Efficient attenuation of 17α-ethynylestradiol (EE2) and tetracycline using novel hybrid materials: batch and column reactor studies
Thanhminglianaa,
Seung Mok Leeb,
Diwakar Tiwari*a and
Shailesh K. Prasadc
aDepartment of Chemistry, School of Physical Sciences, Mizoram University, Aizawl-796004, India, +91-389-2330834. E-mail: diw_tiwari@yahoo.com; Tel: +91-389-2301806
bDepartment of Health and Environment, Catholic Kwandong University, 522 Naegok-dong, Gangneung, Gangwon-do 210-701, Korea
cDepartment of Chemistry, National Institute of Technology, Jamshedpur-831014, India
First published on 11th May 2015
Abstract
Hybrid materials were obtained by modifying natural bentonite (B) or locally collected clay (LC) using hexadecyltrimethylammonium bromide (HDTMA) to obtain bentonite-HDTMA (BH) and local clay-HDTMA (LCH), or by simultaneous pillaring with aluminum and modification with HDTMA to obtain BAH and LCAH. The hybrid materials were used for the efficient attenuation of micro-pollutants, 17α-ethynylestradiol (EE2) and tetracycline (TC), in aqueous solutions under batch and fixed-bed column reactor experiments. Batch data indicated that the uptake of EE2 and TC by the hybrid materials was slightly affected at low and high pH within the pH range of 4.0–10.0. The uptake was not affected by varying the initial sorptive concentration (1.0–10.0 mg L−1 for EE2 and 1.0–20.0 mg L−1 for TC) and the background electrolyte (NaCl) concentrations (0.0001–0.1 mol L−1). Moreover, the attenuation of EE2 and TC by these hybrid materials was fairly efficient. Within a contact time of 60 min for EE2 and 240 min for TC, an apparent equilibrium between the clay and solution was achieved. Kinetic modeling showed that the data were fitted well to the pseudo-second order (PSO) and fractal-like-pseudo-second order (FL-PSO) kinetic models compared with the pseudo-first order (PFO) model because a low value of the least square sum was obtained for these two models. The fixed-bed column results showed that a high breakthrough volume was obtained for attenuation of EE2 and TC using the hybrid materials. Furthermore, the breakthrough data were fitted well to the Thomas equation; therefore, a very high loading capacity was estimated for EE2 and TC for the hybrid materials. These hybrid materials are useful materials in the remediation of aquatic environments contaminated with these two micro-pollutants.
1. Introduction
Clay minerals are composed of fine particles of hydrous aluminosilicates, and develop plasticity when mixed with water. A common characteristic of clay minerals is their layer structure, although they have diverse chemical, mineralogical, and physical characteristics. Clays possess a permanent net negative charge because of isomorphous substitution, which is responsible for the presence of exchangeable cations in the interspace region.1–3 Therefore, the electrical charge possessed by clay minerals and their microscale porosity makes them suitable as natural sorbing materials for several pollutants in the treatment of contaminated water. Although clay minerals are used widely to decontaminate inorganic pollutants from wastewater, pristine clay minerals possesses low sorption capacity for several hydrophobic and low- or nonpolar organic pollutants owing to the hydrophilic nature of these materials.4 Moreover, most clays show low settling capacity, limiting their wider practical application in wastewater treatment.5 The exchange of clay cations with organic cations produces useful hybrid materials for the attenuation of several non-polar organic contaminants (NOC) from aqueous solutions.6–10 Likewise, hybrid materials obtained by pillaring with poly(hydroxo-metal) cations and simultaneous intercalation of suitable organic cations are particularly suitable for wastewater treatment, because these materials show good affinity for organic impurities, and possess satisfactory settling capacity, allowing easy separation of solid and aqueous components.3The presence of organic micro-pollutants, particularly pharmaceuticals and personal care products (PPCPs) in the aquatic environment is a serious environmental concern because several micro-pollutants are persistent, non-biodegradable and toxic even at low levels. The widespread distribution of PPCPs has been demonstrated by a number of monitoring studies and measurable concentrations of many PPCPs have been found in wastewater, surface water, sediments, groundwater, and even in drinking water.11–13 PPCPs enter the terrestrial environment through direct runoff and excretion as unmetabolized drugs or active metabolites and degradation products.14 Around 70% of pharmaceuticals consumed are excreted in urine as active ingredients or metabolites.15–17 The normal sewage treatment system cannot eliminate antibiotics completely, which results in the presence of residual antibiotics in effluent.18
17α-ethynylestradiol (EE2) is a synthetic estrogen. It is a derivative of the natural hormone, estradiol (E2). EE2 is used in almost all modern formulations of combined oral contraceptive pills and hormone replacement therapy for treating conditions such as osteoporosis, menstrual disorders, and prostate and breast cancer.19–21 EE2 is one of the most potent estrogenic chemicals of the known endocrine disruptors.22 Tetracycline (TC) is a broad-spectrum antibiotic drug commonly prescribed for the treatment of bacterial infections.23 TC is widely used in aquaculture and the livestock industry24,25 as a food additive and growth promoter.26 The consumption of tetracycline for veterinary purposes is higher than for other classes of antibiotics.27
Sorptive removal of PPCPs using natural and synthetic materials is a possible method of decontaminating the aquatic environment contaminated with PPCPs. de Rudder et al.28 removed 17α-ethynylestradiol (EE2) from water in three upstream bioreactors (UBRs), filled with sand, granulated activated carbon (GAC), or MnO2 granules. The removal of EE2 in the sand, GAC and MnO2 reactors were 17.3%, 99.8% and 81.7%, respectively. They reported that the removal in the GAC reactor was mainly due to adsorption, whereas the removal in the MnO2 reactor was possibly through the catalytic properties of MnO2. Han et al.20 studied the adsorption of ethynylestradiol (EE2) from aqueous solutions using industrial-grade polyamide 612 (PA612) particles. They observed that the adsorption of EE2 on PA612 followed pseudo-second order kinetics and the strong binding affinity between EE2 and PA612 was due to hydrophobic partitioning of EE2 solutes and hydrogen bonding interactions on PA612 amide groups. Combined coagulation–adsorption treatment using single-walled carbon nanotubes (SWCNTs), multiwall carbon nanotubes (MWCNTs), and powdered activated carbon (PAC) was studied for the removal of EE2.29 The removal percentages using the combined coagulation–adsorption method were similar to those achieved using only an adsorbent. Al-Khateeb et al.30 reported that the adsorption of EE2 on MWCNTs was a pseudo-second-order kinetic process it was exothermic in nature. Adsorption of tetracycline from aqueous solution by graphene oxide (GO) was investigated by Gao et al.31 They reported that tetracycline strongly adsorbed on the GO surface via a π–π interaction and cation-π bonding. Activated carbon with different chemical and textural properties, sludge-derived materials, and the removal by the combined use of microorganisms and activated carbon (bioadsorption) were assessed for the adsorption of three different tetracyclines (TCs) (tetracycline, oxytetracycline, and chlortetracycline).32 Sludge-derived materials possessed significantly higher removal capacity than the commercial activated carbon sample. Li et al.33 investigated the adsorption of tetracycline on kaolinite with pH-dependent surface charges in aqueous solution by batch tests supplemented by FTIR analyses. The adsorption of TC on kaolinite was mainly on the external surfaces via cation exchange. TC adsorption was more pH dependent and the sorption capacity was much lower compared with that on swelling clays.
The present study uses porous clay materials, commercial bentonite and locally collected clay (local clay), to obtain the novel hybrid materials, hexadecyltrimethylammonium bromide (HDTMA) or aluminum-pillared HDTMA-modified clay. Organic cations with long-chain alkyl groups (e.g., HDTMA) impart hydrophobic properties to the mineral surface, and these hybrid materials provide a hydrophobic core and organophilic properties, which aids hydrophobic bonding with several organic pollutants.34 We use the hybrid materials to attenuate the important micro-pollutants, EE2 and TC, in aqueous solutions. Batch reactor experiments are conducted for physico-chemical parametric studies, which provide a plausible mechanism for the interfaces. Similarly, fixed-bed column reactor experiments are conducted to optimize the loading capacity of contaminants under dynamic conditions.
2. Materials and methods
2.1. Materials
Bentonite was procured from a commercial supplier, and was mined in Bhuj, Gujarat, India. The bentonite clay was used after simple washing with distilled water and drying at 90 °C in an oven for 24 h. Local clay was collected in Phullen, Mizoram, India. Because the local clay contained numerous impurities it was carefully separated using the International Soil Reference and Information Centre (ISRIC) standard procedure, as described elsewhere.35 The bentonite and local clay samples were crushed in a mortar and sieved to obtain 100 British Standard Sieve (BSS) mesh size particles (0.150 mm). The cation exchange capacity (CEC) of bentonite and local clay powder were then obtained by using standard United States Environmental Protection Agency (US EPA) method 9080.36 The CEC of bentonite and local clay were 69.35 and 46.38 meq/100 g of clay, respectively. Hexadecyltrimethylammonium bromide (HDTMA), 17α-ethynylestradiol (EE2) and tetracycline hydrochloride were procured from Sigma-Aldrich, USA. Aluminum(III) chloride was obtained from Merck, India. Sodium chloride (Extrapure) was procured from HiMedia, India. The other chemicals used were of analytical or equivalent grade. The water was purified by using a Millipore water purification system (Milli-Q+).2.2. Methodology
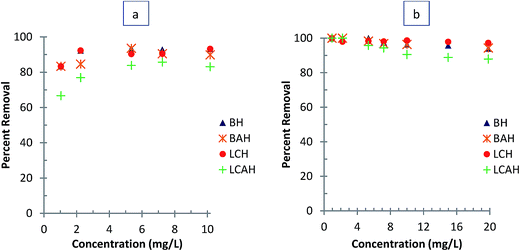
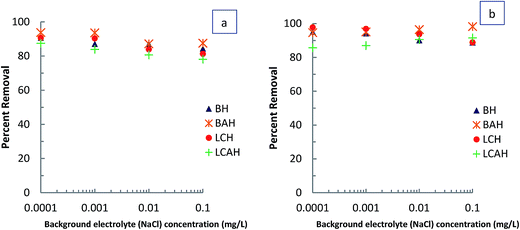
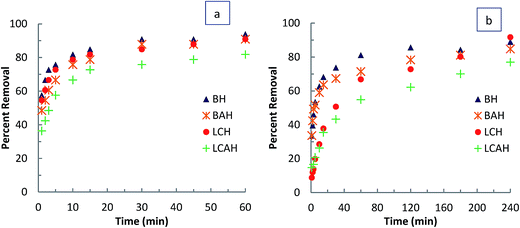
The concentration dependence study was performed by varying the EE2 concentration from 1.0 to 10.0 mg L−1 and the TC concentration from 1.0 to 20.0 mg L−1 at pH 7.0 and 25 ± 1 °C. The adsorption process detailed above was then followed. Results are presented as the percentage removal of EE2 or TC as a function of initial EE2 or TC concentration (mg L−1). Time dependent sorption of EE2 and TC by the hybrid materials was obtained at different time intervals. The initial EE2 concentration of 5.0 mg L−1 and TC concentration of 10.0 mg L−1 for a clay sample of 2.0 g L−1 was taken as constant and the sorption experiments were conducted at pH 7.0 and 25 ± 1 °C. Results are reported as percentage removal of EE2 or TC as a function of time (min). The effect of background electrolyte concentration on sorption of EE2 or TC was studied varying the background electrolyte (NaCl) concentration from 0.0001 to 0.1 mol L−1 with EE2 and TC solution with initial concentrations 5.0 and 10.0 mg L−1, respectively. The solution pH (7.0) and temperature (25 ± 1 °C) was kept constant throughout the experiments. Results are presented as percentage EE2 and TC removed as a function of background electrolyte concentration.
Breakthrough data obtained from the fixed-bed column experiments were used to optimize the loading capacity of EE2 or TC on the hybrid material loaded column under dynamic conditions, using the Thomas equation,41
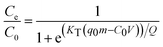 | (1) |
3. Results and discussion
3.1. Characterization of materials
FT-IR data indicated that the hybrid materials, BH, BAH, LCH or LCAH, possess prominent IR stretching bands at 2930 and 2850 cm−1 arising from –CH2 asymmetric and symmetric stretching vibrations respectively. This confirmed the introduction of HDTMA in the clay network for all these hybrid materials. Characteristic peaks in the X-ray diffraction analysis showed that both the clay samples contained quartz, smectite, illite and kaolinite in various proportions. The XRD patterns of the modified clay samples were almost identical to the virgin clay, with slight changes in d-values and peak intensities. The FE-SEM images clearly show that the organo-modified bentonite or LC (BH or LCH) had more heterogeneous and disordered surface structures. BAH and LCAH showed a similar disordered structure, although fine particles were observed on the surface that were possibly aggregated or immobilized as aluminum hydroxide or Al2O3. Detailed characterization of these hybrid materials has been described previously.37,423.2. Batch reactor experiments
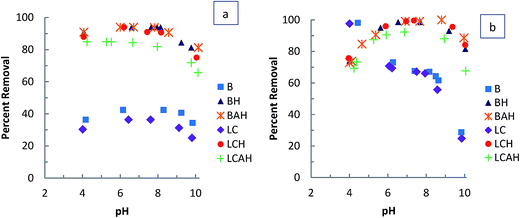
TC has different functional groups with pKa1, pKa2 and pKa3 values of 3.3, 7.7 and 9.7, respectively.47 For the unmodified clays, B and LC, very high sorption of TC was achieved at lower pH values (pH 4), it decreased significantly between pH 4.0 and 6.0, remained nearly constant from pH 6.0–8.0, and then it decreased markedly at pH 10.0. These results agree well with other reports, in which TC adsorption was higher at low pH, and this was attributed to the cation exchange mechanism.33,44 At pH values below 3.3, TC exists as a cation (TCH3+ or TCH3+ 0 0), because of the protonation of the dimethyl-ammonium group. At pH 3.3–7.7, TC exists as a zwitterion (TCH20 or TCH20 + −), because of the loss of a proton from the phenolic diketone moiety. At pHs greater than 7.7, TC exists as an anion (TCH− or TCH+ − −), and at pH 9.7, it exists in di-anionic form (TC2− or TC0 − −) through the loss of another proton from the tri-carbonyl system and phenolic diketone moiety.41 The higher removal at pH 6–8 for TC compared with EE2 for the unmodified clays indicates that the positive part of the TC zwitterions interacted with the clays. Because TCH20 and TCH− both contain a positively charged group in their structure, the molecules are probably arranged at the surface so that the positively charged group is close to the surface and the negatively charged group is furthest from the surface, which reveals that electrostatic attraction may play an important role in the sorption, not only of TCH3+, but also of TCH20 and TCH−.48
A slightly lower uptake of TC was observed for the hybrid material at pH 4.0. The uptake gradually increased at pH 6 (99.5%), and then remained nearly constant from pH 6.0 to 9.0. These results clearly indicate that hydrophobic interactions play a prominent role in TC sorption.49 Above pH 9.0, a slight decrease in the removal of TC by the hybrid materials was observed, owing to the strong electrostatic repulsion. The slightly lower uptake of TC by LCAH may arise from the screening of HDTMA molecule by aluminum oxide/hydroxide particles on the clay surface, restricting the partitioning of TC in the hydrophobic core. Similar results were reported for the uptake of TC by MnFe2O4/activated carbon magnetic composite50 and activated carbons.32 Zhang et al.51 observed similar behavior in the removal of TC by multi-walled carbon nanotubes (MWCNTs) and proposed that adsorption probably occurred via non-electrostatic π–π dispersion interactions between bulk π systems on the MWCNT surface and TC molecules containing benzene rings and double bonds, or via hydrophobic interactions between MWCNTs and TC.
The time dependence sorption data was then used to perform kinetic modelling. The three different kinetic models, pseudo-first order (PFO),55 pseudo-second order (PSO)56 and fractal-like pseudo-second order (FL-PSO)57 models of its non-linear form (eqn (2)–(4)) were used.
| qt = qe(1 − exp(−k1t)) | (2) |
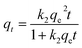 | (3) |
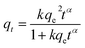 | (4) |
| Systems | Kinetic models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PFO | PSO | FL-PSO | ||||||||
| qe | k1 | s2 | qe | k2 | s2 | qe | k | α | s2 | |
| a s2: least square sum. | ||||||||||
| BH-EE2 | 2.340 | 0.990 | 0.160 | 2.515 | 0.654 | 0.031 | 2.940 | 0.438 | 0.488 | 0.004 |
| BAH-EE2 | 2.203 | 0.671 | 0.276 | 2.411 | 0.415 | 0.083 | 4.038 | 0.126 | 0.340 | 0.003 |
| LCH-EE2 | 2.230 | 0.906 | 0.167 | 2.399 | 0.625 | 0.030 | 2.751 | 0.453 | 0.533 | 0.004 |
| LCAH-EE2 | 2.002 | 0.463 | 0.164 | 2.221 | 0.292 | 0.045 | 2.673 | 0.217 | 0.611 | 0.013 |
| BH-TC | 3.868 | 0.289 | 1.699 | 4.127 | 0.100 | 0.481 | 4.691 | 0.108 | 0.571 | 0.035 |
| BAH-TC | 3.533 | 0.390 | 1.766 | 3.769 | 0.146 | 0.656 | 4.810 | 0.120 | 0.410 | 0.064 |
| LCH-TC | 3.940 | 0.039 | 0.882 | 4.506 | 0.010 | 0.350 | 5.534 | 0.013 | 0.712 | 0.143 |
| LCAH-TC | 3.296 | 0.046 | 1.192 | 3.700 | 0.016 | 0.608 | 7.395 | 0.011 | 0.457 | 0.088 |
3.3. Fixed-bed column reactor experiments
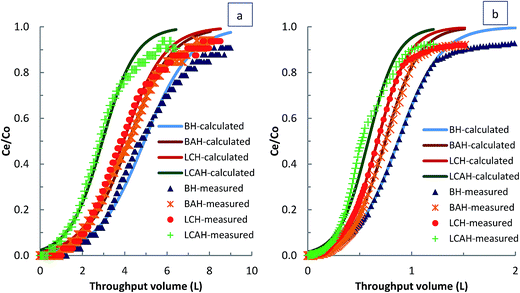
Fixed-bed column experiments were performed to assess the loading capacity of the hybrid materials BH, BAH, LCH and LCAH for EE2 and TC under dynamic conditions. The column experiments were carried out under various column conditions. The breakthrough curves are presented in Fig. 5(a) and (b) for EE2 and TC, respectively. A high breakthrough volume was obtained for EE2 and TC on these clays. A complete breakthrough volume was observed at throughput volumes of 7.68, 6.36, 6.24 and 5.041 L for EE2; and 1.33, 1.09, 1.06 and 0.94 L for TC; for BH, BAH, LCH and LCAH, respectively. The high breakthrough volume indicates the higher removal capacity of EE2 and TC by the modified clays under dynamic conditions. This further shows that the modified clays could be promising sorbing materials for the removal of EE2 and TC.The non-linear least square fitting was conducted for the breakthrough column data employing the Thomas equation (eqn (1)). The fitting was performed to simulate the two unknown parameters, KT and q0. The values of the Thomas constants and the least square sum were estimated (Table 2). A high loading capacity was achieved for EE2 and TC for these clays under dynamic conditions. BH showed a higher removal capacity for EE2 and TC compared with BAH. Similarly, LCH possessed a higher removal capacity than LCAH. The overall removal capacity was in the order BH > BAH ≈ LCH > LCAH. The removal capacity of BAH and LCH for EE2 and TC was comparable hence, the locally collected clay could be a useful natural material for the efficient, effective remediation of wastewater contaminated with the micro-pollutants EE2 and TC. Moreover, the removal capacity of BAH or LCAH for EE2 and TC was comparable with BH or LCH. Although a slight decrease was observed for BAH and LCAH, these materials showed a good settling capacity, and would be a superior alternative in solid/solution separation and wastewater treatment plants. These results were similar to the findings of the batch reactor experiments. The results were consistent with previous reports in which the Thomas equation was used to demonstrate the loading capacity of different sorbing materials.60,61
| Materials | EE2 | TC | ||||
|---|---|---|---|---|---|---|
| Thomas constants | Least square sum (s2) | Thomas constants | Least square sum (s2) | |||
| KT × 10−4 (L min−1 mg−1) | q0 (mg g−1) | KT × 10−4 (L min−1 mg−1) | q0 (mg g−1) | |||
| BH | 1.59 | 111.979 | 10.0 | 4.89 | 34.976 | 12.0 |
| BAH | 1.70 | 97.987 | 11.0 | 6.09 | 29.963 | 12.0 |
| LCH | 1.88 | 86.965 | 11.0 | 6.59 | 26.956 | 12.0 |
| LCAH | 2.30 | 64.996 | 10.0 | 7.09 | 23.478 | 11.0 |
4. Conclusion
Organo-modified clay and inorgano–organo-modified clays were synthesized from natural bentonite and local clay and characterized by IR and XRD. The IR data showed that HDTMA was introduced in the clay network. XRD analysis indicated the presence of quartz, smectite, kaolinite and illite in various proportions in bentonite and local clay. SEM images of the modified clays showed that the structures of HDTMA-modified clay materials were disordered, whereas Al-pillared clays contained fine particles of aluminum hydroxides or Al2O3 aggregated on the clay surface. The clays were used for the remediation of EE2 and TC contaminated waters in batch and column reactor experiments. The batch data implied that the uptake of EE2 and TC by the hybrid materials was slightly affected at low and high pH in the range 4.0–10.0. The uptake was not affected by varying the sorptive concentration (1.0 to 10.0 mg L−1 for EE2 and 1.0 to 20.0 mg L−1 for TC) and the background electrolyte concentration (0.0001 to 0.1 mol L−1 NaCl). Moreover, the attenuation of EE2 and TC by these hybrid materials was efficient; within 60 min of contact time for EE2 and 240 min for TC, an apparent equilibrium between clay and solution was achieved. The fixed-bed column results showed that a high breakthrough volume was obtained for attenuating EE2 and TC using the hybrid materials. Furthermore, the breakthrough data were fitted well to the Thomas equation; therefore, the loading capacity for EE2 was estimated to be 111.979, 97.987, 86.965 and 64.996 mg g−1 and that for TC was 34.976, 29.963, 26.956 and 23.478 mg g−1 for BH, BAH, LCH and LCAH, respectively. Hence, these hybrid materials are promising for the remediation of the aquatic environment contaminated with two important micro-pollutants, EE2 and TC.Acknowledgements
DT wishes to acknowledge CSIR, New Delhi for the financial support through Research Project (no. 01 (2567)/12/EMR-II). The work is also partly supported by UGC (NERO) financial assistance (no. F.5-22/2013-14/(MRP/NERO)/261).References
- Crystal structure of clay minerals and their X-ray identification, ed. S. W. Bailey, G. W. Brindley and G. Brown, Mineralogical Society, London, 1980, pp. 1–113 Search PubMed.
- J. Konta, Appl. Clay Sci., 1995, 10, 275–335 CrossRef CAS.
- S. M. Lee and D. Tiwari, Appl. Clay Sci., 2012, 59–60, 84–102 CrossRef CAS PubMed.
- Y. Park, G. A. Ayoko and R. L. Frost, J. Colloid Interface Sci., 2011, 354, 292–305 CrossRef CAS PubMed.
- D. Tiwari and S. M. Lee, Chem. Eng. J., 2012, 204–206, 23–31 CrossRef CAS PubMed.
- Z. Li and R. S. Bowman, Water Res., 2001, 35, 3771–3776 CrossRef CAS.
- Z. Li, D. Alessi, P. Zhang and R. S. Bowman, J. Environ. Eng., 2002, 128, 583–587 CrossRef CAS.
- G. R. Alther, Waste Manag., 2002, 22, 507–513 CrossRef CAS.
- J. Q. Jiang, C. Cooper and S. Ouki, Chemosphere, 2002, 47, 711–716 CrossRef CAS.
- J. Smith, S. Bartlett-Hunt and S. Burns, J. Hazard. Mater., 2003, 96, 91–97 CrossRef CAS.
- E. Benfenati, D. Barceló, I. Johnson, S. Galassi and K. Levsen, Trends Anal. Chem., 2003, 22, 757–765 CrossRef CAS.
- S. A. Snyder, P. Westerhoff, Y. Yoon and D. L. Sedlak, Environ. Eng. Sci., 2003, 20, 449–469 CrossRef CAS.
- M. Petrović, E. Eljarrat, M. J. L. de Alda and D. Barceló, Anal. Bioanal. Chem., 2004, 378, 549–562 CrossRef PubMed.
- J. Niu, S. Ding, L. Zhang, J. Zhao and C. Feng, Chemosphere, 2013, 93, 1–8 CrossRef CAS PubMed.
- J. Lienert, T. Buerki and B. I. Escher, Water Sci. Technol., 2007, 56, 87–96 CAS.
- A. Joss, E. Keller, A. Alder, A. Gobel, C. McArdell, T. Ternes and H. Siegrist, Water Res., 2005, 39, 3139–3152 CrossRef CAS PubMed.
- K. A. Landry and T. H. Boyer, Water Res., 2013, 47, 6432–6444 CrossRef CAS PubMed.
- S. Larcher and V. Yargeau, Environ. Pollut., 2013, 173, 17–22 CrossRef CAS PubMed.
- Datapharm, EMC Medicine Guides (UK) – Ethinylestradiol, 2008, http://www.medicines.org.uk/guides/ethinylestradiol.
- J. Han, W. Qiu, Z. Cao, J. Hu and W. Gao, Water Res., 2013, 47, 2273–2284 CrossRef CAS PubMed.
- A. Z. Aris, A. S. Shamsuddin and S. M. Praveena, Environ. Int., 2014, 69, 104–119 CrossRef CAS PubMed.
- USFDA, U.S. Food and Drug Administration (USFDA) and National Center for Toxicological Research (NCTR), Endocrine Disruptor Knowledge Base (EDKB), 2012, http://www.fda.gov/scienceresearch/bioinformaticstools/endocrinedisruptorknowledgebase/default.htm.
- P. C. Heaton, S. R. Fenwick and D. E. Brewer, J. Clin. Pharm. Ther., 2007, 32, 483–487 CrossRef CAS PubMed.
- S. Yahiat, F. Fourcade, S. Brosillon and A. Amrane, Int. Biodeterior. Biodegrad., 2011, 65, 997–1003 CrossRef CAS PubMed.
- P. Gao, M. Munir and I. Xagoraraki, Sci. Total Environ., 2012, 173, 421–422 Search PubMed.
- W. Yang, Z. Tang, F. Zhou, W. Zhang and L. Song, Environ. Toxicol. Pharmacol., 2013, 35, 320–324 CrossRef CAS PubMed.
- H. Y. Kim, J. Jeon, J. Hollender, S. Yu and S. D. Kim, J. Hazard. Mater., 2014, 279, 428–435 CrossRef CAS PubMed.
- J. de Rudder, T. V. de Wiele, W. Dhooge, F. Comhaire and W. Verstraete, Water Res., 2004, 38, 184–192 CrossRef CAS PubMed.
- L. Joseph, L. K. Boateng, J. R. V. Flora, Y.-G. Park, A. Son, M. Badawy and Y. Yoon, Sep. Purif. Technol., 2013, 107, 37–47 CrossRef CAS PubMed.
- L. A. Al-Khateeb, A. Y. Obaid, N. A. Asiri and M. A. Salam, J. Ind. Eng. Chem., 2014, 20, 916–924 CAS.
- Y. Gao, Y. Li, L. Zhang, H. Huang, J. Hu, S. M. Shah and X. Su, J. Colloid Interface Sci., 2012, 368, 540–546 CrossRef CAS PubMed.
- J. Rivera-Utrilla, C. V. Gómez-Pacheco, M. Sánchez-Polo, J. J. López-Peñalver and R. Ocampo-Pérez, J. Environ. Manage., 2013, 131, 16–24 CrossRef CAS PubMed.
- Z. Li, L. Schulz, C. Ackley and N. Fenske, J. Colloid Interface Sci., 2010, 351, 254–260 CrossRef CAS PubMed.
- S. A. Boyd, S. Shaobai, J.-F. Lee and M. M. Mortland, Clays Clay Miner., 1988, 36, 125–130 CAS.
- ISRIC (International Soil Reference and Information Centre), Procedures for soil analysis, ed. L. P. van Reeuwijk, FAO, UN, Wageningen, The Netherlands, 6th edn, 2012, pp. 3.1–3.7, http://www.isric.org/isric/webdocs/docs/ISRIC_TechPap09_2002.pdf Search PubMed.
- US EPA (United States Environment Protection Agency), Method 9080, Cation-Exchange Capacity of Soils (Ammonium Acetate), 1986, pp. 1–9 Search PubMed.
- Thanhmingliana and D. Tiwari, Chem. Eng. J., 2015, 263, 364–373 CrossRef CAS PubMed.
- P. C. C. Faria, J. J. M. Órfão and M. F. R. Pereira, Water Res., 2004, 38, 2043–2052 CrossRef CAS PubMed.
- Y. Gao, Y. Li, L. Zhang, H. Huang, J. Hu, S. M. Shah and X. Su, J. Colloid Interface Sci., 2012, 368, 540–546 CrossRef CAS PubMed.
- M. Brigante and P. C. Schulz, J. Hazard. Mater., 2011, 192, 1597–1608 CrossRef CAS PubMed.
- H. C. Thomas, J. Am. Chem. Soc., 1944, 66, 1664–1666 CrossRef CAS.
- S. M. Lee, Lalhmunsiama, Thanhmingliana and D. Tiwari, Chem. Eng. J., 2015, 270, 496–507 CrossRef CAS PubMed.
- X. Yang, R. C. Flowers, H. S. Weinberg and P. C. Singer, Water Res., 2011, 45, 5218–5228 CrossRef CAS PubMed.
- Z. Li and R. S. Bowman, Environ. Sci. Technol., 1997, 31, 2407–2412 CrossRef CAS.
- Y. Park, G. A. Ayoko, R. Kurdi, E. Horváth, J. Kristóf and R. L. Frost, J. Colloid Interface Sci., 2013, 406, 196–208 CrossRef CAS PubMed.
- S. Zheng, Z. Sun, Y. Park, G. A. Ayoko and R. L. Frost, Chem. Eng. J., 2013, 234, 416–422 CrossRef CAS PubMed.
- P. Kulshrestha, R. F. Giese Jr and D. S. Aga, Environ. Sci. Technol., 2004, 38, 4097–4105 CrossRef CAS.
- M. E. Parolo, M. C. Savini, J. M. Vallés, M. T. Baschini and M. J. Avena, Appl. Clay Sci., 2008, 40, 179–186 CrossRef CAS PubMed.
- Thanhmingliana, S. M. Lee and D. Tiwari, RSC Adv., 2014, 4, 43921–43930 CAS.
- L. Shao, Z. Ren, G. Zhang and L. Chen, Mater. Chem. Phys., 2012, 135, 16–24 CrossRef CAS PubMed.
- L. Zhang, X. Song, X. Liu, L. Yang, F. Pan and J. Lv, Chem. Eng. J., 2011, 178, 26–33 CrossRef CAS PubMed.
- K. F. Hayes, C. Papelis and J. O. Leckie, J. Colloid Interface Sci., 1988, 125, 717–726 CrossRef CAS.
- S. M. Lee, Lalhmunsiama and D. Tiwari, Environ. Sci. Pollut. Res. Int., 2014, 21, 3686–3696 CrossRef CAS PubMed.
- L. Joseph, Q. Zaib, I. A. Khan, N. D. Berge, Y.-G. Park, N. B. Saleh and Y. Yoon, Water Res., 2011, 45, 4056–4068 CrossRef CAS PubMed.
- T. Yang, M. L. Chen, L. H. Liu, J. H. Wang and P. K. Dasgupta, Environ. Sci. Technol., 2012, 46, 2251–2256 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Process Saf. Environ. Prot., 1998, 76, 332–340 CrossRef CAS.
- M. Haerifar and S. Azizian, Chem. Eng. J., 2013, 215–216, 65–71 CrossRef CAS PubMed.
- Y. S. Ho, J. Hazard. Mater., 2006, 136, 681–689 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Water Res., 2000, 34, 735–742 CrossRef CAS.
- D. Tiwari, C. Laldawngliana and S. M. Lee, Environmental Engineering Research, 2014, 19, 107–113 CrossRef.
- S. M. Lee, D. Tiwari, K. M. Choi, J. K. Yang, Y. Y. Chang and H. D. Lee, J. Chem. Eng. Data, 2009, 54, 1823–1828 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |