Tandem Click-Suzuki reactions in a novel flow reactor incorporating immobilized and exchangeable reagents†
Noah P.
Tu
*,
Kathy
Sarris
and
Stevan W.
Djuric
Discovery Chemistry & Technology, AbbVie Inc., 1 North Waukegan Road, North Chicago, IL 60064, USA. E-mail: noah.tu@abbvie.com
First published on 10th December 2014
Abstract
A custom-built modular flow reactor featuring immobilized reagents in exchangeable cartridges has been developed. The concept is based on a custom designed flow reactor with easy access to internal components for reagent cartridge exchange. Custom made reagent cartridges filled with different supported catalysts and reagents are used for various reaction conditions. The custom reactor has, as one component, the same footprint as the Accendo Conjure™ reactor and no hardware or software modification to the Conjure unit is required. This reactor opens the way to chemical transformations previously not readily achievable with the Accendo Conjure™ flow chemistry system. In this communication, we demonstrate the applicability of this system to tandem Click-Suzuki reactions.
Performing chemical transformations under flow conditions provides exquisite control over the reaction. With small effective reaction volume, exothermic reactions can be performed more safely compared to batch reactions. Scale-up to larger sample sizes is achieved, not with stepwise transitions to larger vessels, but by running multiple systems in parallel, or adjusting the time a system is in operation.
Previously, we have described the successful integration of a mass-directed preparative HPLC to a Accendo Conjure™ segmented flow reactor.1 The integrated system combined synthesis, purification and compound management in a single continuous operation with full automation. The cycle time for generating small focused libraries of compounds (48–96 members) ready for bioassay and compound registration was reduced to 3 days.2 However, an inherent limitation for carrying out chemical transformations in the integrated system is the requirement of homogeneous reaction conditions. This prerequisite has restricted our integrated platform to performing only a subset of chemical transformations where no solid reagents or catalysts are involved, with the exception of most commonly Click chemistry,3a,b Ullmann condensations, Sonogashira couplings, decarboxylation reactions4 and hydroxylation reaction,5 where the copper reactor is also the catalyst. This limitation has consequently hampered the utilization and application of the integrated flow-purification platform for a wide variety of chemistries typically requested from medicinal chemists.
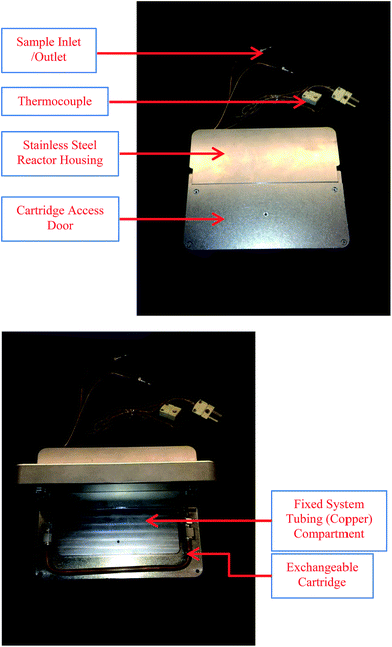
In our efforts towards fully automated compound library production in flow, we have utilized the Accendo Conjure™6 flow reactor as the fluidic device for sample preparation. The Conjure utilized a reactor assembly as shown in Fig. 1. The design of the Conjure includes a coil of hastelloy or copper tubing sandwiched between two stainless steel plates. The thermocouple inside the reactor monitors internal temperature and feedback data to the control software for temperature control.
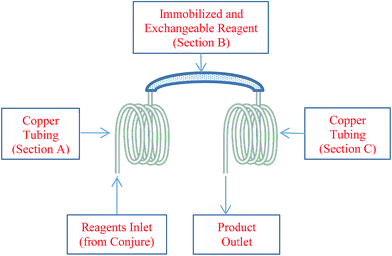
Recently, inspired by previous work from Ley7a,b and Baxendale8 on the use of polymer supported reagents in organic synthesis, we have developed a novel modification to the Conjure system that has an access door to an internal compartment where a disposable/exchangeable cartridge is located (Fig. 2). The reactor was designed in house and manufactured by AutoChem.9 The reactor is designed to fit inside the Conjure platform such that no additional hardware or software modifications are needed. The internal components of the custom reactor (Fig. 3) consist of a copper coil (section A) where individual reagents introduced by Conjure™ reactor are mixed and preheated to form a uniform segment. Upon entering the immobilized and exchangeable reagent (section B), the mobilized segment reacts with the immobilized reagent to form the desired product. Since the integrity of the segment will most likely be interrupted by the solid filled section B, and the integrity of the segment is crucial for proper capturing of the segment to enter HPLC system, the custom reactor incorporates additional copper tubing (section C) to allow the segment to re-assemble prior to HPLC injection. The design of the immobilized reagent and exchangeable cartridge (section B) is such that a variety of solid reagents or catalysts can be filled inside the section and therefore largely expand the scope of chemistry that the Conjure platforms can perform. With its relatively low cost, section B is disposable and may be changed easily (by opening the access door and removing the used section B with a wrench) from library to library to ensure maximum reagent activity and eliminate the possibility of cross contamination between libraries. To date, there are three different immobilized and exchangeable reagent cartridges (section B) available:9 Siliacat DPP-Pd,10 FibreCat 1032 (ref. 11) and a proprietary palladium metal oxide.9 In addition, a variety of commonly used solid supported catalysts, reagents and scavenging material for batch process can be introduced. It is also possible to mix more than one solid supported reagent/catalyst as packing material for section B. For example, a cartridge filled with immobilized Pd catalyst, ligand and base can be used to carry out Buchwald–Hartwig cross coupling reactions in flow requiring only the addition of aryl halide and amine as the mobile reactants.
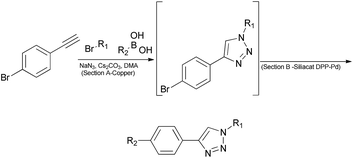
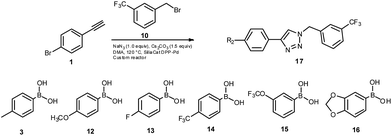
To examine the effectiveness of this custom reactor, we utilized a tandem Click12-Suzuki13 reaction protocol (Scheme 1). Since section A is made of copper, no additional copper source is needed to facilitate the Click chemistry.
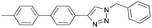
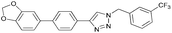
In an initial test reaction (Scheme 2) using bromoalkyne 1, bromoethanol 2 and p-tolyboronic acid 3 gave the desired tandem Click-Suzuki product 4 in moderate yield (28%).
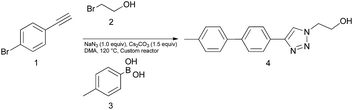 | ||
| Scheme 2 Reaction exploration of tandem Click-Suzuki protocol. Conditions: 1 (0.22 mmol), 2 (0.22 mmol), 3 (0.33 mmol), NaN3 (0.22 mmol), Cs2CO3 (0.33 mmol), DMA (3.0 mL). | ||
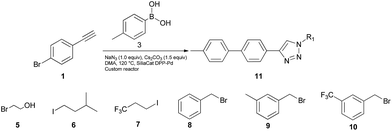
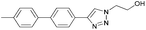
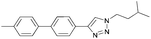
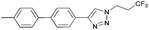
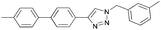
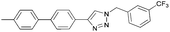
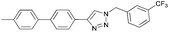
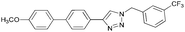
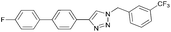
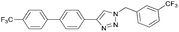
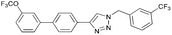
To further examine the effectiveness of this custom reactor for the tandem Click-Suzuki protocol we synthesized two small 6-membered libraries, using bromoalkyne 1 as the common core.14 Boronic acid 3 and 6 different halides (5–10) were used in library 1 (Scheme 3) to yield product 11(a–f), whereas halide 10 and 6 different boronic acids (3, 12–16) were used in library 2 to yield product 17(a–f) (Scheme 4).
When section B is filled with the desired solid reagents, the void volume inside the cartridge is small compared to empty tubing (section A and C). As a result, effective residence time for reactants in section B is low. This translates to lower yield for the Suzuki reaction and the presence of Click chemistry products in the final compound mixture. In order to improve the Suzuki reaction yield, we have repeated the experiment with higher reaction volume to increase the effective residence time in section B. This modification resulted in a moderate improvement in average yields of the two libraries. To further address this issue, we designed a “looped” flow reaction system whereby unreacted reactants can be looped back to the reactor for additional reaction time. Optimization of this system is on-going, as is the development of different chemistries with different solid reagent filled cartridges. These studies will be reported in future communications from our laboratories.
The tandem Click-Suzuki products and their respective isolated yields are reported in Table 1. It should be noted that in a control experiment to compare the yield and success rate of the flow tandem Click-Suzuki reactions with the traditional batch process, library synthesis was repeated under one-pot conditions.15 We observed that yields were uniformly low due to the copper powder and SiliaCat DPP-Pd creating a mixture that was extremely difficult to filter. Breaking up the tandem process into a stepwise protocol with filtering post Click chemistry gave the desired products in yields (30–43%) comparable to the tandem reactions in flow but less efficiently.
Conclusions
We have developed a custom-built flow reactor with immobilized reagents and exchangeable cartridges useful for a variety of chemical transformations previously not readily amenable to flow chemistry. Examples of tandem Click-Suzuki reaction protocols demonstrated the utility of the custom reactor. Further efforts will focus on improving effective residence time of reactants in the exchangeable cartridge.We anticipate the expansion of the concepts presented herein to other reagent systems thereby allowing for further advances in the area of continuous process chemistry in flow systems.16
Acknowledgements
All authors are employees of AbbVie. The design, study conduct, and financial support for this research was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.Notes and references
- J. E. Hochlowski, P. A. Searle, N. P. Tu, J. Y. Pan, S. G. Spanton and S. Djuric, J. Flow Chem., 2011, 2, 56–61 CrossRef.
- J. D. Sutherland, N. P. Tu, T. A. Nemcek, P. A. Searle, J. E. Hochlowski, S. W. Djuric and J. Y. Pan, J. Lab. Autom., 2014, 19(2), 176–182 CrossRef CAS PubMed.
- (a) N. P. Tu, J. E. Hochlowski and S. W. Djuric, Mol. Diversity, 2012, 16, 53–58 CrossRef CAS PubMed; (b) A. R. Bogdan and N. W. Sach, Adv. Synth. Catal., 2009, 351, 849–854 CrossRef CAS.
- P. Cyr and A. B. Charette, Synlett, 2014, 1409–1412 Search PubMed.
- T. N. Andrew and A. J. Musacchio, Org. Lett., 2011, 13(19), 5180–5183 CrossRef PubMed.
- Accendo Corporation, http://www.accendocorporation.com.
- (a) M. D. Hopkin, I. R. Baxendale and S. V. Ley, Chem. Commun., 2010, 46, 2450–2452 RSC; (b) Z. Qian, I. R. Baxendale and S. Ley, Chem.–Eur. J., 2010, 16, 12342–12348 CrossRef CAS PubMed.
- M. Baumann and I. Baxendale, Beilstein J. Org. Chem., 2013, 9, 1613–1619 CrossRef PubMed.
- AutoChem Private Limited, http://www.autochem.com.sg.
- Silicycle, http://www.cilicycle.com.
- Johnson Matthey Catalysis and Chiral Technologies, http://www.jmcct.com.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed. Engl., 2001, 40, 2004–2021 CrossRef CAS.
- Y. Zhang, T. F. Jamison, S. Patel and N. Mainolfi, Org. Lett., 2011, 13(2), 280–283 CrossRef CAS PubMed.
- General procedure: alkyl halide (0.1 mmol in DMA, 1.0 equiv., 100 μL), acetylene (1.0 equiv. in DMA, 100 μL), NaN3 (1.0 equiv. in DMA, 100 μL), boronic acid (1.5 equiv. in DMA, 100 μL) caesium carbonate (1.5 equiv. in water, 100 μL) were aspirated from their respective reagent vials using Conjure feed module, mixed through a PFA mixing tube (0.2 mm ID), loaded into an injection loop and injected into the Conjure reactor equipped with the custom reactor filled with 0.7 g Siliacat DPP-Pd at a flow rate of 146 μL min−1 (10 min residence time). Reaction segments were directed injected into a preparative mass-directed-HPLC system and collection of fraction trigged by detection of molecular weight of desired product.
- All reagents (with the exception of Siliacat DPP-Pd) and copper powder were mixed in 4 mL vial and heated to 120 °C for 10 min followed by the addition of SiliaCat DPP-Pd and heated for another 10 min at 120 °C. The mixture was filtered and purified by reverse phased HPLC system.
- M. Peplow, Nature, 2014, 512, 20–22 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure and characterization data of all compounds. See DOI: 10.1039/c4ra13931c |
| This journal is © The Royal Society of Chemistry 2015 |