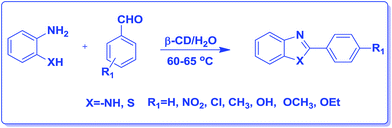
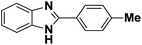
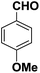
An efficient aqueous phase synthesis of benzimidazoles/benzothiazoles in the presence of β-cyclodextrin†
Ramesh Katlaa,
Rakhi Chowrasiab,
Padma Sunitha Manjarib and
Nelson Luís C. Domingues*a
aOrganic Catalysis and Biocatalysis Laboratory OCBL/FACET, Federal University of Grande Dourados—UFGD, Dourados/Itahúm rod. km 12 s/n, Zip Code 79804-970, Dourados, MS, Brazil. E-mail: nelsondomingues@ufgd.edu.br
bDepartment of Chemistry, Osmania University, Hyderabad, India 500004
First published on 24th April 2015
Abstract
Benzimidazoles/benzothiazoles were synthesized in water under neutral conditions by the reaction of aromatic aldehydes, o-phenylenediamine/2-amino thiophenol mediated by β-cyclodextrin in high yields. β-Cyclodextrin can be recovered and reused without significant loss of activity.
Introduction
Benzimidazole/benzothiazole moieties are important scaffolds in pharmaceutical applications, associated with a wide variety of medicinal, biological activities such as antifungal, antiviral, antibacterial, anticancer, anti-inflammatory, antiulcer, antihypertensive, antihistaminic, anticonvulsant, and antiparkinsonian activities.1–3 In addition, their analogues exhibit significant activity against several viruses, such as HIV, herpes (HSV-1), RNA influenza and human cytomegalovirus (HCMV).4 They are also widely used in organic synthesis as intermediates. Especially benzimidazoles are useful in controlling the diseases such as hypertension,5 ischemia-reperfusion injury,6 as well as obesity.7 There are numerous drugs containing benzimidazole/benzothiazole skeletons as shown in Fig. 1. Several methodologies have been developed for the synthesis of benzimidazole/benzothiazoles. Mirkhani et al. has reported the synthesis of 2-imidazolines and bis-imidazolines by the reaction of ethylenediamine, and nitriles in the presence of sulfur under ultrasonic irradiation.8 Das et al. described benzimidazoles from 1,2-phenylenediamine, with aldehydes by using (bromodimethyl)sulfonium bromide at room temperature.9 Hornberger has developed one-pot synthesis of disubstituted benzimidazoles from 2-nitro anilines with palladium charcoal as a catalyst in the presence of trimethyl orthoformate and catalytic pyridinium p-toluenesulfonate (PPTS) at rt.10 Lin and co-workers synthesized some benzimidazoles from phenylenediamines, and aldehydes, in the presence of molecular iodine.11 Pierre L. Beaulieu et al. demonstrated the oxone mediated benzimidazoles, benzoxazoles and benzothiazoles by using phenylene diamines/2-amino thiophenol/2-amino thiol and acid one-pot synthesis of benzimidazoles using 1,2-phenylenediamines, and aldehydes in wet DMF at rt.12 Srinivasan and co-workers developed the synthesis of 2-aryl chlorides under ambient conditions using ionic liquids, 1-butylimidazolium tetraflouroborate [Hbim]BF4) and 1,3-di-n-butylimidazolium tetrafluoroborate ([bbim]BF4).13Ranu et al. described the synthesis of 2-substituted benzimidazoles by using o-phenylenediamine with aromatic aldehydes in the presence of an ionic liquid, 1-methyl-3-methylimidazolium tetrafluoroborate, [pmim]BF4 at room temperature.14
However, the above mentioned methods have been associated with different draw backs such as the use of hazardous organic solvents, strongly acidic conditions, expensive moisture-sensitive catalysts, or tedious workup conditions as well as low yields. In continuation of our efforts towards the development of novel environmentally benign methodologies,15 herein we report an efficient one-pot protocol for the synthesis of benzimidazole/benzothiazole derivatives by a two-component reaction, involving 1,2-diamino benzene/2-amino thiophenol for the first time promoted by recyclable β-CD in aqueous medium (Scheme 1). Presently organic reactions in aqueous phase have attracted the attention of researchers because of the added advantages of water, as an eco-friendly and economically affordable solvent. However, the fundamental problem in performing the reactions in water is that many organic substrates are hydrophobic and are insoluble in aqueous medium.
Results and discussion
Cyclodextrins (CDs) are cyclic oligosaccharides possessing hydrophobic cavities, which bind substrates selectively and catalyze the chemical reactions with high selectivity. They promote the reactions by supramolecular catalysis involving reversible formation of host-guest complexation by non-covalent bonding. We describe, herein, the synthesis of 2-substituted benzimidazoles/benzothiazoles demonstrating the remarkable catalytic activity of β-cyclodextrin (Scheme 1). In general, the reaction was carried out by the in situ formation of the β-CD complex of the aldehyde in water followed by the addition of 2-aminothiophenol and stirring at 60–65 °C to gave the corresponding 2-phenylbenzo[d] thiazole in high yield (81%) (Table 1, entry 1). These reactions proceeded efficiently without the need of any metal or acid catalyst. The reaction goes to completion in a short time (4–10 h). The reactions also take place with α-CD and γ-CD, with lesser yields, however, β-CD has been chosen as the mediator as it is inexpensive and easily accessible. Several examples illustrating this simple and practical methodology are summarized in Table 1. All the compounds were characterized by 1H NMR, IR, and mass spectrometry. The catalytic activity of cyclodextrins for these reactions is established by the fact that no reaction was observed in the absence of cyclodextrin. Evidence for the complexation between the p-hydroxy benzaldehyde and cyclodextrin is supported by 1H NMR spectroscopy. A comparison of the 1H NMR spectra (DMSO-d6) of β-CD, β-CD p-hydroxy benzaldehyde complex was studied and as indicated in Fig. 3. All the reactions β-CD was recovered and reused.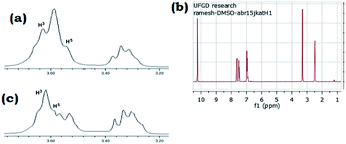 | ||
| Fig. 3 1H NMR (300 MHz, DMSO-d6) spectrum of (a) β-CD (b) p-hydroxy benzaldehyde (c) β-CD–p-hydroxy benzaldehyde inclusion complex. | ||
After the reaction, the reaction mass was cooled and β-CD was filtered and washed with ice-cold water and dried. The recovered β-CD was further used with the same substrates as a catalyst and checked for the yields as well as the catalytic activity of the recovered β-CD, as shown in Fig. 2. As we observed that the yields of benzimidazoles/benzothiazoles slightly decreased after three to fourth cycle as indicated in Fig. 2.
Conclusion
In summary, we have developed an eco-friendly, one-pot protocol for the synthesis of 2-substituted benzothiazoles/benzimidazoles in good to excellent yields under neutral conditions promoted by β-cyclodextrin in aqueous medium. This simple and novel methodology will be useful to green chemistry with some advantage that the reaction excludes non-toxic, moisture sensitive or hazardous catalysts and elevated reaction temperatures, longer reaction times, and the catalyst β-CD is economically viable, readily available, easily handling.Experimental section
General experimental procedure for the synthesis of 2-substituted/benzothiazoles using β-cyclodextrin
β-Cyclodextrin (10 mol%) was dissolved in water (10 ml), and to this clear solution, aldehyde was added, stirred for 15 min, followed by the addition of 1,2-diamino benzene/2-amino thiophenol (1.0 mmol). The reaction mixture was heated at 60–65 °C until completion of the reaction as indicated by TLC. The reaction mixture was cooled to 5 °C and β-cyclodextrin was filtered. The aqueous layer was extracted with ethyl acetate (3 × 10 ml). The combined organic layers were washed with water, saturated brine solution, and dried over anhydrous Na2SO4. The combined organic layers were evaporated under reduced pressure and the resulting crude product was purified by column chromatography using ethyl acetate and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as an eluent. The identity and purity of the products were confirmed by 1H, 13C NMR, and mass spectra.
9) as an eluent. The identity and purity of the products were confirmed by 1H, 13C NMR, and mass spectra.
Acknowledgements
Brazilian authors (R.K. and N.L.C.D) thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico for BJT fellowship and the financial support Processos: 314140/2014-0 and 400706/2014-8 CNPq – Brazil). The authors thanks to Dr Y. V. D. Nageswar, Chief Scientist in Indian Institute of Chemical Technology (IICT) Hyderabad, India for the spectroscopic analysis.References
- (a) O. I. El-Sabbagh, M. M. Baraka, S. M. Ibrahim, C. Pannecouque, G. Andrei, R. Snoeck, J. Balzarini and A. A. Rashad, Eur. J. Med. Chem., 2009, 44, 3746–3753 CrossRef CAS PubMed; (b) V. Zaharia, A. Ignat, N. Palibroda, B. Ngameni, V. Kuete, C. N. Fokunang, M. L. Moungang and B. T. Ngadjui, Eur. J. Med. Chem., 2010, 45, 5080–5085 CrossRef CAS PubMed; (c) I. Hutchinson, S. A. Jennings, B. R. Vishnuvajjala, A. D. Westwell and M. F. G. Stevens, J. Med. Chem., 2002, 45, 744–747 CrossRef CAS PubMed.
- (a) J. S. Kim, B. Gatto, C. Yu, A. Liu, L. F. Liu and E. J. LaVoie, J. Med. Chem., 1996, 39, 992 CrossRef CAS PubMed; (b) A. Kamal, M. N. A. Khan, K. S. Reddy and K. Rohini, Bioorg. Med. Chem., 2007, 15, 1004–1013 CrossRef CAS PubMed; (c) R. B. Pathak, B. Jahan and S. C. Bahel, Bokin Bobai, 1981, 9, 477–480 CAS; (d) D. D. Erol, U. Calis, R. Demirdamar, N. Yulug and M. Ertan, J. Pharm. Sci., 1995, 84, 462–465 CrossRef CAS PubMed.
- (a) T. Roth, M. L. Morningstar, P. L. Boyer, S. H. Hughes, R. W. Buckheit Jr and C. J. Michejda, J. Med. Chem., 1997, 40, 4199 CrossRef CAS PubMed; (b) F. Azam, B. A. El-Gnidi, I. A. Alkskas and M. A. Ahmed, J. Enzyme Inhib. Med. Chem., 2010, 25, 818–826 CrossRef CAS PubMed; (c) Y. Kumar, R. Green, K. Z. Boryska, D. D. Wise, L. L. Wotring and L. B. Townsend, J. Med. Chem., 1993, 36, 3843–3848 CrossRef CAS.
- M. J. Tebbe, W. A. Spitzer, F. Victor, S. C. Miller, C. C. Lee, T. R. Sattelberg, E. Mckinney and C. J. Tang, J. Med. Chem., 1997, 40, 3937 CrossRef CAS PubMed.
- (a) D. I. Shah, M. Sharma, Y. Bansal, G. Bansal and M. Singh, Eur. J. Med. Chem., 2008, 43, 1808 CrossRef CAS PubMed; (b) R. N. Sharma, F. P. Xavier, K. K. Vasu, S. C. Chaturvedi and S. S. Pancholi, J. Enzyme Inhib. Med. Chem., 2009, 24, 890–897 CrossRef CAS PubMed.
- Y. Ogino, N. Ohtake, Y. Nagae, K. Matsuda, M. Moriya, M. Suga, M. Ishikawa, Y. Kanesaka, J. Ito Mitobe, T. Kanno, T. A. Ishiara, H. Iwaasa, T. Ohe, A. Kanatani and T. Fukami, Bioorg. Med. Chem. Lett., 2008, 18, 5010 CrossRef CAS PubMed.
- P. Ghosh and A. Mandal, Catal. Commun., 2011, 12, 744 CrossRef CAS PubMed.
- V. Mirkhani, M. Moghadam, S. Tangestaninejad and H. Kargar, Tetrahedron Lett., 2006, 47, 2129 CrossRef CAS PubMed.
- B. Das, H. Holla and Y. Srinivas, Tetrahedron Lett., 2007, 48, 61 CrossRef CAS PubMed.
- K. R. Hornberger, G. M. Adjabeng, H. D. Dickson and R. G. Davis-Ward, Tetrahedron Lett., 2006, 47, 5359 CrossRef CAS PubMed.
- S. Lin and L. Yang, Tetrahedron Lett., 2005, 46, 4315 CrossRef CAS PubMed.
- P. L. Beaulieu, B. Haché and E. V. Moos, Synthesis, 2003, 11, 1683 CrossRef PubMed.
- R. N. Nadaf, S. A. Siddiqui, T. Daniel, R. J. Lahoti and K. V. Srinivasan, J. Mol. Catal. A: Chem., 2004, 214, 155 CrossRef CAS PubMed.
- D. Saha, A. Saha and B. C. Ranu, Green Chem., 2009, 11, 733 RSC.
- (a) S. N. Murthy, B. Madhav, A. V. Kumar, K. R. Rao and Y. V. D. Nageswar, Helv. Chim. Acta, 2009, 92, 2118 CrossRef CAS PubMed; (b) S. N. Murthy, B. Madhav, A. V. Kumar, K. R. Rao and Y. V. D. Nageswar, Tetrahedron, 2009, 65, 5251 CrossRef PubMed; (c) B. Madhav, S. N. Murthy, V. P. Reddy, K. R. Rao and Y. V. D. Nageswar, Tetrahedron Lett., 2009, 50, 6025 CrossRef CAS PubMed; (d) S. N. Murthy, B. Madhav, V. P. Reddy and Y. V. D. Nageswar, Tetrahedron Lett., 2010, 51, 3649 CrossRef CAS PubMed; (e) J. Shankar, K. Karnakar, B. Srinivas and Y. V. D. Nageswar, Tetrahedron Lett., 2010, 51, 3938 CrossRef CAS PubMed; (f) S. N. Murthy, B. Madhav and Y. V. D. Nageswar, Tetrahedron Lett., 2010, 51, 5252 CrossRef CAS PubMed; (g) K. Ramesh, S. N. Murthy, K. Karnakar and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 3937 CrossRef CAS PubMed; (h) K. Ramesh, S. N. Murthy, K. Karnakar and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 4734 CrossRef CAS PubMed; (i) B. S. P. Anil Kumar, B. Madhav, K. Harsha Vardhan Reddy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 2862 CrossRef PubMed; (j) K. Ramesh, S. N. Murthy, K. Karnakar, Y. V. D. Nageswar, K. Vijayalakhshmi, B. L. A. Prabhavathi Devi and R. B. N. Prasad, Tetrahedron Lett., 2012, 53, 1126 CrossRef CAS PubMed; (k) K. Ramesh, B. Madhav, S. N. Murthy and Y. V. D. Nageswar, Synth. Commun., 2012, 42, 258 CrossRef CAS PubMed; (l) K. Ramesh, S. N. Murthy and Y. V. D. Nageswar, Synth. Commun., 2012, 42, 2471 CrossRef CAS PubMed; (m) K. Ramesh, S. N. Murthy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 2362 CrossRef CAS PubMed; (n) S. S. Panda and S. C. Jain, Synth. Commun., 2011, 41, 729 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra16222f |
| This journal is © The Royal Society of Chemistry 2015 |