Synthesis, structure and magnetic properties of graphite carbon encapsulated Fe3C nanoparticles for applications as adsorbents
Xiaobai Wang,
Peng Zhang,
Wei Wang,
Xiang Lei,
Bo Zou and
Hua Yang*
College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: huayang86@sina.com
First published on 13th March 2015
Abstract
This paper reports the synthesis of graphite carbon encapsulated Fe3C nanoparticles (Fe3C/GC NPs). Melamine is used as a carbon source for the generation of Fe3C, and hexadecyltrimethylammonium bromide (CTAB) is used as a templating agent for the generation of mesoporous carbon and conversion of Fe to Fe3C in the synthetic process. The Fe3C/GC NPs exhibited a high saturation magnetization value (61.5 emu g−1). Due to their mesoporous structure, the Fe3C/GC NPs are employed to remove methylene blue from waste water, and they exhibited excellent adsorption properties. The current synthetic approach is versatile and scalable, and thus opens the door towards the larger-scale synthesis of mesoporous and graphite carbon-based metal materials.
Introduction
Magnetic NPs are significant and ubiquitous compounds that have been utilized in various fields.1–6 Applications include catalysts,7 anode materials,8 data storage media,9 environmental remediation10 and drug delivery.11 Many suitable methods have been developed for the synthesis of magnetic NPs of different compositions and phases with distinct magnetic properties. Normally, magnetic NPs which possess the strongest magnetic properties for the above applications could be achieved by the use of metals12 (Co, Fe, saturation magnetization Ms,bulk ∼ 220 emu g−1) or metal alloys13,14 (CoFe, CoNiFe, Ms,bulk ∼ 245 emu g−1).Unfortunately, naked metal NPs have high chemical activity and are easily oxidized upon contact with air,15 generally leading to loss of magnetism and some unique properties. Therefore, this has promoted the widespread use of moderately magnetic oxide NPs, such as iron(III) oxide (α-Fe2O3, γ-Fe2O3 and Fe3O4, Ms,bulk ∼ 92 emu g−1).16,17
However, due to relatively low saturation, the applications of iron(III) oxide are still restricted. In the last few years, scientists have directed their attention to iron carbides (Fe3C and Fe5C2, Ms,bulk ∼ 140 emu g−1).7,18 Primarily, iron carbides consist of carbon atoms occupying the interstices between close-packed iron atoms, and the presence of carbon atoms provides iron carbides with excellent properties compared with naked metal or iron oxide NPs. For instance, iron carbides exhibit the elevated Ms, air-stability and low toxicity. However, in the practical applications, the magnetic NPs require stability under a wide range of different conditions (high temperatures, and acidic/basic media). The magnetic core–shell structured materials are therefore attracting great interest because they combine fascinating properties of the magnetic (ideally metallic) core with a protective polymer,19 silica,20 precious-metal21 or carbon shell.22 In addition, these shells may not only act as protection for the chemically highly active metal core from oxidative degradation and acid erosion, but may also give the magnetic cores many novel features in magnetic fields, such as superparamagnetic behavior,23 high coercivity24 and high magnetic susceptibility.25
On the other hand, the drawback of polymer-coated magnetic NPs is the relatively low intrinsic stability of the coating at higher temperature, a problem which is even enhanced by a possible catalytic action of the metallic cores. Gold seems to be an ideal coating owing to its low reactivity. However, it was found that the direct coating of magnetic particles with gold is very difficult, because of the dissimilar nature of the two surfaces.26,27 Silica coating of magnetic oxide NPs is a fairly controllable process. However, silica is unstable under basic conditions, in addition, silica may contain pores through which oxygen or other species could diffuse. Most recently, carbon-protected magnetic NPs have received great attention,28,29 due to the carbon shells having significant advantages such as remarkably high chemical and thermal stability as well as biocompatibility.30
To combine a magnetic core and carbon shell into a targeted magnetic NPs, several methods, such as arc technique, laser decomposition, high-temperature annealing, catalytic chemical vapor deposition, catalytic decomposition, explosion, and spraying methods, have been developed.31–34 Several years ago, Luo Ning and coworkers fabricated carbon-encapsulated iron-based NPs (Fe@C, Fe2C@C and Fe3C@C) by detonation decomposition of explosive mixture precursors containing iron ion components.35 Herrmann et al. prepared iron-based NPs, by systematically modifying the degree of reduction during flame spray synthesis under a controlled atmosphere; meanwhile, the authors demonstrated that the carbon shell could be covalently surface functionalized with chloro, nitro, and amino groups.36 However, these approaches result in unstructured material, a high amount of impurities, or complicated procedures.
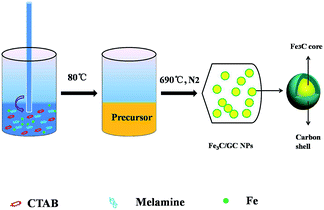
This work reports a facile route for the synthesis of Fe3C/GC NPs, that involved the reaction of FeCl3·6H2O with melamine and CTAB under mild temperatures (up to 690 °C). The Fe3C/GC NPs exhibited ferrimagnetic behavior at room temperature, with high value of Ms (61.5 emu g−1). When the Fe3C/GC NPs are employed to remove organic dyes such as methylene blue (MB) from contaminated water, they exhibited excellent adsorption properties.
Experimental
Synthesis of the precursors
All of the reagents were obtained from commercial sources and used as received without further purification. First, FeCl3·6H2O (0.01 mol), CTAB (0.008 mol) and melamine (0.02 mol) were added into 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol/distilled water in any order (distilled water, 40 mL and ethanol, 40 mL) in a small beaker. Immediately, the beaker was transferred to an 80 °C water bath, stirring was needed. During this process, the mixture became drier (more viscous). Three hours later, the mixture was dried in the oven at 80 °C under an air atmosphere for 12 hours. Finally, the precursor was collected from the beaker and the ground up into a fine powder by an agate mortar and pestle.
1 ethanol/distilled water in any order (distilled water, 40 mL and ethanol, 40 mL) in a small beaker. Immediately, the beaker was transferred to an 80 °C water bath, stirring was needed. During this process, the mixture became drier (more viscous). Three hours later, the mixture was dried in the oven at 80 °C under an air atmosphere for 12 hours. Finally, the precursor was collected from the beaker and the ground up into a fine powder by an agate mortar and pestle.
Synthesis of Fe3C/GC NPs
A certain amount of the above prepared precursor was loaded into an alumina boat. After that, put the boat into the center of a quartz glass tube. Then, the tube was placed into a horizontal electric tube furnace. A thermocouple was used to measure its exact temperature. High-purity nitrogen was introduced into the tube at a controlled flow rate. The furnace was heated at 20 °C min−1 to 690 °C, kept for three hours and cooled to room temperature naturally. Finally, the black powders were collected from the alumina boat and ground up into a fine powder. The preparation is illustrated in Scheme 1.Adsorption experiments
Adsorption experiments were carried out in a small vial. 8 mg of Fe3C/GC NPs were added into 20 mL of MB aqueous solution (the concentrations were between 2 and 300 mg L−1). Next, the vial with suspension was transferred into an ultrasonic bath at the room temperature. UV-vis spectra were measured at various times after removing the MB adsorbed Fe3C/GC NPs. The corresponding data were baseline subtracted. The amount of MB adsorbed at equilibrium qe (mg g−1) was calculated by the following equation:
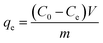 | (1) |
Characterization
The X-ray powder diffraction (XRD) patterns were collected on a Shimadzu diffractometer with Cu Kα radiation (λ = 0.15405 nm). The samples were examined to obtain size/shape images of nanostructured materials by and Philips Tecnai G2 F20 High Resolution Transmission Electron Microscopy (HRTEM). Raman spectrum was measured with a JobinYvon/HORIBA LabRam ARAMIS Raman spectrometer with the radiation from an air-cooled HeNe laser (633![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm). Thermogravimetric Analysis (TGA) measurement was carried out using a DTG-60 (Shimadzu). A certain amount of Fe3C/GC NPs were heated from 25 to 800 °C under an air atmosphere. A vibrating sample magnetometer (VSM, LakeShore 7404) was used to characterize the magnetic properties of as-synthesized samples. The magnetic hysteresis curve was obtained by changing H between +18
nm). Thermogravimetric Analysis (TGA) measurement was carried out using a DTG-60 (Shimadzu). A certain amount of Fe3C/GC NPs were heated from 25 to 800 °C under an air atmosphere. A vibrating sample magnetometer (VSM, LakeShore 7404) was used to characterize the magnetic properties of as-synthesized samples. The magnetic hysteresis curve was obtained by changing H between +18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe and −18
000 Oe and −18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. A Micromeritics ASAP 2020 adsorption porosimeter was used to measure the N2 adsorption isotherms at 77 K. Finally, a spectrophotometer (Maya 2000 Pro, Ocean Optics) was used to analysis the adsorption properties of Fe3C/GC NPs.
000 Oe. A Micromeritics ASAP 2020 adsorption porosimeter was used to measure the N2 adsorption isotherms at 77 K. Finally, a spectrophotometer (Maya 2000 Pro, Ocean Optics) was used to analysis the adsorption properties of Fe3C/GC NPs.
Results and discussion
Structure and morphology of Fe3C/GC NPs
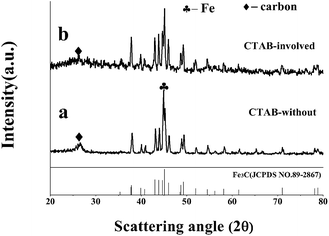
This article reports a procedure for synthesis of Fe3C/GC NPs using CTAB as the template. Fig. 1 shows the XRD patterns of two samples prepared with (b) and without (a) CTAB.In Fig. 1(a), the peaks at around 2θ = 45.0°, 44.6°, 44.3°, can be indexed to Fe3C (JCPDS no. 89-2867) and the peak at around 2θ = 44.7° can be indexed to Fe (JCPDS no. 3-1050). The peak at around 2θ = 26°, can be indexed to carbon (JCPDS no. 41-1487). This indicates that superfluous Fe was created and some melamine decomposed to form carbon in the absence of CTAB. During this process, Fe and Fe3C NPs are coexisting phases (denoted as Fe/Fe3C/GC). From Fig. 1(b) we can see that more than ten characteristic peaks can be perfectly indexed to Fe3C, and no other peaks exist excepting graphite carbon, which indicates that single phase Fe3C was synthesized and the appropriate amount of CTAB was the key factor in the conversion of Fe to Fe3C in this process. It can be concluded that CTAB was necessary to form the Fe3C/GC NPs.
TEM and HRTEM are performed to investigate the detailed information about the morphology of the samples.
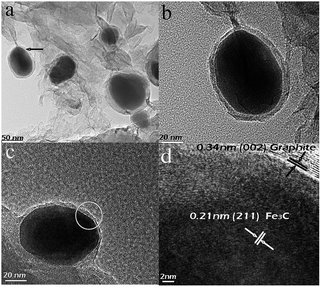
Fig. 2(a) shows the Fe3C/GC NPs embedded in an amorphous carbon. A typical higher-magnification image of a particle is shown in Fig. 2(b), which indicates that the particle has a core–shell structure and the size of the particle is about 63 nm. Fig. 2(c) represents an enlarged image of an isolated encapsulated nanoparticle. The size of the particle is 61 nm. The image clearly indicates that the quasi-spherical nanoparticle has a core–shell structure with a complete and tight encapsulation. Fig. 2(d) shows the HRTEM image of the circle region in Fig. 2(c). It is confirmed that the Fe3C nanoparticle is encapsulated by a crystalline shell. It also can be seen that the well-crystallized structures with lattice fringes of about 0.34 and 0.21 nm, corresponding to an interplanar spacing of graphite (002) and Fe3C (211) crystal plane, respectively.
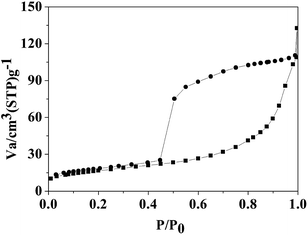
The N2 sorption isotherm of the Fe3C/GC NPs is shown in Fig. 3.
Brunauer–Emmet–Teller (BET) analysis yields a surface area of 60.2 m2 g−1. This is the characteristic of type IV isotherm, which indicates that the existed of mesopores without a significant fraction of micropores.37 Most of the mesopores should be in the amorphous carbon.38,39 It also can be confirmed by the hysteresis loop that the unrestricted adsorption in the high pressure regime and the increased gas uptake at P/P0 > 0.90 indicates that there may be coexistence of slit-like and bottle-like pores. The mesopores may be emptied by the cavitation mechanism, as indicated by the spontaneous desorption at P/P0 ∼ 0.45.38 In addition, the pore-blocking effect may be also exist.40
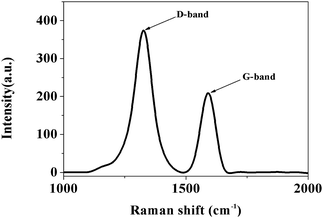
In the Raman spectrum (Fig. 4), there are two peaks at 1600 cm−1 and at 1328 cm−1. The peak at 1600 cm−1 can be indexed to graphitic carbon, and the peak at 1328 cm−1 can be indexed to amorphous carbon,41,42 which indicates that both graphite carbon and amorphous carbon are exist in Fe3C/GC NPs, the result is consistent with the XRD pattern and TEM images analysis. Furthermore, this is also consistent with previous reports claiming the coexistence of amorphous carbon and graphitic carbon on the iron carbide surface.43,44
To quantify the content of carbon in Fe3C/GC NPs, thermogravimetric analysis (TGA) was performed on a sample (Fig. 5). Any weight reduction between 200 and 485 °C should be ascribed to dehydration and the decomposition of functional groups adsorbed on the surface of carbon; weight reductions between 485 and 710 °C should be ascribed to the oxidation of carbon and Fe3C. The carbon became CO2 gas and Fe3C nanoparticles were oxidized into Fe2O3. The sample was pure, therefore, the carbon loading (a%) can be calculated by the following eqn (2).
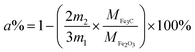 | (2) |
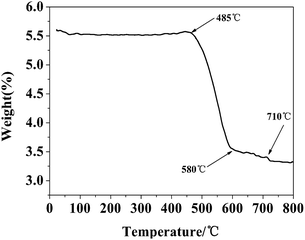 | ||
| Fig. 5 TGA curve of Fe3C/GC NPs measured from 25 to 800 °C in atmospheric air with a heating rate of 20 °C min−1. | ||
The m1 is the mass of Fe3C/GC NPs and m2 is the mass of residual Fe2O3. MFe3C and MFe2O3 is the molecular mass of Fe3C and Fe2O3, respectively. It is concluded that the carbon content is 56%.
Magnetic properties
The magnetization hysteresis loops obtained with a maximum field of ±18 kOe, for the Fe3C/GC NPs are shown in Fig. 6, which displays ferrimagnetic behavior. Fig. 6(a) shows that the measured maximum Ms of the without CTAB samples is 82.1 emu g−1, the remanent magnetization (Mr) value is 3.09 emu g−1 and he coercivity (Hc) value is 122 Oe. Fig. 6(b) shows that the measured Ms of the Fe3C/GC NPs is 61.5 emu g−1. The measured maximum Ms of the sample is much lower than reported bulk Fe3C (140 emu g−1),45 indicating that the reduction of the nanoparticles' Ms value due to the formation of a surface shell and the small particle size. The picture in the lower right corner of Fig. 6(b) demonstrates that the magnetic nanoparticles can be easily recovered from suspensions by applying a magnetic field induced by a neodymium permanent magnet placed next to the vial. Fig. 6(c) shows the Mr and Hc values of Fe3C/GC NPs, the Mr value is 4.32 emu g−1 and the Hc value is 186 Oe.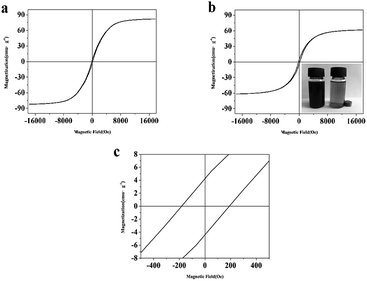 | ||
| Fig. 6 M–H plots of Fe3C/GC NPs (a) and Fe/Fe3C/GC NPs (b). Inset in (b) shows the Fe3C/GC NPs are easily recovered from suspensions. (c) Magnification image of (b). | ||
Adsorption properties
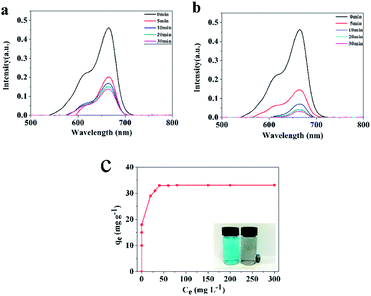
The application of Fe/Fe3C/GC and Fe3C/GC NPs as adsorbents for the separation of waste from aqueous solutions was investigated using MB as waste. UV-vis spectra measured at various times after mixing a MB aqueous solution and adsorbents revealed a fast adsorption capacity of Fe/Fe3C/GC and Fe3C/GC NPs. It took approximately 30 min to reach equilibrium, as shown in the adsorption curves in Fig. 7(a) and (b).Finally, MB was clearly and easily removed from the aqueous solution by using the magnetic properties of Fe3C/GC NPs, as shown in a photograph within Fig. 7(c). The adsorption isotherm of MB on Fe3C/GC NPs is obtained with different initial concentrations of MB (Fig. 7(c)). The adsorption capacity of MB on Fe3C/GC NPs is calculated to be 33.1 mg g−1.
Conclusions
In summary, magnetic Fe3C/GC NPs were fabricated. In the synthetic procedure, the introduction of CTAB was found to be the template for the generation of mesoporous carbon and conversion of Fe to Fe3C in the synthetic process. The Fe3C/GC NPs exhibit ferrimagnetic behavior at room temperature, with high value of Ms (61.5 emu g−1). Furthermore, Fe3C/GC NPs have a large number of mesopores and possess a surface area of 60.2 m2 g−1. They were investigated as adsorbent for the separation of waste from aqueous solutions using methylene blue as waste. The adsorption capacity of MB on Fe3C/GC NPs was calculated a 33.1 mg g−1. Generally, this study not only provided a facile method for the synthesis of Fe3C/GC NPs but also proposed a scheme could be extended to prepare many other important graphite carbon-encapsulated metal nanomaterials.Acknowledgements
This work was supported by the National Natural Science Foundation of China.Notes and references
- S. Wu, P. Wang, Y. Cai, D. Liang, Y. Ye, Z. Tian, J. Liu and C. Liang, RSC Adv., 2015, 5, 9069–9074 RSC.
- A. Maleki, R. Rahimi, S. Maleki and N. Hamidi, RSC Adv., 2014, 4, 29765–29771 RSC.
- M. Biswas, A. Saha, M. Dule and T. K. Mandal, J. Phys. Chem. C, 2014, 118, 22156–22165 CAS.
- S. Yu, X. Gao, H. Jing, R. Zhang, X. Gao and H. Su, CrystEngComm, 2014, 16, 6645–6653 RSC.
- V. M. Khot, A. B. Salunkhe, N. D. Thorat, R. S. Ningthoujam and S. H. Pawar, Dalton Trans., 2013, 1249–1258 RSC.
- J. Isaad and A. El Achari, Dyes Pigm., 2013, 99, 878–886 CrossRef CAS PubMed.
- C. Yang, H. Zhao, Y. Hou and D. Ma, J. Am. Chem. Soc., 2012, 134, 15814–15821 CrossRef CAS PubMed.
- X. Zhao, D. Xia, J. Yue and S. Liu, Electrochim. Acta, 2014, 116, 292–299 CrossRef CAS PubMed.
- D. Saini, R. P. Chauhan and S. Kumar, J. Mater. Sci.: Mater. Electron., 2014, 25, 124–127 CrossRef CAS PubMed.
- B. H. X. Che, S. P. Yeap, A. L. Ahmad and J. Lim, Chem. Eng. J., 2014, 243, 68–78 CrossRef CAS PubMed.
- K. Lin, L. Chen, P. Liu, Z. Zou, M. Zhang, Y. Shen, Y. Qiao, X. Liu and J. Chang, CrystEngComm, 2013, 15, 2999–3008 RSC.
- X. L. Dong, Z. D. Zhang, S. R. Jin and B. K. Kim, J. Appl. Phys., 1999, 86, 6701–6706 CrossRef CAS PubMed.
- S. Yang, L. Wang, S. Yue, Y. Lu, J. He and D. Zhao, Dalton Trans., 2014, 43, 8254–8260 RSC.
- L. Ma, X. Shen, G. Zhu, Z. Ji and H. Zhou, Carbon, 2014, 77, 255–265 CrossRef CAS PubMed.
- E. E. Carpenter, S. Calvin, R. M. Stroud and V. G. Harris, Chem. Mater., 2003, 15, 3245–3246 CrossRef CAS.
- D. Sarkar, G. G. Khan, A. K. Singh and K. Mandal, J. Phys. Chem. C, 2013, 117, 15523–15531 CAS.
- Y. Zhang, L. Li, W. Ma, Y. Zhang, M. Yu, J. Guo, H. Lu and C. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 614–621 CAS.
- Y. Liao, K. Pan, L. Wang, Q. Pan, W. Zhou, X. Miao, B. Jiang, C. Tian, G. Tian, G. Wang and H. Fu, ACS Appl. Mater. Interfaces, 2013, 5, 3663–3670 CAS.
- M. Andrés-Vergés, M. del Puerto Morales, S. Veintemillas-Verdaguer, F. J. Palomares and C. J. Serna, Chem. Mater., 2011, 24, 319–324 CrossRef.
- H. L. Ding, Y. X. Zhang, S. Wang, J. M. Xu, S. C. Xu and G. H. Li, Chem. Mater., 2012, 24, 4572–4580 CrossRef CAS.
- F. Chen, Q. Chen, S. Fang, Y. A. Sun, Z. Chen, G. Xie and Y. Du, Dalton Trans., 2011, 40, 10857–10864 RSC.
- S. Zhu, D. Zhang, Z. Chen and Y. Zhang, J. Mater. Chem., 2009, 19, 7710–7715 RSC.
- D. Li, W. Y. Teoh, C. Selomulya, R. C. Woodward, R. Amal and B. Rosche, Chem. Mater., 2006, 18, 6403–6413 CrossRef CAS.
- M. Li, X. Chen, J. Guan, X. Wang, J. Wang, C. T. Williams and C. Liang, J. Mater. Chem., 2012, 22, 609–616 RSC.
- C. Pereira, A. M. Pereira, P. Quaresma, P. B. Tavares, E. Pereira, J. P. Araujo and C. Freire, Dalton Trans., 2010, 39, 2842–2854 RSC.
- S.-J. Cho, J.-C. Idrobo, J. Olamit, K. Liu, N. D. Browning and S. M. Kauzlarich, Chem. Mater., 2005, 17, 3181–3186 CrossRef CAS.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. Sun, Nano Lett., 2005, 5, 379–382 CrossRef CAS PubMed.
- J. Gong, J. Liu, X. Chen, Z. Jiang, X. Wen, E. Mijowska and T. Tang, J. Mater. Chem. A, 2014, 2, 7461–7470 CAS.
- Q. Li, Z. Zhang, Z. Guo, Y. Lai, K. Zhang and J. Li, Carbon, 2014, 78, 1–9 CrossRef CAS PubMed.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- F. Zhang, L. Cui, K. Lin, F.-M. Jin, B. Wang, S.-X. Shi, D.-A. Yang, H. Wang, F. He, X.-P. Chen and S. Cui, J. Alloys Compd., 2013, 553, 367–374 CrossRef CAS PubMed.
- T. Hayashi, S. Hirono, M. Tomita and S. Umemura, Nature, 1996, 381, 772–774 CrossRef.
- Y. Lu, Z. Zhu and Z. Liu, Carbon, 2005, 43, 369–374 CrossRef CAS PubMed.
- J. Zhu, S. Pallavkar, M. Chen, N. Yerra, Z. Luo, H. A. Colorado, H. Lin, N. Haldolaarachchige, A. Khasanov, T. C. Ho, D. P. Young, S. Wei and Z. Guo, Chem. Commun., 2013, 49, 258–260 RSC.
- N. Luo, X. Li, X. Wang, H. Yan, C. Zhang and H. Wang, Carbon, 2010, 48, 3858–3863 CrossRef CAS PubMed.
- I. K. Herrmann, R. N. Grass, D. Mazunin and W. J. Stark, Chem. Mater., 2009, 21, 3275–3281 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- Z.-L. Xie, X. Huang, M.-M. Titirici and A. Taubert, RSC Adv., 2014, 4, 37423–37430 RSC.
- H. J. Lee, W. Cho, E. Lim and M. Oh, Chemical Commun., 2014, 50, 5476–5479 RSC.
- P. T. M. Nguyen, C. Fan, D. D. Do and D. Nicholson, J. Phys. Chem. C, 2013, 117, 5475–5484 CAS.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS.
- H. Fjellvaag and P. Karen, Inorg. Chem., 1992, 31, 3260–3263 CrossRef CAS.
- N. B. Jackson, A. K. Datye, L. Mansker, R. J. O'Brien and B. H. Davis, in Studies in Surface Science and Catalysis, ed. C. H. Bartholomew and G. A. Fuentes, Elsevier, 1997, vol. 111, pp. 501–516 Search PubMed.
- J. Xu and C. H. Bartholomew, J. Phys. Chem. B, 2004, 109, 2392–2403 CrossRef PubMed.
- E. P. Sajitha, V. Prasad, S. V. Subramanyam, M. Ajay Kumar, S. Subhajit and B. Chandrahaas, J. Phys.: Condens. Matter, 2007, 19, 046214 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |