easier response to an external electric field compared to conventional ferroelectric smectic liquid crystals; therefore, the discovery of such a phase could open new avenues in electro-optic device technology. First, Francescangeli et al. reported the ferroelectric response to a switching electric field in the nematic phase exhibited by 1,2,4-oxadiazole derivatives.16 This is the first demonstration of ferro-nematic phase in low molar mass thermotropic nematic liquid crystal and this is connected with field induced biaxiality. Later Shanker et al. reported the ferro-electric like switching in the four series of new 1,2,4-oxadiazole derived bent-core liquid crystals.17 They showed that this is a general feature of the nematic phases of structurally different 3,5-diphenyl-1,2,4-oxadiazole derivatives. However, recently Ghosh et al. reported ferroelectric-like switching in the nematic phase of unsymmetrical achiral four-ring bent-core liquid crystals.18 These bent-core molecules, exhibit a large nematic phase range (>70 °C). It consists of two unequal lengths in two wings and possesses a polar moiety at one end and alkyloxy chain at the other end. They observed a distinct single polarization current peak under a triangular wave voltage and a low frequency relaxation mode in the dielectric spectra, indicating the presence of clusters with higher ordering, not only in the entire nematic phase, but also extended in the isotropic phase.
In this report, a four-ring bent-core liquid crystal with similar chemical structure as studied by Ghosh et al. but with minor modification in end substituent is studied. The only difference is the presence of a polar fluoro or chloro substituent at one end and an alkyloxy group (C6H13O) at the other end in their compounds. However, in our compounds there is a methoxy group at one end and an alkyloxy group (C7H15O, C8H17O) at the other end of the molecule. The advantages of these four-ring compounds viz., negative bend-splay anisotropy to formulate broad range blue phases, positive birefringence, positive dielectric anisotropy and relatively low viscosities comparable to rod-like liquid crystals, are beneficial for electro-optic devices.20 In the present work, the relaxation modes using dielectric measurements, spontaneous polarization studies by triangular wave method, texture properties using polarizing microscope and texture of the liquid crystal under the ac electric field were studied.
2 Experimental
2.1 Synthesis
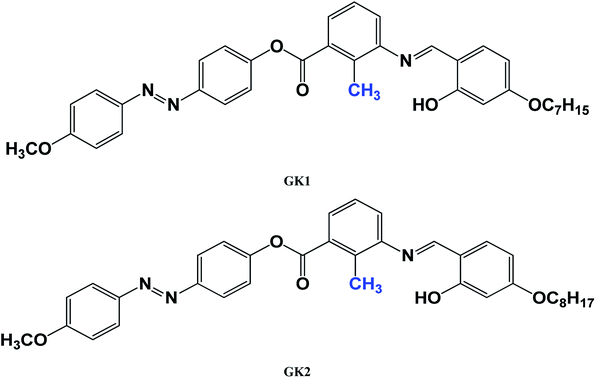
4-Hydroxy 4′-methoxy azobenzene (4) and 2-methyl 3-N-(4-n-alkyloxysalicylidene)-amino benzoicacid (5) were synthesized using the appropriate starting materials following the procedures reported earlier as described in Scheme 1. The synthesis and characterization of the compounds GK1 and GK2 are presented in the ESI.† Elemental analyses of all the compounds were consistent with the proposed molecular formulae. All the compounds were characterized by FTIR, UV-vis and 1H NMR studies.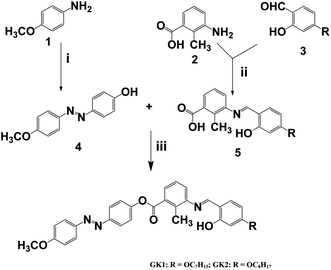 | ||
| Scheme 1 Synthetic details of compounds GK1 and GK2: (i) HCl, NaNO2, 0–5 °C, phenol, NaOH; (ii) abs EtOH, AcOH, Δ, 6 h; (iii) DCC, DMAP, DCM, stirring 48 h. | ||
2.2 Investigation
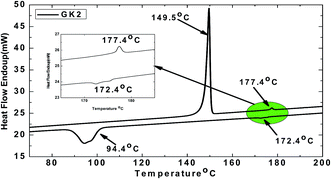
The two GK-series samples with an alkyloxy group at one end and methoxy group at the other end of the molecule are studied. The phase sequence of these two samples is obtained from differential scanning calorimetry (DSC) (PerkinElmer Pyris-1 system) and further confirmed by polarizing optical microscopy (POM) studies. The phase transition temperature and molecular structures of the GK1 and GK2 compounds are summarised in Table 1. The DSC curve of GK2 is shown in Fig. 1 as a representative example. Both the samples are found to exhibit nematic phase. The longitudinal polarizability of these lengthy molecules aided by the kink-shape of the molecules promotes the formation of layered phase. Since these compounds do not exhibit smectic phases it is implied that the lateral attraction between molecules is not large enough to form a layer. However, the optical observations revealed nematic phase with smectic C type cybotactic clusters (NcybC) which are described below. The introduction of lateral dipole in the form of oxygen atom in methoxy moiety at one end of the molecule increases the liquid crystalline range of nematic phase as well as clearing temperature.An indium tin oxide (ITO) coated planar aligned homemade cell with the thickness of 3.2 μm was used for the study of given bent-core liquid crystal. The sample was introduced into the cell using capillary action in its isotropic state 5 °C above the clearing temperature. The temperature of the cell was controlled by an Instec HCS302 hot stage attached to the STC200 temperature controller. The dielectric measurements were carried out using an Agilent (E4980A) precision LCR meter in the frequency range of 20 Hz to 2 MHz. The texture of the liquid crystal was studied using the Olympus (BX51P) Polarizing microscope. The polarizer and analyzer of the microscope were at 90° with respect to each other. For electro-optical measurement, a Tektronix AFG3021 function generator, TPS2024 oscilloscopes were used. The spontaneous polarization was measured using polarization reversal triangular wave method. The electro-convection patterns of the liquid crystal were observed simultaneously under the polarizing microscope with the application of ac electric field.
3 Results and discussion
3.1 Texture observations
The mesomorphic behaviour of compounds GK1 and GK2 was characterized by polarized optical microscopy (POM) with polarizer and analyser assembled at 90° with each other. Upon cooling from the isotropic phase, both the samples exhibited wide range nematic phase, which cooled to crystalline phase without low temperature smectic phase. Thin films of both the samples sandwiched between a nylon-6/6 treated glass plate and a coverslip exhibited characteristic schlieren and marble textures with fluidity under crossed polarizers confirming the nematic phase (see Fig. S1 in the ESI†). This indicates a predominantly homogeneous alignment of the sample with the nematic director being on average parallel to the substrate surface. The characteristic feature of this four-ring bent-core liquid crystal is a strong increase of the birefringence immediately below the isotropic-to-nematic transition. Upon decreasing the temperature, the texture changes the birefringence colour (Fig. 2), indicating a strong increase of birefringence on cooling; most probably due to an increase of the order parameter as a result of the growth of the cybotactic clusters. Similar observations of such textural changes are reported with respect to nematic phases exhibited by bent-core compounds as a signature of the growth of cybotactic SmC clusters in the cybotactic nematic phase.21 Chakraborty et al. studied another similar compound, with an identical bent-core molecular structure possessing terminal alkyloxy chains. A small angle X-ray investigations on this sample confirmed the cybotactic nematic phase and the existence of SmC-type cybotactic clusters with a more obvious four-spot diffraction pattern in the entire nematic phase range.22 The colours of the texture continuously change with decreasing temperature, but not as rapidly as near the transition temperature.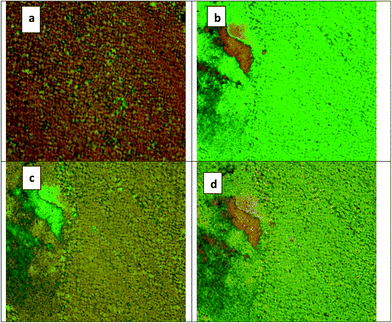 | ||
| Fig. 2 Microphotographs showing the change in texture colour with decrease in temperature of GK1; (a) at 180.3 °C, (b) 165 °C, (c) 161 °C, and (d) 132.7 °C. | ||
3.2 Dielectric spectroscopy
Dielectric spectroscopy of both the compounds was carried out using the sample sandwiched in a planar aligned cell of thickness 3.2 μm in the frequency range 20 Hz to 2 MHz. The measuring voltage was 0.1 Vpp. The complex dielectric permittivity in terms of real (ε′) and imaginary (ε′′) parts is given by the relation:| ε*(f) = ε′(f) − iε′′(f) | (1) |
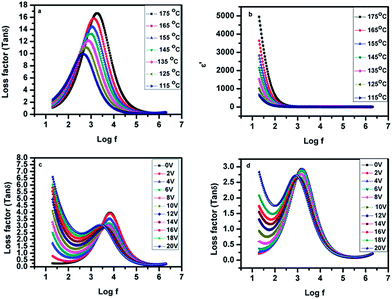
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at different temperature for GK1 samples is shown in Fig. 3. Fig. 3(a) shows that there is only one relaxation peak and it appeared in the low frequency region. To further confirm the single relaxation peak we also carried out frequency dependence of the loss factor (tan
δ) at different temperature for GK1 samples is shown in Fig. 3. Fig. 3(a) shows that there is only one relaxation peak and it appeared in the low frequency region. To further confirm the single relaxation peak we also carried out frequency dependence of the loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at different temperatures in a thicker cell of dimension 7.7 μm and the results are presented in ESI (see Fig. S2 in ESI†). The appearance of single peak in the low frequency domain suggests a collective mode. The peak of the curve shifted towards the higher frequency side as the temperature is increased. This implies that the relaxation frequency increased with an increase in the temperature. The increase in relaxation frequency seen with an increase in temperature is due to a reduction of the viscosity of the material. The frequency dependence of the real part of dielectric permittivity of the samples GK1 at different temperatures is shown in Fig. 3(b). Fig. 3(b) shows that the dielectric permittivity is very large for the samples. The large dielectric permittivity is a characteristic of the ferro-electric cluster, as reported previously for other bent-core materials.17 Similar kinds of loss factor (tan
δ) at different temperatures in a thicker cell of dimension 7.7 μm and the results are presented in ESI (see Fig. S2 in ESI†). The appearance of single peak in the low frequency domain suggests a collective mode. The peak of the curve shifted towards the higher frequency side as the temperature is increased. This implies that the relaxation frequency increased with an increase in the temperature. The increase in relaxation frequency seen with an increase in temperature is due to a reduction of the viscosity of the material. The frequency dependence of the real part of dielectric permittivity of the samples GK1 at different temperatures is shown in Fig. 3(b). Fig. 3(b) shows that the dielectric permittivity is very large for the samples. The large dielectric permittivity is a characteristic of the ferro-electric cluster, as reported previously for other bent-core materials.17 Similar kinds of loss factor (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) peaks and large dielectric permittivity are also observed in the dielectric spectra of the sample GK2 (see Fig. S3 in ESI†). The influence of the bias voltage on the relaxation mode is also studied. The peak is suppressed under the applied voltage (Fig. 3(c) and (d)) which shows that this is the collective process.
δ) peaks and large dielectric permittivity are also observed in the dielectric spectra of the sample GK2 (see Fig. S3 in ESI†). The influence of the bias voltage on the relaxation mode is also studied. The peak is suppressed under the applied voltage (Fig. 3(c) and (d)) which shows that this is the collective process.
3.3 Electro-optical study
To verify the presence of polar ordering, the electrical response and switching behaviour of GK1 and GK2 are measured in the entire nematic range by means of the repolarization current technique. A triangular wave with peak-to-peak voltage of up to 80 Vpp was applied across the sample. No peak corresponding to spontaneous polarization was observed in both the samples from the frequency range 1 Hz to 50 Hz. The response curve obtained in both the samples at frequency 10 Hz is shown in ESI (Fig. S4†). This observation could be explained by considering the symmetry-allowed twist and splay instability which often destroy the polar structure and cancel the macroscopic polarization. Thus, in general, in bent-core materials exhibiting polarization in nematic phase, current peaks are found to be absent when the electric current is measured through the sample under triangular electric fields. However, if an applied electric field is strong enough to cooperatively orient the dipoles and increase the correlation length of polar order in the cybotactic domains, ferroelectric-like switching may occur.20 To study the electro-convection pattern an ac electric field is applied across the sample, but no electro-convection pattern was observed for the sample at applied voltage from 10 Vpp to 80 Vpp or in the frequency range from 1 Hz to 100 Hz. There is no change in the texture of liquid crystal under the applied ac electric field (Fig. S5 in ESI†).3.4 Density functional theory calculations
The quantum mechanical calculations of molecular properties were performed using density functional theory (DFT),23 by employing the combination of Becke3-Lee-Yang-Parr (B3LYP) hybrid functional and 6-311G (d,p) basis set using the Gaussian 09 package, to obtain the information related to molecular conformation, bend angle and dipole moment of the compounds with fluoro (7-F), chloro (7-Cl), methyl (7-Me) and methoxy (7-MeO) substituents at one end of the molecule. Full geometry optimizations have been carried out without imposing any constraints.23 Spin-restricted DFT calculations were carried out in the framework of the generalized gradient approximation (GGA) using B3LYP hybrid functional, exchange-correlation functional and the 6-311G (d,p) standard basis set24,25 due to its successful application for larger organic molecules, as well as hydrogen bonded systems in past,26–28 and bent core molecules29–32 recently. The results are summarized in Table 2.| Compounds | Dipole moment (Debye) | Bend angle (°) Θ | |||
|---|---|---|---|---|---|
| μx | μy | μz | μresultant = (μx2 + μy2 + μz2)1/2 | ||
| a The values relative to angles and dipole moment are expressed in degree (°) and Debye (D) respectively. | |||||
| 7-F | 5.17 | 3.23 | 2.46 | 6.59 | 147 |
| 7-Cl | 5.95 | 3.44 | 2.26 | 7.24 | 146 |
| 7-CH3 | 2.76 | 1.60 | 3.33 | 4.61 | 142 |
| 7-OCH3 | 1.59 | 3.13 | 1.24 | 3.72 | 146 |
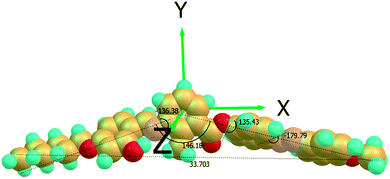
The three dihedral angles as shown in Fig. 4 between the first phenyl and central phenyl moieties with salicylidene imine linkage, central phenyl ring and first phenyl ring of azo moiety with an ester linkage are 136°, 135° respectively reflecting the absence of coplanarity of three phenyl rings with the exception being the two phenyl rings with an azo linkage (∼180°) in the molecule. The analysis shows that the bend-angle is approximately 146° for almost all mesogens and is influenced by the transverse methyl group in 2-position of the central phenyl ring. The calculation of the bending angle using DFT theory gives only a possible estimation. Recently, such relationships were discussed for 2-methyl substituted three-ring bent-core mesogens also.33 For all the mesogens, the dipole points almost in the lateral direction with respect to molecular long axis. All molecules possess a considerably larger dipole along the molecule's long axis (X-axis) except in methoxy homologue (GK1). In GK1 or GK2 the out-board dipole moment of CAr–O–CH3 atom in methoxy moiety reduces the longitudinal dipole moment and hence it may not be sufficient to promote the ferroelectric like polar switching in these compounds. Further the polarizability component αXX is found to be largest along the longitudinal X-axis, which indicates more dispersed electron cloud. Such dispersed electron cloud leads to larger amount of surface contact.
4 Conclusions
In conclusion, the dielectric spectroscopy as well as the electro-optical study of two of the four-ring bent-core liquid crystals has been undertaken. The studied compounds are similar to the compounds studied by Ghosh et al. The only difference is in the polar fluoro substituent at one end of the compound. Ghosh et al. have observed the ferroelectric like polar switching and electro-convection pattern in the entire nematic range. Ferroelectric like polar switching or the electro-convection pattern have not been observed in the present studied compounds. They observed three peaks in the dielectric loss curve and in the present case only one sharp peak in the dielectric loss curve has been observed. The fluoro substituent in organic compounds in particular in an aromatic ring is regarded as so interesting because of the combination of polar and steric effects leading to large terminal attraction. Despite the high polarity, the fluoro substituent has a low polarizability which confers low intermolecular dispersion interactions. The fluoro substituent is the smallest, after hydrogen, of all possible substituents, and like hydrogen it is monoatomic. Even though the methoxy (the outboard dipole) moiety also contributes to the polarizability and lateral terminal attraction, the out-board dipole reduces the longitudinal dipole moment and hence it may not be sufficient to promote the ferroelectric like polar switching in these compounds. This suggests that for the ferro-nematic phase, the polar substituent at one end of the molecule with a large dipole moment is required.Acknowledgements
Department of Science and Technology (SR/S2/CMP-007/2010) and University Grant Commission India are gratefully acknowledged by A. Sinha and A. Nafees respectively. The authors acknowledge Dr P. Tandon, Lucknow University for useful discussions related to DFT studies.References
- D. R. Link, G. Natale, R. Shao, J. E. MacLennan, N. A. Clark, E. Körblova and D. M. Walba, Science, 1997, 278, 1924–1927 CrossRef CAS.
- T. Sekine, T. Niori, J. Watanabe, T. Furukawa, S. W. Choi and H. Takezoe, J. Mater. Chem., 1997, 8, 1307–1309 RSC; L. E. Hough, M. Spannuth, M. Nakata, D. A. Coleman, C. D. Jones, G. Dantlgraber, C. Tschierske, J. Watanabe, E. Körblova, D. M. Walba, J. E. Maclennan, M. A. Glaser and N. A. Clark, Science, 2009, 325, 452–456 CrossRef CAS PubMed; C. Tschierske, in Chirality at the nanoscale, ed. D. B. Amabillino, Wiley-VCH, Weinheim, 2009, p. 271 Search PubMed.
- T. Niori, T. Sekine, J. Watanabe, T. Furukawa and H. Takezoe, J. Mater. Chem., 1996, 6, 1231–1233 RSC.
- J. Matraszek, J. Mieczkowski, J. Szydlowska and E. Gorecka, Liq. Cryst., 2000, 27, 429–436 CrossRef CAS; I. Wirth, S. Diele, A. Eremin, G. Pelzl, S. Grande, L. Kovalenko, N. Pancenko and W. Weissflog, J. Mater. Chem., 2001, 11, 1642–1650 RSC; W. Weissflog, H. Nádasi, U. Dunemann, G. Pelzl, S. Diele, A. Eremin and H. Kresse, J. Mater. Chem., 2001, 11, 2748–2758 RSC.
- E. Mátyus and K. Keser, J. Mol. Struct., 2001, 543, 89 CrossRef.
- T. J. Dingemans and E. T. Samulski, Liq. Cryst., 2000, 27, 131–136 CrossRef CAS.
- A. Roy, N. V. Madhusudana, P. Toledano and A. M. Figureiredo Neto, Phys. Rev. Lett., 1999, 82, 1466–1469 CrossRef CAS.
- H. R. Brand, P. E. Cladis and H. Pleiner, Eur. Phys. J. B, 1998, 6, 347–352 CrossRef CAS.
- T. C. Lubensky and L. Radzihovsky, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 66, 031704 CrossRef CAS; L. Radzihovsky and T. C. Lubensky, Europhys. Lett., 2001, 54, 206–212 CrossRef.
- H. R. Brand, H. Pleiner and P. E. Cladis, Eur. Phys. J. E, 2002, 7, 163–166 CrossRef CAS PubMed.
- C. Tschierske and D. J. Photinos, J. Mater. Chem., 2010, 20, 4263–4294 RSC.
- J. Harden, B. Mbanga, N. Eber, K. Fodor-Csorba, S. Sprunt, J. T. Gleeson and A. Jakli, Phys. Rev. Lett., 2006, 97, 157802 CrossRef CAS.
- D. B. Wiant, J. T. Gleeson, N. Eber, K. Fodor-Csorba, A. Jakli and T. Toth-Katona, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 72, 041712 CrossRef CAS.
- D. B. Wiant, S. Stojadinovic, K. Neupane, S. Sharma, K. Fodor-Csorba, A. Jakli, J. T. Gleeson and S. Sprunt, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 030703 CrossRef CAS.
- C. Bailey, K. Fodor-Csorba, J. T. Gleeson, S. N. Sprunt and A. Jakli, Soft Matter, 2009, 5, 3618–3622 RSC.
- O. Francescangeli, V. Stanic, S. I. Torgova, A. Strigazzi, N. Scaramuzza, C. Ferrero, I. P. Dolbnya, T. M. Weiss, R. Berardi, L. Muccioli, S. Orlandi and C. Zannoni, Adv. Funct. Mater., 2009, 19, 2592–2600 CrossRef CAS.
- G. Shanker, M. Nagaraj, A. Kocot, J. K. Vij, M. Prehm and C. Tschierske, Adv. Funct. Mater., 2012, 22, 1671–1683 CrossRef CAS.
- S. Ghosh, N. Begum, S. Turlapati, S. K. Roy, A. K. Das and N. V. S. Rao, J. Mater. Chem. C, 2014, 2, 425–431 RSC.
- R. Berardi, L. Cuccioli and C. Zannoni, J. Chem. Phys., 2008, 128, 024905 CrossRef PubMed.
- N. Avci, V. Borshch, D. D. Sarkar, R. Deb, G. Venkatesh, T. Turiv, S. Shiyanovskii, N. V. S. Rao and O. D. Lavrentovich, Soft Matter, 2013, 9, 1066–1075 RSC.
- C. Keith, A. Lehmann, U. Baumeister, M. Prehm and C. Tschierske, Soft Matter, 2010, 6, 1704–1721 RSC.
- L. Chakraborty, N. Chakraborty, D. D. Sarkar, N. V. S. Rao, S. Aya, K. V. Le, F. Araoka, K. Ishikawa, D. Pociecha, E. Gorecka and H. Takezoe, J. Mater. Chem. C, 2013, 1, 1562–1566 RSC.
- M. J. Frisch, et al., Gaussian 09, Revision B.01, Gaussian, Inc., CT, Wallingford, 2010 Search PubMed.
- K. Kim and K. D. Jordan, J. Phys. Chem., 1994, 98, 10089–10094 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- A. R. Leach, Molecular modelling–principles and applications, Pearson education limited, England, 2nd edn, 2001 Search PubMed.
- N. H. March, Electron Density Theory of Atoms and Molecules, Academic, London, 1992 Search PubMed.
- E. S. Kryachko and E. V. Ludena, Energy Density Functional Theory of Many-Electron System, Kluwer, Dordrecht, 1990 Search PubMed.
- J. Seltmann, A. Marini, B. Mennucci, S. Dey, S. Kumar and M. Lehmann, Chem. Mater., 2011, 23, 2630–2636 CrossRef CAS.
- A. R. K. Selvaraj, W. Weissflog and R. J. Friedemann, J. Mol. Model., 2007, 13, 907–917 CrossRef CAS PubMed.
- A. R. K. Selvaraj, W. Weissflog, G. Pelzl, S. Diele, H. Kresse, Z. Vakhovskaya and R. J. Friedemann, Phys. Chem. Chem. Phys., 2006, 8, 1170–1177 RSC.
- W. Weissflog, G. Naumann, B. Kosata, M. W. Schroeder, A. Eremin, S. Diele, H. Kresse, R. J. Friedemann, A. R. K. Selvaraj and G. Pelzl, J. Mater. Chem., 2005, 15, 4328–4337 RSC.
- W. Weissflog, U. Baumeister, M. G. Tamba, G. Pelzl, H. Kresse, R. J. Friedemann, G. Hempel, R. Kurz, M. Roos, K. Merzweiler, A. Jakli, C. Zhang, N. Diorio, R. Stannarius, A. Eremin and U. Kornek, Soft Matter, 2012, 8, 2671–2685 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12162g |
| This journal is © The Royal Society of Chemistry 2015 |