2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) formation and fate: an example of the coordinate contribution of lipid oxidation and Maillard reaction to the production and elimination of processing-related food toxicants
Rosario Zamora
and
Francisco J. Hidalgo
*
Instituto de la Grasa, Consejo Superior de Investigaciones Científicas, Carretera de Utrera km 1, Campus Universitario – Edificio 46, 41013 Seville, Spain. E-mail: fhidalgo@ig.csic.es; Fax: +34 954 616 790; Tel: +34 954 611 550
First published on 5th January 2015
Abstract
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is produced by reaction of phenylacetaldehyde, the Strecker aldehyde of phenylalanine, with creati(ni)ne in the presence of formaldehyde and ammonia, which are formed in situ. Traditionally, the carbonyl compounds required for the formation of the PhIP ring were considered to be produced as a consequence of Maillard reaction between phenylalanine and carbohydrates. This review collects all recent evidence suggesting that lipids can also contribute to the Strecker degradation of phenylalanine and the formation of formaldehyde analogously to carbohydrates. Furthermore, lipid-derived reactive carbonyls not only contribute to PhIP formation but they can also be involved in PhIP fate observed in the presence of oxidized oils. This role has not been yet investigated for carbohydrate-derived reactive carbonyls but it might be hypothesised to take place because of the decrease of PhIP observed when an excess of monosaccharides is employed to study PhIP formation. All these results suggest that carbohydrates and lipids can contribute simultaneously to PhIP formation and fate. This is another example of the simultaneous contribution of both lipids and carbohydrates to the carbonyl chemistry that takes place in foods upon processing and/or storage. Furthermore, many of these conclusions can also be extended to the formation of other aminoimidazoazarenes with an imidazopyridine structure.
1. Introduction
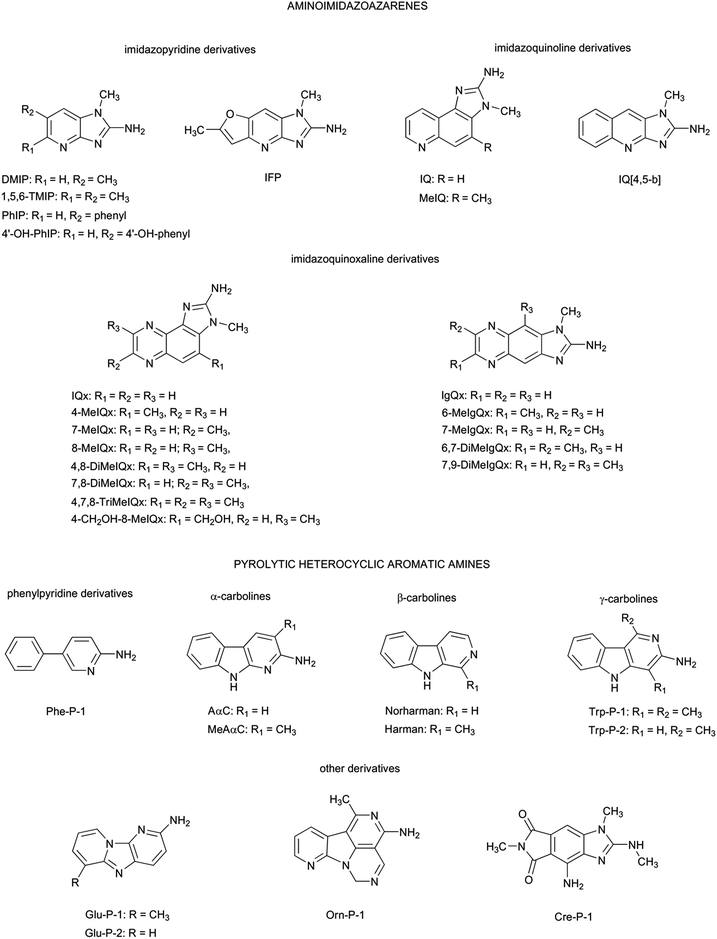
Thermal processing has numerous beneficial consequences for foods, including enhancement of nutritional quality, improved digestibility and bioavailability of nutrients, prolongation of shelf-life, better palatability, taste, texture, flavour, and functional properties, release of bioactive components, generation of beneficial compounds with antioxidant and antimicrobial properties, destruction of anti-nutritional substances, and inactivation of food-borne pathogens.1 However, it also produces a loss of certain nutrients and the formation of potentially mutagenic and carcinogenic molecules.2 In this respect, formation of acrylamide,3 furan,4 acrolein,5 or heterocyclic aromatic amines,6 among others, has received considerable attention in recent years. In particular, heterocyclic aromatic amines have been related to the increased cancer risk associated with the consumption of cooked proteinaceous food products.7Heterocyclic aromatic amines are a complex mixture of compounds formed at ppb levels in muscle foods cooked at high temperature.8 They are characterized for having a planar, multi-ring aromatic structure with one or more nitrogen atoms in their ring system and, usually, an exocyclic amino group, although there are also some few exceptions.9 Chemical structures of the main heterocyclic aromatic amines isolated to present are collected in Fig. 1.
Heterocyclic aromatic amines can be classified in two groups: aminoimidazoazarenes, which are usually formed at temperatures typical of cooking/frying (∼200 °C), and pyrolitic heterocyclic aromatic amines, which are formed by pyrolysis of amino acids and proteins at temperatures higher than 250 °C. Among them, and based on evidences from animal experiments, the International Agency for Research on Cancer (IARC) has classified three of these amines [2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (8-MeIQx), and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)] in the class 2B as possible human carcinogens, and one of them [2-amino-3-methylimidazo[4,5-f]quinoline (IQ)] in the class 2A as a probable human carcinogen.10 These four amines have also been listed by the National Toxicology Program (NTP) in its 11th Report on Carcinogens (RoC) as reasonably anticipated to be human carcinogens.11 All these four compounds are aminoimidazoazarenes.
Although more than 20 aminoimidazoazarenes have been isolated and characterized to present, only three basic skeletons are repeated in the identified compounds: imidazopyridine, imidazoquinoline, and imidazoquinoxaline. This similarity among all these compounds suggests that all of them are produced from a limited number of reactants through a reduced number of formation pathways. In fact, these compounds have been traditionally considered to be the result of complex reactions that involve creati(ni)ne, free amino acids, and carbohydrates through the Maillard reaction. However, a recent study has pointed out that some of these compounds can also be formed as a consequence of the carbonyl chemistry initiated by lipid oxidation,12 and previous studies also showed contradictory results on the influence of fats in formation and fate of heterocyclic aromatic amines.13
Maillard reaction and lipid oxidation are not two independent processes. In fact, lipid oxidation products influence Maillard pathway and vice versa, and there are common intermediates and analogous polymerization mechanisms in both routes conducting to food browning.14 The objective of this review is to collect the scattered existing information suggesting that this coordinate contribution of both lipid oxidation and Maillard reaction to food browning is also playing a role in the formation and fate of heterocyclic aromatic amines. This review will be mostly focused on PhIP because it is the heterocyclic aromatic amine that has been mostly studied in this sense.
2. PhIP: a product of carbonyl chemistry in foods
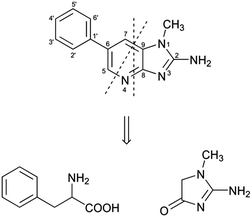
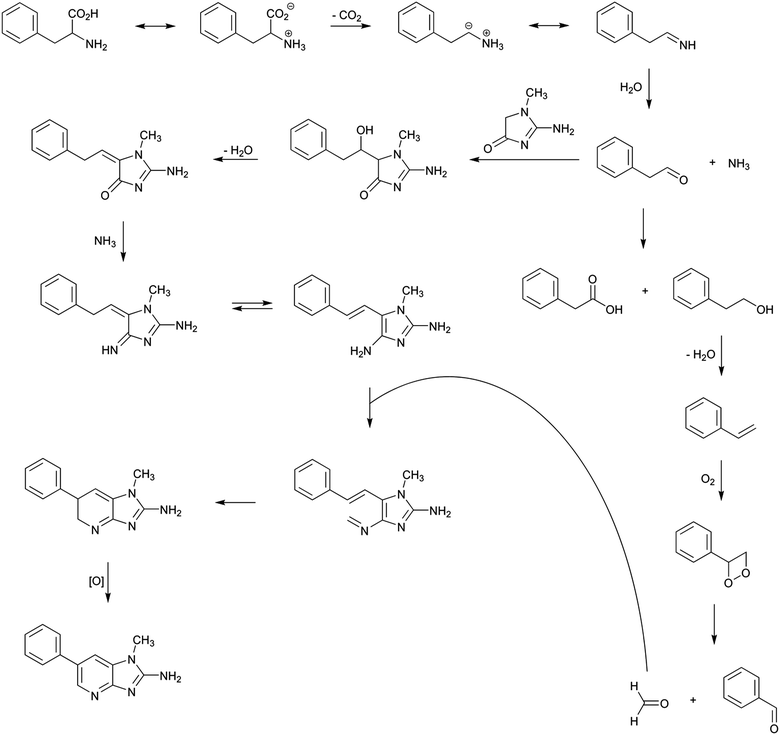
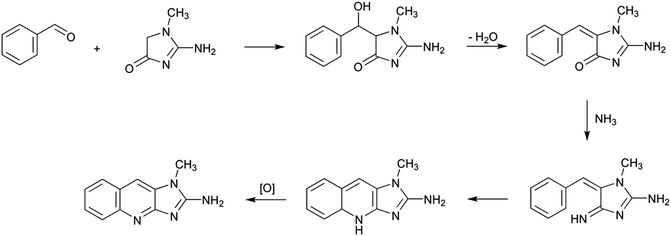
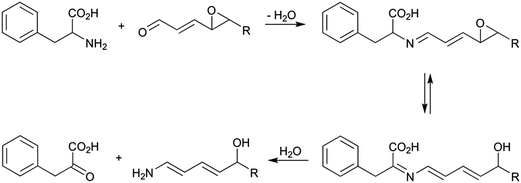
Among the different aminoimidazoazarenes produced under usual cooking conditions, PhIP is generally one of the heterocyclic aromatic amines produced to a highest extent in addition to 2-amino-1,7-dimethylimidazo[4,5-g]quinoxaline (7-MeIgQx), 8-MeIgQx, and 2-amino-1,7,9-trymethylimidazo[4,5-g]quinoxaline (7,9-DiMeIgQx).13,15,16 PhIP is typically found in foods at amounts up to 35 ng g−1,17 but there are reports of higher levels, especially in fried and barbecued chicken.18The mechanism by which PhIP is produced has been the objective of different studies but it has not been fully elucidated until very recently.19 First studies demonstrated that PhIP is produced by reaction of phenylalanine with creati(ni)ne.16,20–23 The atoms of both reactants were easily located on the PhIP molecule by using isotope labelling.23 Thus, phenylalanine is responsible for the phenyl ring and carbons 5, 6, and 7 of the pyridine ring in PhIP (Scheme 1). In addition, creatinine is incorporated almost intact to the PhIP molecule and is responsible for the imidazole ring. This last part is common for all aminoimidazoazarenes where the synthon creatinine can be easily recognized (Fig. 1). Although phenylalanine has 9 carbons and 9 carbons come from the amino acid in PhIP, phenylalanine cannot be incorporated directly to PhIP molecule because the phenyl ring is attached to one end of the alkyl chain in phenylalanine, and the phenyl ring is attached to the centre of the three carbons of the pyridine ring in PhIP that initially belonged to phenylalanine. In addition, and by means of isotopic labelling, the no incorporation of the carboxylic carbon of phenylalanine to PhIP was also demonstrated.23 This fact suggested that phenylalanine suffered a degradation previously to form part of PhIP. The formed compound, which was an intermediate in the reaction, was found to be phenylacetaldehyde.16 Therefore the first step of the formation of PhIP is the Strecker degradation of phenylalanine to produce phenylacetaldehyde (Scheme 2), a step that does not need the presence of creati(ni)ne but is facilitated in the presence of reactive carbonyl compounds derived from carbohydrates,24,25 lipids,26 amino acids,27 or polyphenols28 (see below).
Once phenylacetaldehyde is formed, the reaction between phenylacetaldehyde and creati(ni)ne is produced to form in a first step 2-amino-5-(1-hydroxy-2-phenylethyl)-1-methyl-1H-imidazol-4(5H)-one and then, after dehydration, 2-amino-1-methyl-5-(2-phenylethylidene)-1H-imidazol-4(5H)-one.16 This last compound has already the basic structure of the PhIP, but it still needs the finishing of the pyridine ring with the inclusion of one carbon and one nitrogen atoms.
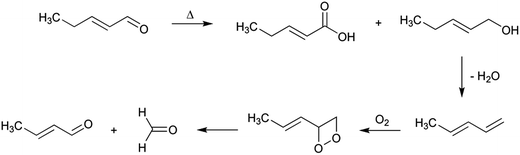
The origin of both atoms has been the objective of different studies. Thus, the nitrogen seemed to come from the thermal decomposition of both phenylalanine and creati(ni)ne, which is known to produce ammonia.29 In addition, Murkovic et al.23 found that the origin of the additional carbon was the carbon 2 of phenylalanine by using isotope labelling. More recently, Zamora et al.19 found that thermal degradation of phenylacetaldehyde produced formaldehyde, and this is the way in which the last carbon is incorporated to complete PhIP molecule. Formaldehyde has been suggested to be produced as shown in Scheme 2, at least to a certain extent.19 Upon heating, a small amount of phenylacetaldehyde suffered a disproportionation reaction to produce phenylacetic acid and phenylethanol (both products were detected in the reaction mixture of heated phenylacetaldehyde). The later dehydration of the alcohol, the oxidation of the produced olefin, and, finally, its breakage would be the origin of formaldehyde. Therefore, the origin of formaldehyde would be the carbon 2 of phenylalanine, which is in agreement with the labelling experiments.23
Once ammonia and formaldehyde have been formed, the PhIP molecule may be completed as suggested in Scheme 2. The reaction between the condensation product of phenylacetaldehyde and creatinine with ammonia would produce the corresponding imine, which later would evolve the amine by tautomerisation. This amine would then react with formaldehyde to produce a new imine, which after electronic rearrangement and oxidation, would be the origin of PhIP.
Therefore, PhIP is produced in four steps:
1 Formation of the Strecker aldehyde from the parent amino acid.
2 Reaction of the phenylacetaldehyde with creati(ni)ne to produce the aldol condensed product.
3 Formation of formaldehyde and ammonia from phenylacetaldehyde, phenylalanine or creati(ni)ne.
4 Final assembly of the molecule by incorporating formaldehyde and ammonia.
Obviously, although steps 1, 2, and 4 have to take place sequentially, step 3 can take place simultaneously to steps 1 or 2. Furthermore, in complex food products, formaldehyde and ammonia can have other origins different to phenylacetaldehyde, phenylalanine, or creati(ni)ne.
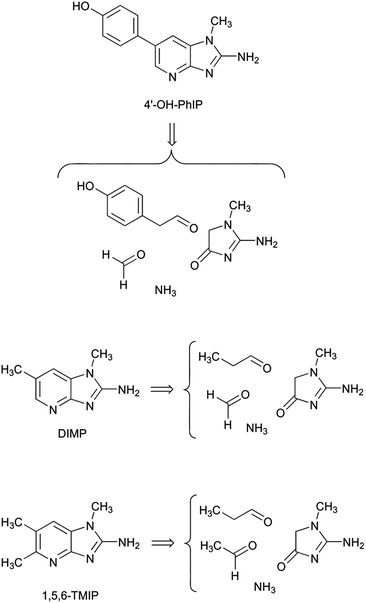
This four-step mechanism described for PhIP might be general for the different imidazopyridine derivatives identified to present. Thus, the formation of 2-amino-1-methyl-6-(4-hydroxyphenyl)imidazo[4,5-b]pyridine (4′-OH-PhIP) should be produced analogously to PhIP with the only difference of the change of phenylacetaldehyde by the Strecker aldehyde of tyrosine 2-(4-hydroxyphenyl)acetaldehyde (Scheme 3).
The formation pathway of 2-amino-1,6-dimethylimidazo[4,5-b]pyridine (DIMP) can also be hypothesized to be produced analogously by starting from propanal in the place of phenylacetaldehyde (Scheme 3). Propanal is not a Strecker aldehyde derived from amino acids, but it is a major oxidation product of n3 fatty acids.30
Analogously, 2-amino-1,5,6-trimethylimidazo[4,5-b]pyridine (1,5,6-TMIP) can be hypothesised to be produced analogously to DIMP but with acetaldehyde in the place of formaldehyde to close of the pyridine ring (Scheme 3). Acetaldehyde is a common component in many foods where it is formed from carbohydrates, lipids or phenols, among other food components.31
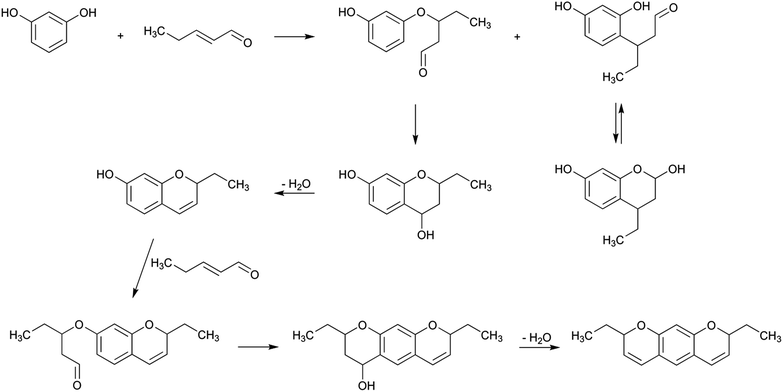
An analogous pathway can also be proposed for the formation of 2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine (IFP) by reaction of 5-methylfurfural, creati(ni)ne and ammonia (Scheme 4). In this case, steps 1 and 3 are fused together with the formation of 5-methylfurfural. This compound has been shown to be both a byproduct of the Maillard reaction32 and a product of lipid oxidation.33 The aldol reaction between 5-methylfurfural and creatinine would produce the corresponding adduct that can later be dehydrated. The carbonyl group of the initial creatinine would then react with ammonia to produce the corresponding imine, which lately would suffer an electronic rearrangement and oxidation to produce IFP.
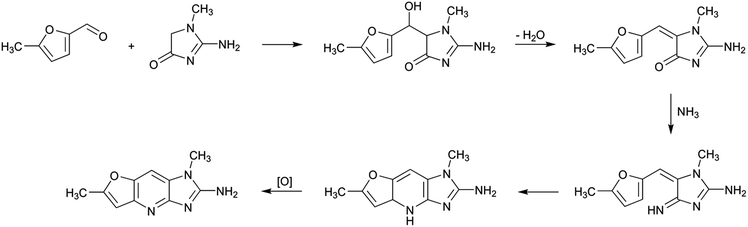 | ||
| Scheme 4 Suggested formation pathway of 2-amino-1,6-dimethylfuro[3,2-e]imidazo[4,5-b]pyridine (IFP). | ||
Although it is not classified under imidazopyridine derivatives, an analogous formation mechanism can also be hypothesized for 2-amino-1-methylimidazo[4,5-b]quinoline (IQ[4,5-b]), the linear tricyclic isomer of the probable human carcinogen IQ. The reaction pathway would be analogous to that of IFP but the initial aldehyde would be benzaldehyde, also a common minor food component.34 The reaction pathway is shown in Scheme 5. The reaction of benzaldehyde and creatinine would produce the corresponding aldol in a first step and then the dehydrated adduct. The reaction of this compound with ammonia followed by an electronic rearrangement and oxidation would produce IQ[4,5-b].
By studying the different proposed reaction pathways it is possible to understand why the different imidazopyridine derivatives are produced to different extents. Thus, the concentration of the reactants and the formation of the final pyridine ring are likely playing a major role in the amount of the heterocyclic aromatic amine produced. Next two sections will be dedicated to the coordinate contribution of both lipid oxidation and Maillard reaction to the formation of the carbonyl compounds required for producing the imidazopyridine skeleton.
3. The Strecker degradation of amino acids and other degradative pathways that produce Strecker aldehydes as a consequence of both Maillard reaction and lipid oxidation
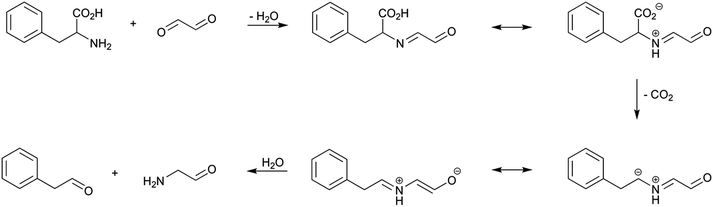
Strecker degradation is part of the oxidative decarboxylation reactions of amino acids that can be effected by a variety of reagents and reaction conditions.24 This reaction is a source of important volatile constituents of food flavours. Thus, in addition to the aldehyde derived from the parent amino acids, usually named Strecker aldehyde, different pyrazines, pyridines, pyrroles, and oxazoles, among other compounds, are produced.25In Maillard chemistry, the term Strecker degradation is usually employed when α-dicarbonyl compounds act as oxidizing agents to effect the decarboxylation of the involved amino acid. Many α-dicarbonyl compounds are produced in the course of Maillard reaction by carbohydrate dehydration or fragmentation, including 1- or 3-deoxyosones, glyoxal, 2,3-butanedione, and 2-oxopropanal. The reaction of phenylalanine, as a model amino acid, with glyoxal, as a model α--dicarbonyl compound, is shown in Scheme 6.
The reaction begins with the formation of the corresponding conjugated imine between the amino group of the amino acid and one of the carbonyl groups of the glyoxal. The formed α-iminocarbonyl compound undergoes then a thermally induced, irreversible decarboxylation. The reason for this loss of carbon dioxide can be better understood from the zwitterionic form of the α-iminocarbonyl compound. The carbon dioxide loss is facilitated by the formation of an azomethine ylide, which is stabilized by resonance because of its conjugation with the carbonyl function. Finally, the azomethine ylide undergoes addition of water to produce the Strecker aldehyde (phenylacetaldehyde) and a 2-oxoamino derivative (2-aminoacetaldehyde).
α-Dicarbonyl compounds are not major lipid oxidation products, although glyoxal and other short-chain α-dicarbonyl compounds are known to be produced to a certain extent as a consequence of lipid oxidation.35 However, in the course of lipid oxidation, many compounds analogous to α-dicarbonyl compounds are produced which are able to degrade amino acids similarly to the α-dicarbonyl compounds derived from carbohydrates.
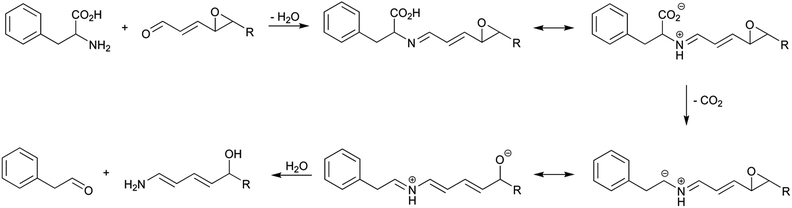
The Strecker degradation of amino acids produced by secondary lipid oxidation products was firstly described in 2004 for the formation of phenylacetaldehyde by phenylalanine degradation in the presence of epoxyalkenals,26 and later extended to other lipid-derived reactive carbonyls.36–38 Strecker aldehyde formation by lipid oxidation products is believed to be produced analogously to amino acid degradation by α-dicarbonyl compounds. Scheme 7 shows the reaction pathway for the degradation of phenylalanine in the presence of 4,5-epoxyalkenals. The reaction begins with the formation of the imine that suffers then the decarboxylation. This loss is facilitated by the extension of the conjugation in the produced azomethine ylide because of the existence of the second oxygenated function. The final addition of water produces the Strecker aldehyde (phenylacetaldehyde) and a hydroxyamino derivative which, for 4,5-epoxyalkenals, evolves to 2-alkypyridines.
This reaction is not exclusive for lipid-derived short chain aldehydes, the corresponding long-chain ketones produced during the lipid oxidation pathway are also able to degrade amino acids.37,39 In fact, the ability of aldehydes and ketones for degrading amino acids was similar in most experiments and differences found might be more related to differences in solubility among the different lipid oxidation products than differences in reactivity.40 In addition, this reaction has also been described for lipid hydroperoxides,41 although a free radical mechanism is likely taking place in this degradation in addition to the contribution of the reactive carbonyls produced by hydroperoxide decomposition.40
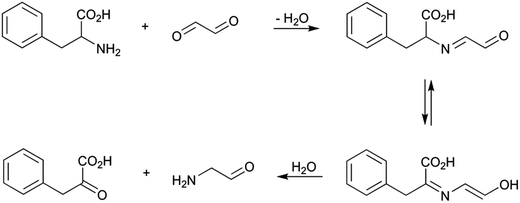
Strecker aldehydes have also been described to be produced by decarboxylation of α-oxoacids,42 and these last compounds can also be formed by amino acid degradation in the presence of both carbohydrates and oxidized lipids. Thus, for example, Ferreira et al.43 found α-oxoacids in wines and related them with the generation of aged wine aroma as a consequence of Maillard reaction. A mechanism for α-oxoacid formation by isomerisation of the Schiff base formed between the amino acid and glyceraldehydes was suggested by Chu and Yaylayan.44 These authors suggested the existence of a transamination. Scheme 8 shows a reaction pathway for the formation of phenylpyruvic acid from phenylalanine in the presence of glyoxal. The reaction begins with the formation of the corresponding imine. This compound suffer then an isomerisation that can be seen as a tautomerism because of the existence of the second carbonyl group. This tautomerism may also be favoured because of the extension of the conjugation with the carboxylic group. The hydrolysis of this new imine would produce the α-oxoacid.
A similar reaction was previously described for both activated amine and amino acid degradations in the presence of lipid oxidation products.45,46 Scheme 9 shows the reaction pathway of phenylalanine degradation in the presence of 4,5-epoxyalkenals. As can be observed, the electronic rearrangement is favoured by both the existence of an epoxy group at one end and the conjugation of the carboxylic group produced as a consequence of the isomerisation. This pathway is also valid for amines, although the reaction will be or not produced depending on the electronic effects of the substituents present at the α-carbon. Thus, long-chain saturated amines, in which the α-carbon is joined to an amino group and an alkyl chain, were converted into carbonyl compounds only to a very low extent.45 On the other hand, 2-phenylglycine methyl ester, in which the α-carbon is joined to an amino group, an aromatic ring, and a methoxycarbonyl group produced the corresponding carbonyl compound with a reaction yield of about 50% in the presence of 4,5-epoxyalkenals.45
4. Origin of other aldehydes needed for ring closure in heterocyclic aromatic amine formation having imidazopyridine structure
The other aldehydes required for ring closure of aminoimidazoazarenes are also produced as both carbohydrate and lipid oxidation products. Thus, the formaldehyde required for ring closure in PhIP has long been known to be a product of carbohydrate cleavage as a consequence of retroaldol reactions.47 In addition, it can be produced by amino acid degradation in the presence of carbohydrates, such as in the Strecker degradation of glycine.48 Furthermore, retroaldol reaction of serine has also been shown to produce formaldehyde.49Formaldehyde is also commonly produced as a consequence of lipid oxidation.50 Recently, Zamora et al.51 have shown its formation during thermal degradation of α,β-unsaturated carbonyls. Formaldehyde might be hypothesised to be produced from these carbonyls analogously to their formation from phenylacetaldehyde discussed above (Scheme 2). The proposed formation mechanism for formaldehyde formation when starting from 2-pentenal is shown in Scheme 10. Upon thermal heating, the α,β-unsaturated aldehyde might suffer a disproportionation reaction to produce the corresponding acid and alcohol derivatives. The later dehydration of the alcohol, the oxidation of the produced olefin, and, finally, its breakage would be the origin of formaldehyde. In the case of 2-pentenal, 2-butenal (crotonaldehyde) would also be produced in addition to formaldehyde. Crotonaldehyde is a well-known aldehyde produced in heat-processed edible fats and oils as well as in food mainly from n3 fatty acids.52 n3 fatty acids are also the origin of 2-pentenal, which agrees with the formation of crotonaldehyde from this kind of fatty acids.
In addition to formaldehyde as the second carbonyl compound responsible for PhIP formation, most reactive carbonyl compounds proposed above as responsible for the formation of the different imidazopyridine derivatives are also produced from both carbohydrates and lipids in a coordinate way. Thus, acetaldehyde is formed from both carbohydrates and lipids, among other food components;31 5-methylfurfural is also a byproduct of the Maillard reaction32 as well as a product of lipid oxidation;33 and benzaldehyde is produced in the degradation of phenylalanine in the presence of both lipids and carbohydrates.34 The only exception would be propanal, which is a major lipid oxidation product, but it does not seem to be produced from carbohydrates to a significant extent.30 If the proposed pathways for 2-amino-1,6-dimethylimidazo[4,5-b]pyridine (DIMP) and 2-amino-1,5,6-trimethylimidazo[4,5-b]pyridine (1,5,6-TMIP) formation in Scheme 3 are correct, the formation of these heterocyclic aromatic amines should be mainly produced in the presence of lipids.
5. Inhibition of PhIP formation by phenolic compounds
Because PhIP is a product of carbonyl chemistry, any compound that can scavenge carbonyl compounds will play a major role in PhIP formation, and this control should be independent of the origin of the carbonyl compound (either carbohydrate or lipid). To this respect, phenolic compounds have been traditionally used to control heterocyclic aromatic amine formation and many authors have shown that the use of phenolic compounds (and plant extracts rich in them) decreases the PhIP formed.53 However, the inhibition of PhIP formation was not well correlated with the antioxidant/free radical-scavenging capacity of phenolic compounds,54 therefore suggesting the existence of an antioxidant-independent mechanism for PhIP inhibition by phenolic compounds. In fact, this mechanism is related to the ability of phenolic compounds to scavenge reactive carbonyls.55 However, not all phenolic compounds exhibited a similar carbonyl scavenging ability.A recent study by Salazar et al.56 has shown that there is a structure/function relationship for the PhIP scavenging ability of phenolic compounds. The obtained results showed that phenols having two hydroxyl groups at meta position of the aromatic ring were the most efficient inhibitors, playing a role in this inhibitory effect the presence of others substituents at the aromatic ring. The positive or negative effect of these other substituents is related to their electronic effects. Thus, the presence of additional hydroxyl and amino groups at the aromatic ring mostly cancelled the carbonyl-scavenging ability of phenolic compounds, which was absent in ortho- and para-dihydroxy derivatives. Furthermore, the presence of several rings with opposite effects in complex phenols produced a reduced inhibitory effect.
All these results are a consequence of the reaction mechanisms involved in phenol/carbonyls reactions. Hidalgo and Zamora recently described the reaction of phenolic compounds with 2-alkenals.57 The reaction pathway is schematized in Scheme 11 for the reaction between resorcinol and 2-pentenal. As can be observed phenolic compounds with two hydroxyl groups at meta positions have two reactive groups toward carbonyl compounds: the hydroxyl groups and the aromatic CH groups in ortho position to one of the hydroxyl groups and in para position to the second of the hydroxyl groups. The aromatic CH group in ortho to both hydroxyl groups was less reactive than the other two CH groups more likely because of steric hindrance. If the reaction is produced by addition of the aromatic CH group to the carbon–carbon double bond of the aldehyde, the produced compound is a cyclic hemiacetal with structure of chroman-2,7-diol. If the reaction is produced by addition of the OH to the carbon–carbon double bond of the aldehyde, the carbonyl of the formed adduct suffer then the addition of the aromatic CH to produce a cyclic structure which is lately dehydrated to produce a 2H-chromen-7-ol. Because this last adduct still has one free OH and an activated aromatic CH, it can add a second molecule of the aldehyde with the formation of an adduct with the structure of 2,8-dihydropyrano[3,2-g]chromene.
No all these derivatives exhibited the same stability and chroman-2,7-diols were the main reaction products at high temperature.57 For this reason, the adducts formed by the addition of the aromatic CH group to the carbonyl group in saturated aldehydes have been the reaction products found by different authors. Thus, Cheng et al.58 found that epigallocatechin gallate inhibited the formation of PhIP via scavenging of phenylacetaldehyde, and the reaction took place by addition of the aromatic CH in ortho to the hydroxyl group in A-ring of the phenol to the carbonyl group. Analogous adducts were found in other reactions involving glyoxal and methylglyoxal.59
Therefore, phenolic compounds have been shown to be able to scavenge the carbonyl compounds that are involved in the Strecker degradation of phenylalanine to produce phenylacetaldehyde and also the phenylacetaldehyde once it has been produced. Furthermore, phenols are known to react with formaldehyde.60 Consequently, phenolic compounds may be acting at the different steps of PhIP formation pathway in which carbonyl compounds are involved. The different reactivity of phenolic compounds for the different carbonyl compounds implied at the various steps still remains to be clarified.
6. PhIP fate in the presence of lipid-derived reactive carbonyls
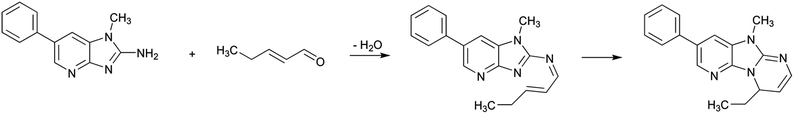
Once they have been produced, heterocyclic aromatic amines may be degraded as a function of heating time and temperature.61 In addition, Randel et al.62 found that different heterocyclic aromatic amines were degraded in oil under storage and frying conditions, a degradation that was parallel to a reduction in their mutagenic potential. Recently, Hidalgo et al.63 showed that this degradation is a consequence of the reaction of PhIP with the lipid-derived reactive carbonyls produced in the course of lipid oxidation. These authors studied the reaction of PhIP and its homologous 2-amino-1-methylbenzimidazole with 2-alkenals, 2,4-alkadienals, 4-oxo-2-alkenals, 4,5-epoxy-2-alkenals, and 4-hydroxy-2-nonenal and found that those reactions produced the formation of stable heterocyclic structures.Scheme 12 shows the reaction pathway between PhIP and 2-pentenal. The reaction seems to take place firstly with the formation of an imine between the free amino group of PhIP and the carbonyl group of 2-pentenal. Then, an electronic rearrangement is produced and a stable adduct having a tricyclic structure is formed. Analogous adducts having all of them similar structures were produced with all assayed reactive carbonyls. However, some carbonyls were more reactive than others and the stability of the produced adducts depended on the other groups present in the reactive carbonyls in addition to the carbonyl group and the carbon–carbon double bond. Thus, the most reactive carbonyl compounds for this reaction were 4-oxo-2-alkenals and 4,5-epoxy-2-alkenals. On the other hand, the less reactive compounds were 2,4-alkadienals. This reactivity is likely related to the activation of the carbon at β-position of the carbonyl carbon to participate in the reaction. In addition, the stability of the adduct was determined by the reactivity of the additional group not involved in the reaction that produced the tricyclic adduct.
The formation of adducts between PhIP and lipid-derived reactive carbonyls is produced much more easily than the formation of PhIP by reaction of creatinine, phenylalanine and lipid-derived reactive carbonyls because the activation energy (Ea) of the formation of the tricyclic adduct is much lower than the Ea of PhIP formation (27.4 kJ mol−1 for PhIP disappearance in the presence of 4-oxo-2-nonenal vs. 80.9 kJ mol−1 for PhIP formation in the reaction between creatinine, phenylalanine and 4-oxo-2-nonenal).63,64 Therefore, once PhIP is produced, lipid-derived carbonyls are likely contributing to PhIP disappearance more than to its formation, at least to a relatively low temperature, which is in agreement with the results of Randel et al.62 Furthermore, these results also provide an explanation for the reduction of the mutagenic potential observed in parallel to PhIP disappearance.62 Thus, the formed adduct has lost the primary amino group of the original PhIP, which seems to be the responsible for its metabolic activation.65
Can carbohydrate-derived reactive carbonyls produce an effect similar to that of lipid-derived reactive carbonyls? Although no studies have been carried out in this sense, previous studies have shown that, although monosaccharides increased the formation of heterocyclic aromatic amines when added to a low extent, they exhibited an inhibitory effect over amine formation when present to a large excess.66 These results seem to be in agreement with a potential role of carbohydrate-derived reactive carbonyls in PhIP fate, analogously to lipid-derived reactive carbonyls. According to the pathway of Scheme 12, the only requisite for a carbonyl compound to react with PhIP is that it is α,β-unsaturated. Carbohydrates also produce this kind of carbonyls to some extent during Maillard reaction.67 Therefore, carbohydrates might also participate in heterocyclic aromatic amine fate to some extent. Additional studies are needed to confirm this potential amine-mitigating power of carbohydrates.
7. Conclusions
Although PhIP has been traditionally considered to be produced as a by-product of the Maillard reaction between phenylalanine, creati(ni)ne, and carbohydrates, recent evidences suggest that it is a product of carbonyl chemistry in foods, and, as such, other carbonyls can also contribute to its formation. In particular, this review has dealt with the role of lipid-derived carbonyl compounds in PhIP formation and fate. Analogously to carbohydrate-derived carbonyls, lipid-derived carbonyls are able to produce the Strecker degradation of phenylalanine to form phenylacetaldehyde, the key molecule that reacts with creatinine to generate the first intermediate in PhIP formation. Furthermore, lipid-derived carbonyls also produce formaldehyde, the molecule responsible for the ring closure in PhIP. This ability is also shared by carbohydrate-derived reactive carbonyls. Moreover, lipid-derived reactive carbonyls are also able to react with PhIP producing stable adducts in which the free amino group of PhIP has disappeared. This last ability has not been yet investigated for carbohydrate-derived reactive carbonyls, although the decreases observed for PhIP formation in the presence of excess of carbohydrates point out to a scavenging role also for these last reactive carbonyls. All these results, which might also be extended to other aminoimidazoazarenes with the structure of imidazopyridine, point out to a similar role of carbohydrate- and lipid-derived reactive carbonyls that are likely contributing to PhIP formation and fate in an analogous way, therefore extending the coordinate contribution of both carbohydrates and lipids to food browning14 also to the production and elimination of process-related food toxicants.Acknowledgements
This study was supported in part by the European Union (FEDER funds) and the Plan Nacional de I + D of the Ministerio de Economía y Competitividad of Spain (project AGL2012-35627).Notes and references
- M. van Boekel, V. Fogliano, N. Pellegrini, C. Stanton, G. Scholz, S. Lalljie, V. Somoza, D. Knorr, P. R. Jasti and G. Eisenbrand, Mol. Nutr. Food Res., 2010, 54, 1215 CAS.
- C. J. Seal, A. de Mul, G. Eisenbrand, A. J. Haverkort, K. Franke, S. P. D. Lalljie, H. Mykkänen, E. Reimerdes, G. Scholz, V. Somoza, S. Tuijtelaars, M. van Boekel, J. van Klaveren, S. J. Wilcockson and L. Wilms, Br. J. Nutr., 2008, 99, S1 CrossRef CAS PubMed.
- (a) B. O. Riboldi, A. M. Vinhas and J. D. Moreira, Food Chem., 2014, 157, 310 CrossRef CAS PubMed; (b) Y. Xu, B. Cui, R. Ran, Y. Liu, H. P. Chen, G. Y. Kai and J. X. Shi, Food Chem. Toxicol., 2014, 69, 1 CrossRef CAS PubMed; (c) B. Matthaus and N. U. Haase, Eur. J. Lipid Sci. Technol., 2014, 116, 675 CrossRef; (d) F. J. van de Brug, N. B. L. Luijckx, H. J. Cnossen and G. F. Houben, Food Control, 2014, 39, 75 CrossRef PubMed; (e) P. Pedreschi, M. S. Mariotti and K. Granby, J. Sci. Food Agric., 2014, 94, 9 CrossRef PubMed; (f) V. Gokmen, Qual. Assur. Saf. Crops Foods, 2014, 6, 319 CrossRef CAS; (g) I. S. Arvanitoyannis and N. Dionisopoulou, Crit. Rev. Food Sci. Nutr., 2014, 54, 708 CrossRef CAS PubMed.
- (a) T. Y. Curtis, J. Postles and N. G. Halford, J. Cereal Sci., 2014, 59, 382 CrossRef CAS PubMed; (b) M. Anese, L. Manzocco, S. Calligaris and M. C. Nicoli, J. Agric. Food Chem., 2013, 61, 10209 CrossRef CAS PubMed; (c) M. S. Mariotti, K. Granby, J. Rozowski and F. Pedreschi, Food Funct., 2013, 4, 1001 RSC; (d) M. Anese and M. Suman, Food Res. Int., 2013, 51, 257 CrossRef CAS PubMed; (e) R. Seljasen, H. L. Kristensen, C. Lauridsen, G. S. Wyss, U. Kretzschmar, I. Birlouez-Aragone and J. Kahl, J. Sci. Food Agric., 2013, 93, 2611 CrossRef CAS PubMed; (f) S. Moro, J. K. Chipman, J. W. Wegener, C. Hamberger, W. Dekant and A. Mally, Mol. Nutr. Food Res., 2012, 56, 1197 CrossRef CAS PubMed.
- (a) K. Abraham, S. Andres, R. Palavinskas, K. Berg, K. E. Appel and A. Lampen, Mol. Nutr. Food Res., 2011, 55, 1277 CrossRef CAS PubMed; (b) Q. Zhu, Z. Sun, Y. Jiang, F. Chen and M. F. Wang, Mol. Nutr. Food Res., 2011, 55, 1375 CrossRef CAS PubMed; (c) H.-J. C. Chen, Mol. Nutr. Food Res., 2011, 55, 1391 CrossRef CAS PubMed; (d) M. S. Tang, H. T. Wang, Y. Hu, W. S. Chen, M. Akao, Z. H. Feng and W. W. Hu, Mol. Nutr. Food Res., 2011, 55, 1291 CrossRef CAS PubMed; (e) G. Aldini, M. Orioli and M. Carini, Mol. Nutr. Food Res., 2011, 55, 1301 CrossRef CAS PubMed; (f) R. Y. Shi, T. Rickett and W. J. Sun, Mol. Nutr. Food Res., 2011, 55, 1320 CrossRef CAS PubMed; (g) K. Bein and G. D. Leikauf, Mol. Nutr. Food Res., 2011, 55, 1342 CrossRef CAS PubMed.
- (a) M. A. Shabbir, A. Raza, F. M. Anjum, M. R. Khan and H. A. R. Suleria, Crit. Rev. Food Sci. Nutr., 2015, 55, 82 CrossRef CAS PubMed; (b) U. U. Rahman, A. Sahar, M. I. Khan and M. Nadeem, LWT--Food Sci. Technol., 2014, 59, 229 CrossRef CAS PubMed; (c) J. Trafialek and W. Kalanowski, Int. J. Food Sci. Nutr., 2014, 65, 774 CrossRef CAS PubMed; (d) M. Friedman, J. Agric. Food Chem., 2014, 62, 6025 CrossRef CAS PubMed; (e) K. Sloczynska, B. Powroznik, E. Pekala and A. M. Waszkielewicz, J. Appl. Genet., 2014, 55, 273 CrossRef CAS PubMed; (f) D. Behsnilian, P. Butz, R. Greiner and R. Lautenschlaeger, Meat Sci., 2014, 98, 392 CrossRef CAS PubMed; (g) Z. Abid, A. J. Cross and R. Sinha, Am. J. Clin. Nutr., 2014, 100, 386S CrossRef CAS PubMed; (h) E. Kim, D. Coelho and F. Blachier, Nutr. Res., 2013, 33, 983 CrossRef CAS PubMed.
- T. Sugimura, K. Wakabayashi, H. Nakagama and M. Nagao, Cancer Sci., 2004, 95, 290–299 CrossRef CAS PubMed.
- (a) M. S. Alaejos, V. Gonzalez and A. M. Alonso, Food Addit. Contam., Part A, 2008, 25, 2 CrossRef CAS PubMed; (b) H. S. Shin and H. S. Shin, Food Sci. Biotechnol., 2003, 12, 588 CAS; (c) K. G. Lee and T. Shibamoto, Food Rev. Int., 2002, 18, 151 CrossRef CAS PubMed; (d) B. Stavric, Food Chem. Toxicol., 1994, 32, 977 CrossRef CAS.
- M. S. Alaejos and A. M. Afonso, Compr. Rev. Food Sci. Food Saf., 2011, 10, 52 CrossRef CAS PubMed.
- International Agency for Research on Cancer, IARC Monographs of the Evaluation of the Carcinogenic Risk of Chemicals to Humans, World Health Organization, International Agency for Research on Cancer, Lyon, vol. 56, pp. 163–242, available at http://monographs.iarc.fr/ENG/Monographs/vol56/, accessed 06.10.14 Search PubMed.
- National Toxicology Program, in 12th Report on Carcinogens, US Department of Health and Human Services, Public Health Service, National Toxicology Program, pp. 221–224, available at: http://ntp.niehs.nih.gov/?objectid=03C9AF75-E1BF-FF40-DBA9EC0928DF8B15, accessed 06.10.14 Search PubMed.
- R. Zamora, E. Alcon and F. J. Hidalgo, Food Chem., 2012, 135, 2569 CrossRef CAS PubMed.
- K. I. Skog, M. A. E. Johansson and M. I. Jagerstad, Food Chem. Toxicol., 1998, 36, 879 CrossRef CAS.
- R. Zamora and F. J. Hidalgo, Crit. Rev. Food Sci. Nutr., 2005, 45, 49 CrossRef CAS PubMed.
- W. Ni, L. McNaughton, D. M. LeMaster, R. Sinha and R. J. Turesky, J. Agric. Food Chem., 2008, 56, 68 CrossRef CAS PubMed.
- S. Zöchling and M. Murkovic, Food Chem., 2002, 79, 125 CrossRef.
- K. Puangsombat, P. Gadgil, T. A. Houser, M. C. Hunt and J. S. Smith, Meat Sci., 2011, 88, 227 CrossRef CAS PubMed.
- A. Solyakov and K. Skog, Food Chem. Toxicol., 2002, 40, 1205 CrossRef CAS.
- R. Zamora, E. Alcon and F. J. Hidalgo, Food Chem., 2014, 155, 74 CrossRef CAS PubMed.
- K.-W. Cheng, C. C. Wong, C. K. Cho, I. K. Chu, K. H. Sze, C. Lo, F. Chen and M. Wang, Chem. Res. Toxicol., 2008, 21, 2026 CrossRef CAS PubMed.
- J. S. Felton, M. K. Knize, F. T. Hatch, M. J. Tanga and M. E. Colvin, Cancer Lett., 1999, 143, 127 CrossRef CAS.
- M. Murkovic, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 802, 3 CrossRef CAS PubMed.
- M. Murkovic, H.-J. Weber, S. Geiszler, K. Fröhlich and W. Pfannhauser, Food Chem., 1999, 65, 233 CrossRef CAS.
- V. A. Yaylayan, Food Sci. Technol. Res., 2003, 1, 1 CrossRef.
- G. P. Rizzi, Food Rev. Int., 2008, 24, 416 CrossRef CAS.
- F. J. Hidalgo and R. Zamora, J. Agric. Food Chem., 2004, 52, 7126 CrossRef CAS PubMed.
- F. J. Hidalgo, E. Alcon and R. Zamora, Food Res. Int., 2013, 54, 1394 CrossRef CAS PubMed.
- G. P. Rizzi, J. Agric. Food Chem., 2006, 54, 1893 CrossRef CAS PubMed.
- (a) M. Sohn and C.-T. Ho, J. Agric. Food Chem., 1995, 43, 3001 CrossRef CAS; (b) V. A. Yaylayan, C. P. Locas, A. Wnorowski and J. O'Brien, J. Agric. Food Chem., 2004, 52, 5569 CrossRef PubMed.
- (a) M. S. Katsuda, D. J. McClements, L. H. S. Miglioranza and E. A. Decker, J. Agric. Food Chem., 2008, 56, 5926 CrossRef CAS PubMed; (b) H. Moriya, T. Kuniminato, M. Hosokawa, K. Fukonaga, T. Nishiyama and K. Miyashita, Fish. Sci., 2007, 73, 668 CrossRef CAS PubMed; (c) F. Nacka, M. Cansell, P. Meleard and N. Combe, Lipids, 2001, 36, 1313 CrossRef CAS; (d) E. N. Frankel, E. J. Parks, R. Xu, B. O. Schneeman, P. A. Davis and J. B. German, Lipids, 1994, 29, 233 CrossRef CAS.
- (a) A. Ott, J. E. Germond and A. Chaintreau, J. Agric. Food Chem., 2000, 48, 1512 CrossRef CAS PubMed; (b) S.-Q. Liu and G. J. Pilone, J. Food Sci. Technol., 2000, 35, 49 CrossRef CAS; (c) T. A. Miyake and T. Shibamoto, J. Agric. Food Chem., 1998, 46, 3694 CrossRef CAS; (d) T. Miyake and T. Shibamoto, J. Agric. Food Chem., 1995, 43, 1669 CrossRef CAS.
- L. A. Ameur, B. Rega, P. Giampaoli, G. Trystram and I. Birlouez-Aragon, Food Chem., 2008, 111, 758 CrossRef PubMed.
- G. Takeoka, C. Perrino Jr and R. Buttery, J. Agric. Food Chem., 1996, 44, 654 CrossRef CAS.
- (a) Y. Q. Wen, F. He, B. Q. Zhu, Y. B. Lan, Q. H. Pan, C. Y. Li, M. J. Reeves and J. Wang, Food Chem., 2014, 152, 29 CrossRef CAS PubMed; (b) L. Xiao, J. Lee, G. Zhang, S. E. Ebeler, N. Wickramasinghe, J. Seiber and A. E. Mitchel, Food Chem., 2014, 151, 31 CrossRef CAS PubMed; (c) W. Wu, N. P. Tao and S. Q. Gu, Eur. Food Res. Technol., 2014, 238, 237 CrossRef CAS PubMed; (d) S. G. Cho and S. J. Kays, Food Res. Int., 2013, 54, 1463 CrossRef CAS PubMed.
- (a) L. P. Han, L. Li, B. Li, D. Zhao, Y. T. Li, Z. B. Xu and G. Q. Liu, Food Res. Int., 2013, 51, 836 CrossRef CAS PubMed; (b) Y. P. Jiang, M. Hengel, C. P. Pan, J. N. Seiber and T. Shibamoto, J. Agric. Food Chem., 2013, 61, 1067 CrossRef CAS PubMed; (c) A. N. Onyango, Chem. Phys. Lipids, 2012, 165, 777 CrossRef CAS PubMed.
- F. J. Hidalgo, E. Gallardo and R. Zamora, J. Agric. Food Chem., 2005, 53, 10254 CrossRef CAS PubMed.
- R. Zamora, E. Gallardo and F. J. Hidalgo, J. Agric. Food Chem., 2007, 55, 1308 CrossRef CAS PubMed.
- R. Zamora, E. Alcon and F. J. Hidalgo, J. Agric. Food Chem., 2013, 61, 10231 CrossRef CAS PubMed.
- R. Zamora, E. Gallardo, J. L. Navarro and F. J. Hidalgo, J. Agric. Food Chem., 2005, 53, 4583 CrossRef CAS PubMed.
- F. J. Hidalgo and R. Zamora, Crit. Rev. Food Sci. Nutr. DOI:10.1080/10408398.2012.761173 , in press.
- R. Zamora, E. Gallardo and F. J. Hidalgo, J. Agric. Food Chem., 2008, 56, 7970 CrossRef CAS PubMed.
- F. L. Chu and V. A. Yaylayan, J. Agric. Food Chem., 2008, 56, 10697 CrossRef CAS PubMed.
- A. C. D. S. Ferreira, S. Reis, C. Rodrigues, C. Oliveira and P. G. D. Pinho, J. Food Sci., 2007, 72, S314 CrossRef CAS PubMed.
- F. L. Chu and V. A. Yaylayan, Ann. N. Y. Acad. Sci., 2008, 1126, 30 CrossRef CAS PubMed.
- R. Zamora, E. Gallardo and F. J. Hidalgo, J. Agric. Food Chem., 2006, 54, 2398 CrossRef CAS PubMed.
- R. Zamora, J. L. Navarro, E. Gallardo and F. J. Hidalgo, J. Agric. Food Chem., 2006, 54, 6101 CrossRef CAS PubMed.
- F. Ledl and E. Schleicher, Angew. Chem., Int. Ed. Engl., 1990, 29, 565 CrossRef.
- I. Blank and L. B. Fay, J. Agric. Food Chem., 1996, 44, 531 CrossRef CAS.
- V. A. Yaylayan, A. Keyhani and A. Wnorowski, J. Agric. Food Chem., 2000, 48, 636 CrossRef CAS PubMed.
- (a) S. Matthew, C. Grey, K. Rumpunen and P. Adlercreutz, Food Chem., 2011, 126, 1399 CrossRef PubMed; (b) T. C. Huang, C. T. Ho and H. Y. Fu, J. Agric. Food Chem., 2004, 52, 2924 CrossRef CAS PubMed.
- R. Zamora, J. L. Navarro, I. Aguilar and F. J. Hidalgo, Food Chem., 2015, 174, 89 CrossRef CAS PubMed.
- (a) M. Granvogl, J. Agric. Food Chem., 2014, 62, 1272 CrossRef CAS PubMed; (b) G. Luna, M. T. Morales and R. Aparicio, J. Agric. Food Chem., 2006, 54, 4790 CrossRef CAS PubMed; (c) M. Qian and G. Reineccius, J. Dairy Sci., 2002, 85, 1362 CrossRef CAS; (d) W. E. Neff and E. Selke, J. Am. Oil Chem. Soc., 1993, 70, 157 CrossRef CAS.
- (a) M. Murkovic, S. Steinberger and W. Pfannhauser, Z. Lebensm.-Unters. Forsch., 1998, 207, 477 CrossRef CAS; (b) I. Quelhas, C. Petisca, O. Viegas, A. Melo, O. Pinho and I. M. P. L. V. O. Ferreira, Food Chem., 2010, 122, 98 CrossRef CAS PubMed; (c) B. Janoszka, Food Chem., 2010, 120, 463 CrossRef CAS PubMed; (d) M. Gibis and J. Weiss, Food Chem., 2012, 134, 766 CrossRef CAS PubMed.
- (a) J. Damasius, P. R. Venskutonis, R. Ferracane and V. Fogliano, Food Chem., 2011, 126, 149 CrossRef CAS PubMed; (b) K. W. Cheng, F. Chen and M. Wang, Mol. Nutr. Food Res., 2007, 51, 969 CrossRef CAS PubMed.
- (a) V. N. Totlani and D. G. Peterson, J. Agric. Food Chem., 2005, 53, 4130 CrossRef CAS PubMed; (b) X. Peng, K.-W. Cheng, J. Ma, B. Chen, C. T. Ho, C. Lo, F. Chen and M. Wang, J. Agric. Food Chem., 2008, 56, 1907 CrossRef CAS PubMed; (c) C.-Y. Lo, W.-T. Hsiao and X.-Y. Chen, J. Food Sci., 2011, 76, H90 CrossRef CAS PubMed.
- R. Salazar, G. Arámbula-Villa, F. J. Hidalgo and R. Zamora, Food Chem., 2014, 151, 480 CrossRef CAS PubMed.
- F. J. Hidalgo and R. Zamora, Food Chem., 2014, 160, 118 CrossRef CAS PubMed.
- K.-W. Cheng, C. C. Wong, J. Chao, C. Lo, F. Chen, I. K. Chu, C.-M. Che, C.-T. Ho and M. Wang, Mol. Nutr. Food Res., 2009, 53, 716 CAS.
- (a) V. M. Totlani and D. G. Peterson, J. Agric. Food Chem., 2006, 54, 7311 CrossRef CAS PubMed; (b) C.-Y. Lo, S. Li, D. Tan, M.-H. Pan, S. Sang and C.-T. Ho, Mol. Nutr. Food Res., 2006, 50, 1118 CrossRef CAS PubMed; (c) H. Liu, H. Liu, W. Wang, C. Khoo, J. Taylor and L. Gu, Food Funct., 2011, 2, 475 RSC; (d) S. Kokkinidou and D. G. Peterson, Food Funct., 2013, 4, 1093 RSC.
- G. Casiraghi, G. Casnati, A. Pochini, G. Puglia, R. Ungaro and G. Sartori, Synthesis, 1981, 143 CrossRef CAS.
- C. P. Chiu and B. H. Chen, Food Chem., 2000, 68, 267 CrossRef CAS.
- G. Randel, M. Balzer, S. Grupe, S. Drusch, B. Kaina, K.-L. Platt and K. Schwarz, Food Chem. Toxicol., 2007, 45, 2245 CrossRef CAS PubMed.
- F. J. Hidalgo, E. Alcon and R. Zamora, J. Agric. Food Chem., 2014, 62, 12045 CrossRef CAS PubMed.
- R. Zamora, E. Alcon and F. J. Hidalgo, Food Chem., 2013, 138, 180 CrossRef CAS PubMed.
- C. Cheung, X. Ma, K. W. Krausz, S. Kimura, L. Feigenbaum, T. P. Dalton, D. W. Nebert, J. R. Idle and F. J. Gonzalez, Chem. Res. Toxicol., 2005, 18, 1471 CrossRef CAS PubMed.
- (a) K. Skog, M. Jägerstad and L. Reuterswärd, Food Chem. Toxicol., 1992, 30, 681 CrossRef CAS; (b) K. Skog, Food Chem. Toxicol., 1993, 31, 665 CrossRef; (c) M. Bordas, E. Moyano, L. Puignou and M. T. Galceran, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 802, 11 CrossRef CAS PubMed.
- S. Estendorfer, F. Ledl and T. Severin, Angew. Chem., Int. Ed. Engl., 1990, 29, 536 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |