Synthesis of furo[3,4-c]quinolin-3(1H)-one derivatives through TMG catalyzed intramolecular aza-MBH reaction based on the furanones†
Dawei Yin‡
,
Wei Wang‡,
Yangqiu Peng,
Zemei Ge,
Tieming Cheng,
Xin Wang* and
Runtao Li*
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Science, Peking University, Beijing 100191, China. E-mail: xinwang@bjmu.edu.cn; lirt@bjmu.edu.cn
First published on 15th December 2014
Abstract
The first TMG catalyzed intramolecular aza-MBH reaction based on furanone derivatives was developed and successfully applied in the synthesis of a novel fused heterocyclic scaffold, furo[3,4-c]quinolin-3(1H)-ones in moderate to good yields.
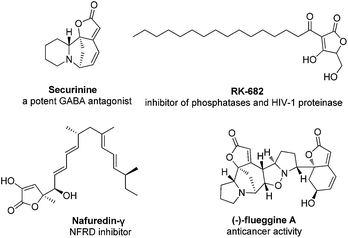
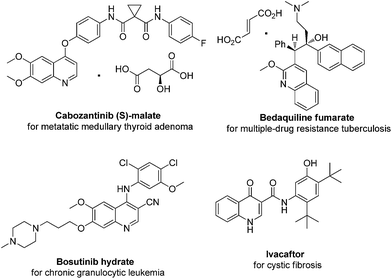
The 2(5H)-furanone structural motif is found in a number of natural products which exhibits a wide range of biological activities, including antifungal, antibacterial, antiprotozoal, anti-cancer, anti-virus, anti-inflammatory, and so on (Fig. 1).1,2 Quinoline is one of the most prevalent scaffolds encountered in medicinal chemistry.3 For example, there are four drugs with quinolone scaffold among twenty eight NCEs newly approved by the U.S. Food and Drug Administration (FDA) in 2012 (Fig. 2).4 Thus, we envisaged the combination of quinoline derivatives with 2(5H)-furanone moiety which would lead to a new kind of scaffold with potential biological activity. However, there is only one patent involving the synthesis of such kind of compounds, in which the author used harsh conditions and multi-step reaction. (US005604235) Therefore, the need for the development of more convenient methods remains.
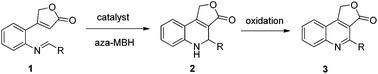
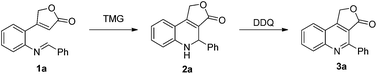
The Morita–Baylis–Hillman (MBH) reaction is one of the most useful and popular carbon–carbon bond forming reactions since its atom economy and functional group generation.5 Over the last two decades, this reaction and its applications have received remarkably growing interest. However, comparing with the intermolecular Morita–Baylis–Hillman (MBH) reaction,6 the intramolecular MBH reaction is still not fruitful.7 As a continuation of our ongoing effort in developing the reactions based on 2(5H)-furanone,8 we herein disclose the first tetramethyl guanidine (TMG) catalyzed intramolecular aza-MBH reaction of 2(5H)-furanone derivatives 1 to construct the key intermediate dihydrofuro[3,4-c] quinolin-3(1H)-ones 2, which was easily transformed into furo[3,4-c] quinolin-3(1H)-one derivatives 3 by oxidation (Scheme 1).
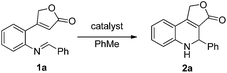
At the beginning of our study, the starting materials 1 were conveniently obtained according to the method reported by Patel (detailed information shown in ESI†).9 Then, 4-(2-(benzylideneamino)phenyl)furan-2(5H)-one (1a) was chosen as model substrate to optimize the reaction conditions for intramolecular aza-MBH reaction. Various catalysts, including organic bases and Lewis acids which are commonly used in the MBH reaction, were firstly screened. As shown in Table 1, only two non-nucleophilic organic bases, DBU and TMG, gave the desired product dihydrofuro[3,4-c] quinolin-3(1H)-one 2a in low yields of 11% and 19%, respectively (Entries 7 and 8, Table 1). Much to our delight, when using TMG as the catalyst and increasing the reaction temperature, the yield was greatly improved and the reaction time was significantly shortened. When the reaction was carried out at 120 °C, compound 2a was obtained in 63% yield in 20 h (Entry 10, Table 1). However, it is noteworthy that compound 2a was vulnerable to oxidation even under room temperature and DDQ could oxidize 2a to furo[3,4-c] quinolin-3(1H)-one 3a quantitatively within 5 minutes. Considering the unstability, compounds 2 were directly oxidized to 3 in the subsequent study. Encouraged by this promising finding, we continued to explore the influence of other high boiling point solvents on the reaction, which included o-xylene, ethylene glycol, diethylene glycol, N-methyl-2-pyrrolidone (NMP) and n-octane (Entries 1–5, Table 2). The results reveal that o-xylene is the best choice for the improvement of yields and shorten of reaction time (Entry 5, Table 2). Furthermore, the screening of catalyst loading reveals that lower catalyst loading was unfavourable for the reaction (Entries 6–7, Table 2). Thus, 20 mol% of TMG catalyst, o-xylene as solvent and 140 °C were chosen to be the most suitable reaction conditions.
| Entry | Catalyst | Temp. (°C) | Time | Yielda |
|---|---|---|---|---|
| a Isolated yields by silica-gel column purification. | ||||
| 1 | Triethylamine 20 mol% | 50 | 48 h | Trace |
| 2 | DIPEA 20 mol% | 50 | 48 h | Trace |
| 3 | DABCO 20 mol% | 50 | 48 h | Trace |
| 4 | TMEDA 20 mol% | 50 | 48 h | Trace |
| 5 | CuTc 20 mol% | 50 | 48 h | N.R. |
| 6 | ZnCl2 20 mol% | 50 | 48 h | N.R. |
| 7 | DBU 20 mol% | 50 | 48 h | 11% |
| 8 | TMG 20 mol% | 50 | 48 h | 19% |
| 9 | TMG 20 mol% | 100 | 24 h | 34% |
| 10 | TMG 20 mol% | 120 | 20 h | 63% |
| Entry | Catalyst | Solvent | Temp.(°C) | Time | Yielda |
|---|---|---|---|---|---|
| a Overall yield after DDQ oxidation. | |||||
| 1 | TMG 20 mol% | Diethylene glycol | 140 | 1 h | 29% |
| 2 | TMG 20 mol% | Ethylene glycol | 140 | 2 h | 19% |
| 3 | TMG 20 mol% | NMP | 140 | 2 h | 42% |
| 4 | TMG 20 mol% | n-Octane | 140 | 2 h | 11% |
| 5 | TMG 20 mol% | o-Xylene | 140 | 7 h | 69% |
| 6 | TMG 10 mol% | o-Xylene | 140 | 24 h | 47% |
| 7 | TMG 5 mol% | o-Xylene | 140 | 24 h | 31% |
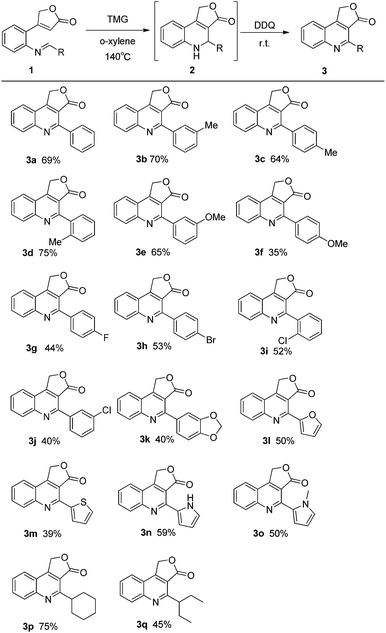
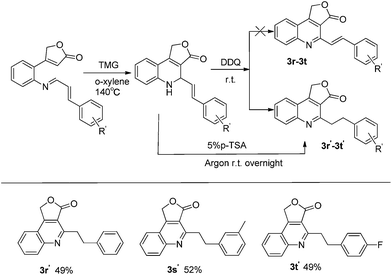
With the optimal reaction conditions in hand, the scope of the reaction was explored (Fig. 3). In most cases, the reactions proceeded smoothly to generate furo[3,4-c] quinolin-3(1H)-one 3 in moderate to good yields. For imines 1 derived from substituted aromatic aldehydes, both the position and the properties of substituted group would affect the yield of aza-MBH reaction. Usually, electron donating substituents afforded good yields (yields from 64%–75%, 1b–1f) except the one with para-methoxy group whose strong donating effect may reduce the electrophilicity of imine carbon. Simultaneously, electron withdrawing substituents also afforded the acceptable results (yields 40–53%, 1g–1j). Besides, the substrates from heterocyclic aromatic aldehydes (1l–1o) and aliphatic aldehydes (1p, 1q) could yield the reaction and provide the corresponding aza-MBH products. To further expand the substrate scope of this methodology, we turned our attention to the substrates derived from cinnamaldehyde and its derivatives. Much to our surprise, under optimized reaction conditions, these substrates afforded the products 4-phenethylfuro[3,4-c]quinolin-3(1H)-ones (3r′–3t′) instead of the expected product 4-styrylfuro[3,4-c]quinolin-3(1H)-ones (3r–3t) (Fig. 4), suggesting that the final aromatization step was derived from hydrogen migration rather than DDQ oxidation. This proposal was supported by the experimental result that the product (3r′) was also obtained by stirring the intermediate compound 2r in 5% p-toluene sulfonic acid (PSA) overnight under argon atmosphere.
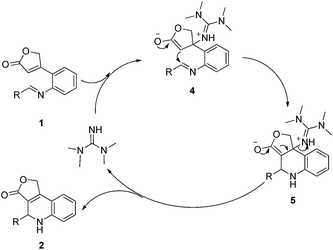
According to the literature,10 a possible mechanism of this intramolecular aza-MBH reaction is proposed in Scheme 2. Through 1,4-addition reaction, TMG attacks the activated alkene 1 to generate the zwitterionic aza-enolate 4. Then 4 undergoes the intramolecular aldol-like reaction affording the cyclized intermediate 5. Finally, releasing TMG from 5 yields the product 2.
Conclusions
In summary, we have developed the first TMG catalyzed intramolecular aza-MBH reaction based on the furanone derivatives and a novel series of furo[3,4-c] quinolin-3(1H)-one derivatives were synthesized in moderate to good overall yields. This reaction could tolerate a wide range of substituents including aryl, heterocyclic aryl, alkyl and vinyl groups. Furo[3,4-c] quinolin-3(1H)-one represents a new kind of fused heterocyclic scaffold containing furanone and quinoline, which would become a more potential privileged structure in the drug discovery.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (no. 81172915, 20972005).Notes and references
- For examples, see (a) V. E. J. Hikawczuk, J. R. Saad, O. S. Giordano, C. García, T. Martín, V. S. Martín, M. E. Sosa and C. E. Tonn, J. Nat. Prod., 2008, 71, 190 CrossRef PubMed; (b) E. Gondela and K. Z. Walczak, Eur. J. Med. Chem., 2010, 45, 3993 CrossRef CAS PubMed; (c) R. Surmont, G. Verniest and N. De Kimpe, J. Org. Chem., 2010, 75, 5750 CrossRef CAS PubMed; (d) Z. P. Xiao, T. W. Ma, M. L. Liao, Y. T. Feng, X. C. Peng, J. L. Li, Z. P. Li, Y. Wu, Q. Luo, Y. Deng, X. Liang and H. L. Zhu, Eur. J. Med. Chem., 2011, 46, 4904 CrossRef CAS PubMed.
- (a) A. M. ElSohly, D. A. Wespe, T. J. Poore and S. A. Snyder, Angew. Chem., Int. Ed., 2013, 52, 5789 CrossRef CAS PubMed; (b) D. Rognan, T. Boulanger, R. Hoffmann, D. P. Vercauteren, J. M. Andre, F. Durant and C. G. Wermuth, J. Med. Chem., 1992, 35, 1969 CrossRef CAS; (c) Y. Sun, F. Hahn, Y. Demydchuk, J. Chettle, M. Tosin, H. Osada and P. F. Leadlay, Nat. Chem. Biol., 2010, 6, 99 CrossRef CAS PubMed; (d) T. Nagamistu, D. Takano, M. Seki, S. Arima, M. Othtawa, K. Shiomi, Y. Harigaya and S. Omura, Tetrahedron, 2008, 64, 8117 CrossRef PubMed; (e) B. X. Zhao, Y. Wang, D. M. Zhang, R. W. Jiang, G. C. Wang, J. M. Shi, X. J. Huang, W. M. Chen, C. T. Che and W. C. Ye, Org. Lett., 2011, 13, 3888 CrossRef CAS PubMed.
- (a) I. Weissbuch and L. Leiserowitz, Chem. Rev., 2008, 108, 4899 CrossRef CAS PubMed; (b) S. Kumar, S. Bawa and H. Gupta, Mini-Rev. Med. Chem., 2009, 9, 1648 CrossRef CAS; (c) V. R. Solomon and H. Lee, Curr. Med. Chem., 2011, 18, 1488 CrossRef CAS; (d) A. P. Gorka, A. de Dios and P. D. Roepe, J. Med. Chem., 2013, 56, 5231 CrossRef CAS PubMed.
- (a) C. D. Hart and R. H. De Boer, OncoTargets Ther., 2013, 6, 1 CAS; (b) D. Jones, Nat. Rev. Drug Discovery, 2013, 12, 175 CrossRef CAS PubMed; (c) D. H. Boschelli, Y. D. Wang, S. Johnson, B. Wu, F. Ye, A. C. B. Sosa, J. M. Golas and F. Boschelli, J. Med. Chem., 2004, 47, 1599 CrossRef CAS PubMed; (d) F. V. Goor, S. Hadida, P. D. J. Grootenhuis, B. Burton, J. H. Stack, K. S. Straley, C. J. Decker, M. Miller, J. McCartney, E. R. Olson, J. J. Wine, R. A. Frizzell, M. Ashlock and P. A. Negulescu, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 18843 CrossRef PubMed.
- (a) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447 CrossRef CAS PubMed; (b) G. Masson, C. Housseman and J. P. Zhu, Angew. Chem., Int. Ed., 2007, 46, 4614 CrossRef CAS PubMed; (c) V. Dederck, J. Mattinez and F. Lamaty, Chem. Rev., 2009, 109, 1 CrossRef PubMed; (d) D. Basavaiah and G. Veeraraghavaiah, Chem. Soc. Rev., 2012, 41, 68 RSC.
- (a) G. N. Ma, J. J. Jiang, M. Shi and Y. Wei, Chem. Commun., 2009, 5496 RSC; (b) V. Singh and S. Batra, Tetrahedron, 2008, 64, 4511 CrossRef CAS PubMed; (c) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS PubMed; (d) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (e) D. Basavaiah, K. V. Rao and R. J. Reddy, Chem. Soc. Rev., 2007, 36, 1581 RSC; (f) Y. Lu, S. X. Wang, X. Han, F. Zhou and Y. Wang, Synlett, 2011, 2766 CrossRef PubMed; (g) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (h) Y. L. Shi and M. Shi, Eur. J. Org. Chem., 2007, 2905 CrossRef CAS.
- (a) H. Mandai, K. Shimowaki, K. Mitsudo and S. Suga, Asian J. Org. Chem., 2014, 3, 437 CrossRef CAS; (b) H. L. Song, K. Yuan and X. Y. Wu, Chem. Commun., 2011, 47, 1012 RSC; (c) P. Langer, Angew. Chem., Int. Ed., 2000, 39, 3049 CrossRef CAS; (d) F. C. Pigge, R. Dhanya and E. R. Hoefgen, Angew. Chem., Int. Ed., 2007, 46, 2887 CrossRef CAS PubMed; (e) L. R. Reddy, P. Saravanan and E. J. Corey, J. Am. Chem. Soc., 2004, 126, 6230 CrossRef CAS PubMed; (f) P. R. Krishna, V. Kannan and G. V. M. Sharma, J. Org. Chem., 2004, 69, 6467 CrossRef CAS PubMed.
- (a) W. Wang, J. Wang, S. Zhou, Q. Sun, Z. Ge, X. Wang and R. Li, Chem. Commun., 2013, 49, 1333 RSC; (b) J. Wang, X. Wang, Z. Ge, T. Cheng and R. Li, Chem. Commun., 2010, 46, 1751 RSC.
- P. Thombare, J. Desai, A. Argade, S. Gite and P. Patel, Synth. Commun., 2009, 39, 2423 CrossRef CAS.
- N. E. Leadbeater and C. van der Pol, J. Chem. Soc., Perkin Trans. 1, 2001, 2831 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra13796e |
| ‡ These authors contributed equally to this research. |
| This journal is © The Royal Society of Chemistry 2015 |