A novel AgNPs-based colorimetric sensor for rapid detection of Cu2+ or Mn2+ via pH control†
Genhua Wua,
Chen Dongab,
Yonglong Lib,
Zhuqing Wangab,
Yuexia Gaob,
Zheyu Shen*b and
Aiguo Wu*b
aSchool of Chemistry and Chemical Engineering, Anqing Normal College, Anqing, Anhui 246001, China
bKey Laboratory of Magnetic Materials and Devices, Chinese Academy of Sciences & Division of Functional Materials and Nano Devices, Ningbo Institute of Materials Technology & Engineering, Chinese Academy of Sciences, Ningbo, Zhejiang 315201, China. E-mail: aiguo@nimte.ac.cn; shenzheyu@nimte.ac.cn; Fax: +86-574-86685163; Tel: +86-574-86685039 Tel: +86-574-87617278
First published on 10th February 2015
Abstract
In this study, we propose a new silver nanoparticles (AgNPs)-based colorimetric sensor for rapid detection of Cu2+ or Mn2+ at pH 1.9 or 12.0, respectively. At pH 1.9, the AgNPs stabilized with sodium pyrophosphate (Na4P2O7) and hydroxypropylmethylcellulose (HPMC) gradually become smaller in the presence of Cu2+ resulting in a color change of the solution from yellow to colorless. At pH 12.0, however, the AgNPs aggregate immediately in the presence of Mn2+, which induces a color change of the solution from yellow to brown. The incubation time between the detection system and metal ion (Cu2+ or Mn2+) is fixed at 10 min. This mechanism is confirmed using UV-vis spectroscopy, Transmission electron microscopy (TEM), Dynamic light scattering (DLS) and Fourier-Transform Infrared Spectrometry (FT-IR). At the optimized experimental conditions, the selectivity of our AgNPs-based detection system is excellent for Cu2+ or Mn2+ compared with other metal ions. The limit of detections (LODs) of Cu2+ and Mn2+ by the naked eye are respectively 0.05 and 0.5 μM, and those by UV-vis spectroscopy are respectively 2.0 and 20 nM. The above LODs are all lower than the corresponding national drinking water standards (16 μM of Cu2+ and 1.8 μM of Mn2+). The applicability of our AgNPs-based colorimetric sensor is also validated by detection of Cu2+ and Mn2+ in tap water and lake water. Therefore, these results reinforce that our AgNPs-based colorimetric sensor is applicable to rapid colorimetric detection of Cu2+ and Mn2+ in complicated real water samples with excellent selectivity and high sensitivity.
1. Introduction
Manganese and copper are essential trace elements for the human body, both of them play important roles in life processes, such as enzymatic conversion, bone growth, fat metabolism and so on. However, excessive manganese or copper in the body can lead to many serious human afflictions, including hepatic necrosis, liver degeneration, arthritis, cramps and so forth. Thus, detection of manganese and copper ions in various samples prior to their entrance to the human body has great significance. As is well known, there are many techniques employed for the detection of manganese ions (Mn2+) and copper ions (Cu2+) in aqueous solution, such as ultraviolet-visible spectroscopy (UV-vis),1 atomic absorption spectrometry (AAS),2 atomic fluorescence spectrometry (AFS),3 inductively coupled plasma-atomic emission spectrometry (ICP-MS)4 and X-ray fluorescence spectroscopy (XRF).5 These methods own relatively high accuracy and anti-interference ability. However, the high operating costs, time-consuming and complicated pre-treatment processes limit their applications for on-site detection.The colorimetric assay method has drawn much attention in recent years due to its ease of operation, low cost and high sensitivity. Colorimetric sensors based on precious metal nanoparticles, such as gold nanoparticles (AuNPs) or silver nanoparticles (AgNPs), can offer an attractive approach for simple, rapid, highly sensitive and selective recognition of metal ions,6–8 nitrite,9 organic molecules10 and proteins.11 Compared with AuNPs, AgNPs have been more widely used because of its strong surface plasmon resonance (SPR), highly stable dispersions and catalytic activity.12 To the best of our knowledge, there are many published works focusing on colorimetric detection of Cu2+ or Mn2+ based on functionalized AgNPs system.6,13–17 Ma et al. proposed a facile and eco-friendly colorimetirc sensor based on dopamine-stabilized AgNPs for Cu2+ detection at pH 10.8.6 This sensor shows good sensitivity and selectivity for Cu2+ detection and can be applied for Cu2+ detection in tap water. However, the cost of this sensor is relatively high because the dopamine needs to be used for the stabilization of the AgNPs. Zhou et al. reported a colorimetirc sensor based on 4-mercaptobenzoic acid-modified AgNPs for Cu2+ detection at pH 7.4,17 whose limit of detection (LOD) is 100 nM by eye vision and 25 nM by UV-vis spectra. Although the LODs are all lower than the national standard of table-water (16 μM), the AgNPs preparation process is complicated because ice bath is necessary. Annadhasan et al. reported a simple and green synthesis method of L-tyrosine-stabilized AgNPs, which can be used for detection of Mn2+ at pH 11.18 The LOD is 16 nM, which is much lower than the national standard of table-water (1.8 μM). However, the selectivity is not good because Hg2+ has interference to the Mn2+ detection. Hu et al. proposed a highly selective colorimetric sensor for Mn2+ detection based on the aggregation of functionalized AgNPs at pH 9.14 The LOD is 5.0 μM by eye vision, which is higher than the national standard of table-water (1.8 μM). Furthermore, all the reported colorimetirc sensors based on AgNPs can only be used for detection of single metal ion.
In this study, we developed a new AgNPs-based colorimetric sensor, which could be used for the detection of Cu2+ or Mn2+ at pH 1.9 or 12.0, respectively. The AgNPs are stabilized with sodium pyrophosphate (Na4P2O7) and hydroxypropylmethylcellulose (HPMC). At pH 1.9, the AgNPs gradually become smaller in the presence of Cu2+ resulting in a color change of the solution from yellow to colorless and a decrease of the surface plasmon absorption intensity. At pH 12.0, however, the AgNPs aggregate immediately in the presence of Mn2+, which induces a color change of the solution from yellow to brown and a decrease of the surface plasmon absorption intensity. In addition, compared to other published articles,6,13–17 our proposed new AgNPs-based colorimetric sensor has more improvements as follows: (1) the cost of the sensor is relatively low; (2) the AgNPs preparation process is simple; (3) the selectivity is excellent for Cu2+ and Mn2+; (4) the sensitivity is very high for Cu2+ and Mn2+. Furthermore, compared to our previous work,13,16 the LODs of Cu2+ and Mn2+ in this work are improved significantly. That's because the detection of Cu2+ or Mn2+ in this work is respectively carried out at pH 1.9 or 12.0 and only controlled by different pH value, however our previous work is carried out at pH 2.3 or 10.8 in different detection systems. Moreover, the AgNPs-based sensor in this work is enough stable at pH 1.9 or 12.0 due to the stabilizer HPMC.
2. Experimental
2.1 Materials
Na4P2O7·10H2O, AgNO3, NaBH4, NaOH, HCl, NaCl, KCl, MgCl2·6H2O, CaCl2, BaCl2·2H2O, ZnCl2, MnCl2·4H2O, CdCl2·2.5H2O, Pb(NO3)2, CuCl2·2H2O, HgCl2, NiCl2·6H2O, CoCl2·6H2O, FeSO4·7H2O, FeCl3, CrCl3·6H2O, AlCl3, K2Cr2O7, Na2SO4, NaNO3, Na2CO3, Na3PO4 were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Hydroxypropylmethylcellulose (HPMC) was obtained from Aladdin Reagent Co. Ltd (Shanghai, China). The glasswares were washed with aqua-regia (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 3
HNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) and well rinsed with MilliQ water prior to use.
1 (v/v)) and well rinsed with MilliQ water prior to use.
2.2 Methods
TEM images were recorded from a JEOL 2100 instrument (JEOL, Japan) which was operated at 200 kv. UV-vis spectroscopy was performed using a T10CS instrument (Beijing Purkinje General Instrument Co., Ltd, China). IR data were obtained on a Nicolet 6700 Fourier-Transform Infrared Spectrometer (Thermo scientific, USA). Size distribution of AgNPs was measured by DLS using a Zeta particle size analyzer (Nano-ZS, Malvern Instrument Ltd, UK). As controls, the Cu2+ and Mn2+ concentrations in real water samples were detected by inductively coupled plasma mass spectrometry (ICP-MS, Optima 2100DV, Perkin Elmer, USA). MilliQ water was used for all experiments.2.3 Preparation of the functionalized AgNPs
The functionalized AgNPs were prepared by reducing AgNO3 with NaBH4 in the presence of Na4P2O7. Briefly, 1.0 mL of AgNO3 aqueous solution (20 mM) was rapidly added into 100 mL of Na4P2O7 aqueous solution (500 μM). After that, 1.0 mL of fresh NaBH4 solution (0.1 M) was slowly injected into the mixture under vigorous stirring. The reaction was kept for 20 min (the color of the solution turned into a vivid yellow). The AgNP dispersion was stored at room temperature for 24 h. Finally, 1.0 mL of HPMC aqueous solution (1.0 mg mL−1) was slowly added into 19 mL of the above AgNP dispersion under stirring. After 10 min, the functionalized AgNP dispersion was stored at room temperature until use.2.4 Colorimetric detection of Cu2+ and Mn2+
The colorimetric detection of Cu2+ or Mn2+ using our AgNPs-based detection system was respectively operated under an acidic or alkaline condition. Typically, 200 μL of Cu2+ or Mn2+ aqueous solutions with various concentrations were added into 800 μL of AgNPs-based detection system, whose pH value was respectively tuned to 1.5–2.3 or 11.8–12.7. The mixtures were maintained at room temperature (25 °C controlled by the air conditioner) for 1.0–30 min. The color change and the SPR absorption spectra were observed.2.5 Detection of real water samples
Real water samples of tap water obtained from our institution and lake water collected from a pond in our institution were first filtered through syringe filters with 0.2 μm of membrane. After that, a series of Cu2+ or Mn2+ standard solutions were respectively added into the tap or lake water. The samples were then detected utilizing our AgNPs-based detection system or ICP-MS.3. Results and discussion
3.1 Detection mechanism
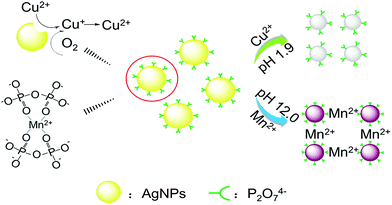
The detection mechanism of our AgNPs-based colorimetric sensor for Cu2+ and Mn2+ (Scheme 1) is verified by FT-IR, UV-vis spectroscopy, TEM and DLS.In the FT-IR spectra of our AgNPs-based colorimetric sensor (Fig. S1†), the strong peaks at 1110 cm−1 and 924 cm−1 are assigned to P–O stretches in Na4P2O7, which indicates that the AgNPs are successfully modified by P2O74−.
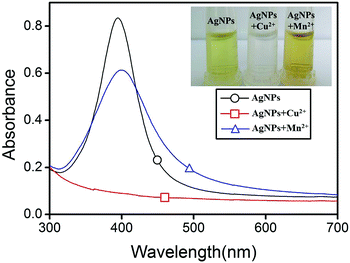
Fig. 1 shows the UV-vis absorption spectra and photographic image of the AgNPs-based detection system in the presence of Cu2+ (10 μM) at pH 1.9 or Mn2+ (10 μM) at pH 12.0. It can be seen that the AgNPs-based detection system is yellow and has obvious UV-vis absorption with a peak wavelength at 395 nm. However, the color of the detection system with Cu2+ (10 μM) at pH 1.9 changes from yellow to colorless, and the UV-vis absorption decreases significantly. That's because the standard potential of Ag+/Ag reduces from 0.79 V to 0.22 V in the presence of Cl− and that of CuCl2/CuCl is 0.57 V.16 Cu2+ can oxidize AgNPs forming Cu+, which is unstable and easily oxidized to be Cu2+ by oxygen dissolved in the solution. Therefore, Cu2+ plays a catalytic role in the entire reaction and slowly etches the AgNPs.
From Fig. 1, it is also found that the color of the detection system with Mn2+ (10 μM) at pH 12.0 changes from yellow to brown and red shift happens in the UV-vis absorption. This result indicates that the AgNPs aggregate in the presence of Mn2+ at an alkaline condition. That's because P2O74− on the surface of AgNPs can bind to Mn2+ to form a four-coordinated structure (Scheme 1).19
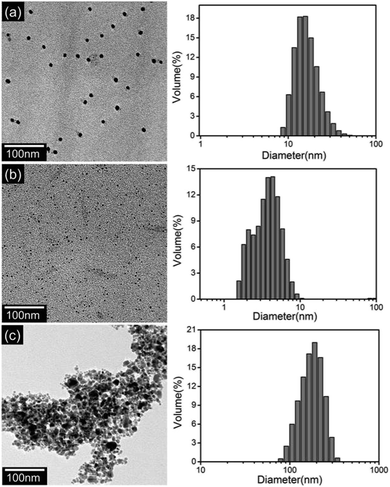
Fig. 2 shows TEM images and corresponding particle size distributions of the AgNPs at different conditions. From Fig. 2(a), it can be seen that the AgNPs without Cu2+ and Mn2+ are well dispersed and the size is around 15 nm. However, the AgNPs in the presence of Cu2+ at pH 1.9 become smaller (Fig. 2(b)). This result confirms the mechanism that AgNPs can be etched by Cu2+ (Scheme 1). Fig. 2(c) shows TEM images and size distributions of the AgNPs in the presence of Mn2+ at pH 12.0. It can be observed that the AgNPs aggregate significantly. This result indicates that the addition of Mn2+ could readily lead to the aggregation of AgNPs (Scheme 1).
3.2 Optimization of experimental conditions
The concentration of two modifiers (Na4P2O7 and HPMC), incubation time of AgNPs with metal ions, pH value of the AgNPs dispersions are optimized according to the sensing effect of our AgNPs-based detection system.As mentioned in methods section, our AgNPs-based detection system is stabilized by P2O74− and HPMC and used to detect Cu2+ under acidic conditions and Mn2+ under alkaline conditions. P2O74− as a modifier of the AgNPs can form chelates with Mn2+ under an alkaline condition. HPMC as a stabilizer of the AgNPs can increase the stability of the AgNPs under an acidic or an alkaline condition. From the photographic image and corresponding plot of A0/A (A is the absorbance value at 414 nm in the UV-vis spectra of the detection systems incubated with 10 μM of Cu2+, and A0 is that of the detection systems without Cu2+) of the detection systems with different P2O74− concentrations at pH 1.9 (Fig. S2(a)†), it is found that the sensing effect of Cu2+ is best when the P2O74− concentration is 500 μM. From the photographic image and corresponding plot of A0/A (A is the absorbance value at 395 nm in the UV-vis spectra of the detection systems incubated with 10 μM of Mn2+, and A0 is that of the detection systems without Mn2+) of the detection systems with different P2O74− concentrations at pH 12.0 (Fig. S2(b)†), we can see that the sensing effect of Mn2+ is best when the P2O74− concentration is larger than 100 μM. In view of the sensing effect of our AgNPs-based detection system for both Cu2+ and Mn2+, the P2O74− concentration is fixed at 500 μM for the subsequent experiments.
Influence of the HPMC concentration in the AgNPs-based detection system on the sensing effect of Cu2+ or Mn2+ is also studied. From the photographic image and corresponding plot of A0/A of the detection systems with different HPMC concentrations at pH 1.9 (Fig. S3(a)†), it can be concluded that the sensing effect of Cu2+ is best at 50 mg L−1 of HPMC. From the photographic image and corresponding plot of A0/A of the detection systems with different HPMC concentrations at pH 12.0 (Fig. S3(b)†), we can see that the HPMC concentration should be ≤50 mg L−1 according to the sensing effect of Mn2+. Therefore, taking into account the sensing effect of our AgNPs-based detection system for both Cu2+ and Mn2+, the HPMC concentration is fixed at 50 mg L−1 as an optimized value for the subsequent study.
Influence of the incubation time between the detection system and Cu2+ (0.2 μM) at pH 1.9 or between the detection system and Mn2+ (5.0 μM) at pH 12.0 on the sensing effect is also investigated. From the changing of the A/A0 values (Fig. S4†), we can conclude that 10 min is enough to reach the reaction equilibrium. Thus, the incubation time between the detection system and metal ion (Cu2+ or Mn2+) is fixed at 10 min for the following experiments.
Influence of the pH value on the sensing effect of metal ions (Cu2+ or Mn2+) is also studied. The pH value ranges from 1.5 to 2.3 for Cu2+ detection, and ranges from 11.8 to 12.7 for Mn2+ detection (Fig. S5†) because our sensing system is not sensitive (the color change is not obvious) for Cu2+ or Mn2+ detection when pH value is within the range of neutral pH (pH 5–8). The intensity of the SPR absorption increases significantly with increasing of the pH value under an acidic condition for Cu2+ detection, but decreased with increasing of the pH value under an alkaline condition for Mn2+ detection. The AgNPs is unstable when pH value is lower than 1.6 (for Cu2+ sensing) or higher than 12.5 (for Mn2+ sensing). According to the sensing effect and the AgNPs's stability, the pH value is optimized to be 1.9 for Cu2+ detection and 12.0 for Mn2+ detection.
From Fig. S5,† it seems the pH value has a very great influence on the UV-vis absorbance of the detection systems. The difference of the UV-vis absorbance between pH 2.0 and 1.9 is very big because the [H+] difference is 2.6 mM between pH 2.0 and 1.9, which is also very big compared with the [Cu2+] (0.5 μM). Furthermore, Mn2+ and some of other metal ions should not present as ion form in normal solutions at pH 12. In this study, the detection result of our AgNPs-based system at pH 12 is positive for Mn2+ and negative for other metal ions. Forming of hydroxide for other metal ions cannot result in a positive result because their hydroxide cannot induce the aggregation of AgNPs. In addition, in our Mn2+ detection system based on P2O74−-modified AgNPs at pH 12, Mn2+ should prefer to binding with P2O74− to form a four-coordinated structure rather than forming hydroxide.
Based on the optimized conditions as mentioned above, we studied the stability of AgNPs-based detection system at pH 1.9 or 12.0 compared with the control without pH tuning (pH 9.0). It is found that the UV-absorption of the detection system at pH 1.9 or 12.0 is very stable (Fig. S6†), which indicates that the detection system at pH 1.9 or 12.0 is very stable during storage at room temperature. Our sensor is also photostable because all of our experiments were carried out under bright environment and the detection results are repeatable. The lifespan of our sensor at room temperature is at least 24 h. Other studies confirm that our sensor is not reversible.
3.3 Selectivity of the detection system for Cu2+ and Mn2+
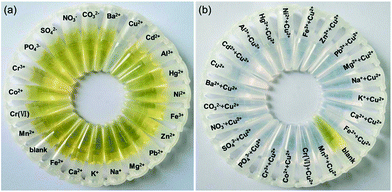
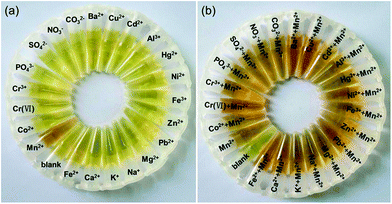
The selectivity of the detection system for Cu2+ and Mn2+ is evaluated by eye vision and UV-vis spectra compared with other ions including Ba2+, Cu2+, Cd2+, Al3+, Hg2+, Ni2+, Fe3+, Zn2+, Pb2+, Mg2+, Na+, K+, Ca2+, Fe2+, Mn2+, Cr(VI), Co2+, Cr3+, PO43−, SO42−, NO3−, and CO32−. Fig. 3(a) shows the photographic image of the detection systems incubated with Cu2+ or other ions at pH 1.9. It is found that Cu2+ can etch the AgNPs resulting in the color change of the detection systems from yellow to colorless, but all other ions cannot. Fig. 3(b) shows the photographic image of the detection systems incubated with Cu2+, or mixture of Cu2+ and other ions at pH 1.9. We can see the colour of the detection system incubated with Cu2+ is very similar with that incubated with the mixture of Cu2+ and other ions. The result indicates that other ions have no interference to the Cu2+ detection because they cannot inhibit the etching of the AgNPs by Cu2+.Fig. 4(a) shows the photographic image of the detection systems incubated with different ions at pH 12.0. It can be seen that Mn2+ can induce the aggregation of the AgNPs resulting in the color change of the detection systems from yellow to brown, but all other ions cannot. Fig. 4(b) shows the photographic image of the detection systems incubated with Mn2+, or mixture of Mn2+ and other ions at pH 12.0. It is obvious that the colour of the detection system incubated with Mn2+ is similar with that incubated with the mixture of Mn2+ and other ions. The result indicates that other ions have no interference to the Mn2+ detection because they cannot inhibit the aggregation of the AgNPs induced by Mn2+.
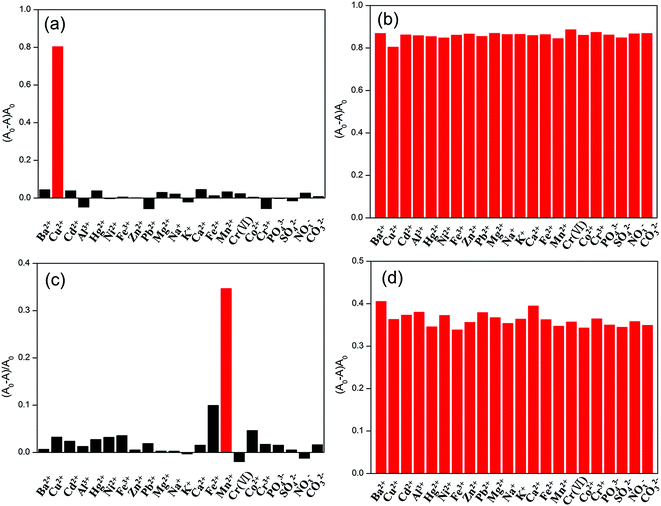
Fig. 5(a) shows (A0 − A)/A0 of the detection systems incubated with Cu2+ (1.0 μM), Hg2+ (1.0 μM), Cr(VI) (1.0 μM) or other ions (10 μM) at pH 1.9. Fig. 5(b) shows (A0 − A)/A0 of the detection systems incubated with Cu2+ (1.0 μM) and other ions (10 μM) at pH 1.9. A and A0 are respectively the absorbance value at 414 nm in the UV-vis absorption spectra of the detection systems incubated with or without Cu2+. From Fig. 5(a), it is found that a dramatic increase in the absorbance ratio ((A0 − A)/A0) is clearly observed for Cu2+, whereas no obvious change is obtained for other ions. From Fig. 5(b), we can find that all other ions have no big interference to the detection of Cu2+. The Fe3+ and Cr2O72− have little influence on the detection because they can also etch the AgNPs due to their oxidation ability. The interference is not big because their reaction rates are relatively low compared with the catalytic reaction of Cu2+.
Fig. 5(c) shows the (A0 − A)/A0 of the detection systems incubated with 10 μM of various ions at pH 12.0. Fig. 5(d) shows the (A0 − A)/A0 of the detection systems incubated with Mn2+ (10 μM) and other ions (10 μM) at pH 12.0. A and A0 are respectively the absorbance value at 395 nm in the UV-vis absorption spectra of the detection systems incubated with or without Mn2+. It is obvious that the (A0 − A)/A0 of the detection system incubated Mn2+ is much larger than those of the detection systems incubated with other ions, and comparable to those of the detection systems incubated with Mn2+ and other ions.
In this work, we also studied the influence of potentially interfering ions and their tolerance ratios on the detection of Cu2+ or Mn2+ (Table S1†). The result indicates that the detection of Cu2+ or Mn2+ have a high tolerance on other metal ions and the ionic strength has no influence on the performances of the colorimetric sensor.
Therefore, the selectivity our AgNPs-based detection system is excellent for Cu2+ and Mn2+ compared with other ions including Ba2+, Cu2+, Cd2+, Al3+, Hg2+, Ni2+, Fe3+, Zn2+, Pb2+, Mg2+, Na+, K+, Ca2+, Fe2+, Mn2+, Cr(VI), Co2+, Cr3+, PO43−, SO42−, NO3−, and CO32−.
3.4 Sensitivity of the detection system for Cu2+ and Mn2+
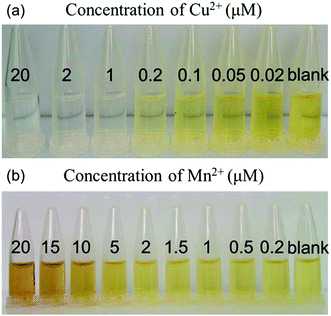
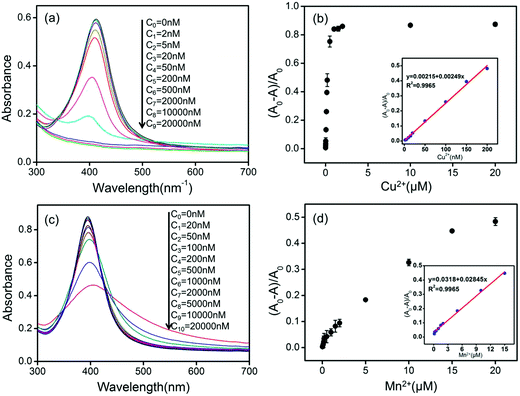
The colorimetric response and UV-vis spectra are used to further evaluate the sensitivity of our proposed AgNPs-based detection system. The photographic image of the AgNPs-based detection systems at pH 1.9 in the presence of Cu2+ with various concentrations is shown in Fig. 6(a). With decreasing of the Cu2+ concentration from 20 to 0.02 μM, the color of the AgNPs dispersions changes from colorless to yellow. The limit of detection (LOD) of Cu2+ is observed to be about 0.05 μM by the naked eyes. The final results are almost same with different concentrations of Cu2+ in the reaction system because the catalytic reaction of Cu2+ proceeds until the AgNPs are etched completely. However, the reaction rates are different with different concentrations of Cu2+. Our results are reasonable because reaction time is fixed at 10 min. Fig. 6(b) shows the photographic image of the AgNPs-based detection systems at pH 12.0 containing various Mn2+ concentrations from 20 to 0.2 μM. With decreasing of the Mn2+ concentration, the color of the detection systems changes from brown to yellow. The LOD of Mn2+ by eye vision is observed to be about 0.5 μM. The color gradient in Fig. 6, which also depends on the camera and photographic technology, seems not significant. However, it's enough obvious to be distinguished the naked eyes.UV-vis spectra of our AgNPs-based detection systems with various Cu2+ concentrations at pH 1.9 are shown in Fig. 7(a). It is found that the SPR absorption gradually decreases with increasing of Cu2+ concentration. Fig. 7(b) shows plot of (A0 − A)/A0 of different AgNPs dispersions versus Cu2+ concentrations ranging from 20 to 0.002 μM (A0 and A are respectively the absorbance of the AgNPs dispersions at 414 nm without or with Cu2+). The inset of Fig. 7(b) shows a good linear relationship (R2 = 0.9965) between (A0 − A)/A0 and Cu2+ concentrations from 2.00 to 200 nM with the LOD of 2 nM.
Fig. 7(c) shows UV-vis spectra of the detection systems with various Mn2+ concentrations. It can be seen that the SPR absorption gradually decreases with increasing of Mn2+ concentration. Fig. 7(d) shows plot of (A0 − A)/A0 of different AgNPs dispersions versus Mn2+ concentrations ranging from 20 to 0.02 μM (A0 and A are respectively the absorbance of the AgNPs dispersions at 395 nm without or with Mn2+). The inset of Fig. 7(d) shows a plot of (A0 − A)/A0 versus Mn2+ concentration ranging from 0.15 μM to 15 μM. A good linear relationship (R2 = 0.9965) is found between (A0 − A)/A0 and Mn2+ concentrations with the LOD of 20 nM.
The LODs of our AgNPs-based detection system for Cu2+ and Mn2+ are compared with those of other analytical methods using AuNPs or AgNPs-based systems (Table S2†). It is found that the LOD of our detection system for Mn2+ is comparable to that of the lowest value,7,9,16 and the LOD of our detection system for Cu2+ is lowest.8,11,12
3.5 Detection of real water samples
To validate the applicability, our AgNPs-based detection system is used to analyze the real water samples, such as tap water and lake water. Fig. 7(b) and (d) are used as the standard curves for Cu2+ and Mn2+. The analytical results are shown in Table 1 and the colorimetric detection results are shown in Fig. S7.† We can find that the observed Cu2+ or Mn2+ concentrations are very close to those determined by ICP-MS method. These results indicate that our AgNPs-based detection system can be used for analysis of Cu2+ and Mn2+ in the real water samples.| Samples | Cu2+ or Mn2+ added (μM) | Cu2+ or Mn2+ observed (μM) (mean ± E, n = 3) | ICP-MS observed (μM) |
|---|---|---|---|
| Lake water (Cu2+) | 0.05 | 0.06 ± 0.003 | 0.058 |
| 0.10 | 0.09 ± 0.009 | 0.096 | |
| Tap water (Cu2+) | 0.05 | 0.05 ± 0.003 | 0.055 |
| 0.10 | 0.11 ± 0.015 | 0.099 | |
| Lake water (Mn2+) | 0.5 | 0.36 ± 0.068 | 0.393 |
| 1 | 0.72 ± 0.177 | 0.782 | |
| Tap water (Mn2+) | 0.5 | 0.35 ± 0.089 | 0.406 |
| 1 | 0.89 ± 0.254 | 0.810 |
4. Conclusions
A new AgNPs-based colorimetric sensor for rapid detection of Cu2+ or Mn2+ at pH 1.9 or 12.0 is proposed in this study. Under an acidic condition, the AgNPs stabilized with Na4P2O7 and HPMC gradually become smaller in the presence of Cu2+ resulting in a color change of the solution from yellow to colorless. Under an alkaline condition, however, the AgNPs aggregate immediately in the presence of Mn2+, which induces a color change of the solution from yellow to brown. The selectivity of our AgNPs-based detection system by the naked eyes and UV-vis spectra is excellent for Cu2+ or Mn2+ compared with other ions including Ba2+, Cu2+, Cd2+, Al3+, Hg2+, Ni2+, Fe3+, Zn2+, Pb2+, Mg2+, Na+, K+, Ca2+, Fe2+, Mn2+, Cr(VI), Co2+, Cr3+, PO43−, SO42−, NO3−, and CO32−. Furthermore, the limit of detections (LODs) of Cu2+ and Mn2+ by eye vision are respectively 0.05 and 0.5 μM, and those by UV-vis spectroscopy are respectively 2.0 and 20 nM. The above LODs are all lower than the national drinking water standard. In addition, our AgNPs-based detection system is applicable for rapid colorimetric detection of Cu2+ and Mn2+ in complicated real water samples.Acknowledgements
This work was supported by the Program of Zhejiang Provincial Natural Science Foundation of China (no. R5110230, LQ13E030004), Natural Science Foundation of China (Grants nos 31128007, 31170964, 51203175), Hundred Talents Program of Chinese Academy of Sciences (2010-735), and the Project for Science and Technology Service of Chinese Academy of Sciences (KFJ-EW-STS-016). And the aided program for Science and Technology Innovative Research Team of Ningbo Municipality (Grant no. 2014B82010).References
- X. Wen, Q. Yang, Z. Yan and Q. Deng, Microchem. J., 2011, 97, 249–254 CrossRef CAS PubMed.
- İ. Narin, M. Soylak, L. Elçi and M. Doğan, Talanta, 2000, 52, 1041–1046 CrossRef.
- M. J. da Silva, A. P. S. Paim, M. F. Pimentel, M. L. Cervera and M. de la Guardia, Anal. Chim. Acta, 2010, 667, 43–48 CrossRef CAS PubMed.
- A. Milne, W. Landing, M. Bizimis and P. Morton, Anal. Chim. Acta, 2010, 665, 200–207 CrossRef CAS PubMed.
- L. A. Hutton, G. D. O'Neil, T. L. Read, Z. J. Ayres, M. E. Newton and J. V. Macpherson, Anal. Chem., 2014, 9, 4566–4572 CrossRef PubMed.
- Y. Ma, H. Niu, X. Zhang and Y. Cai, Chem. Commun., 2011, 47, 12643–12645 RSC.
- S. Wu, Y. Chen and Y. Sung, Analyst, 2011, 136, 1887–1891 RSC.
- Y. Gao, X. Li, Y. Li, T. Li, Y. Zhao and A. Wu, Chem. Commun., 2014, 50, 6447–6450 RSC.
- J. Zhang, C. Yang, X. Wang and X. Yang, Analyst, 2012, 137, 3286–3292 RSC.
- Z. Wu, H. Zhao, Y. Xue, Q. Cao, J. Yang, Y. He, X. Li and Z. Yuan, Biosens. Bioelectron., 2011, 26, 2574–2578 CrossRef CAS PubMed.
- F. Xia, X. Zuo, R. Yang, Y. Xiao, D. Kang, A. Vallée-Bélisle, X. Gong, J. D. Yuen, B. B. Y. Hsu, A. J. Heeger and K. W. Plaxco, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10837–10841 CrossRef CAS PubMed.
- Z. Zhang, C. Shao, Y. Sun, J. Mu, M. Zhang, P. Zhang, Z. Guo, P. Liang, C. Wang and Y. Liu, J. Mater. Chem., 2012, 22, 1387–1395 RSC.
- Y. Gao, J. Xin, Z. Shen, W. Pan, X. Li and A. Wu, Sens. Actuators, B, 2013, 181, 288–293 CrossRef CAS PubMed.
- R. Hu, L. Zhang and H. Li, New J. Chem., 2014, 38, 2237–2240 RSC.
- Y. Zhou, H. Zhao, C. Li, P. He, W. Peng, L. Yuan, L. Zeng and Y. He, Talanta, 2012, 97, 331–335 CrossRef CAS PubMed.
- L. Miao, J. Xin, Z. Shen, Y. Zhang, H. Wang and A. Wu, Sens. Actuators, B, 2013, 176, 906–912 CrossRef CAS PubMed.
- Y. Zhou, H. Zhao, Y. He, N. Ding and Q. Cao, Colloids Surf., A, 2011, 391, 179–183 CrossRef CAS PubMed.
- M. Annadhasan, T. Muthukumarasamyvel, V. R. Sankar Babu and N. Rajendiran, ACS Sustainable Chem. Eng., 2014, 2, 887–896 CrossRef CAS.
- M. Bobtelsky and S. Kertes, J. Appl. Chem., 1955, 5, 675–686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra00001g |
| This journal is © The Royal Society of Chemistry 2015 |