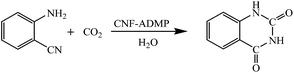
Amino-functionalized carbon nanofibres as an efficient metal free catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitriles†
Subodh Kumara,
Sanny Vermaa,
Efrat Shawatb,
Gilbert Daniel Nessimb and
Suman L. Jain*a
aChemical Sciences Division, CSIR-Indian Institute of Petroleum, Dehradun-248005, India. E-mail: suman@iip.res.in; Fax: +91-135-2660202; Tel: +91-135-2525788
bDepartment of Chemistry, Bar Ilan Institute for Nanotechnology and Advanced Materials (BINA), Bar Ilan University, Ramat Gan, 52900, Israel
First published on 2nd March 2015
Abstract
Carbon nanofibres (CNFs) functionalized with 4-amino-2,6-dimethylpyridine were found to be an efficient metal free catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitriles using water as a reaction medium. The presence of water they exhibited a remarkable enhancement in reaction rates and provided high to excellent yield of the products. After completion of the reaction, the catalyst was easily separated by centrifugation and was successfully reused for several runs without showing any significant decrease in catalytic activity.
Carbon dioxide is the major greenhouse gas responsible for global warming. It is considered to be an ideal C1 feedstock as it is abundant, non-toxic, non-flammable, easily available, and a renewable carbon source.1 The chemical fixation of carbon dioxide to useful chemicals has received tremendous interest in recent decades.2 In this regard, the development of catalytic methodologies to activate carbon dioxide and the resultant formation of valuable products constitutes a good example of sustainable green chemistry. Quinazolines which belong to the N-containing heterocyclic compounds have attracted considerable interest due to their wide and distinct pharmaceutical activities.3 Derivatives of quinazolines such as quinazoline-2,4(1H,3H)-diones are also considered to be an important class of pharmaceutical intermediates.4 The conventional synthesis of these compounds involves the reaction of anthranilic acid derivatives with urea, anthranilamides with phosgene, anthranilic acid with potassium cyanate or chlorosulfonyl isocyanate.5 However, these conventional methodologies are associated with the obvious drawbacks of using specific or/toxic reagents like phosgene.
Thus, the use of carbon dioxide for the synthesis of quinazoline-2,4(1H,3H)-diones has attracted considerable interest in recent years. Ma et al.6 reported the synthesis of quinazoline-2,4(1H,3H)-diones from the reaction of carbon dioxide and 2-aminobenzonitrile in water without using any catalyst. Subsequently, a number of basic catalysts including organic bases, metal oxides and basic ionic liquids have been reported for the synthesis of such compounds.7 Among them, organic bases owing to their higher basicity are of good choice; however, difficult recovery owing to the homogeneous nature of these bases limited the synthetic utility of such processes. Immobilization of organic base moieties to solid supports is one of the logical approaches to transform these homogeneous catalysts to heterogeneous ones. In this regard, Zhao et al.7a reported magnetic base catalysts for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2. Nagai et al.8 reported a polystyrene derivative bearing amidine moieties for the synthesis of quinazoline-2,4(1H,3H)-diones by the reaction of 2-aminobenzonitriles with carbon dioxide. Recently, Zhao et al.9 reported the synthesis of quinazoline-2,4-(1H,3H)-diones from CO2 by using a 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) based protic ionic liquid at atmospheric pressure and room temperature. However, highly energy intensive product recovery from ionic liquid as well as longer reaction times is the major drawbacks of this methodology.
Cluster based carbon materials such as carbon nanotubes (CNTs) and carbon nanofibres (CNFs), which exhibit outstanding physical, electronic, and mechanical properties, can be synthesized in various morphologies and structures.10 In recent years, great interest is being paid towards the development of versatile chemical modification methodologies to obtain carbon nanostructure derivatives with more attractive features.11 In this regard, a number of derivatives have been prepared and fully characterized that exhibit promising applications in nano-biotechnology and catalysis.11,12
In the present paper we report for the first time carbon nanofibres functionalized with 4-amino-2,6-dimethylpyridines (CNFs-ADMP) as efficient metal free catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from the reaction of 2-aminobenzonitrile with CO2 in aqueous media (Scheme 1). In this work, we synthesized CNFs, from CNF mats obtained using a high-yield synthesis process, which is described in detail elsewhere.13
Further functionalization of CNFs to obtain 4-amino-2,6-dimethylpyridine-functionalized carbon nanofibres (CNFs-ADMP) was carried out via diazotization reaction as shown in Scheme 2.14 In a typical synthesis, 50 mg of purified CNFs were dispersed in 100 ml N,N-dimethylformamide using bath sonicator for 2 h and probe sonicator for 30 min. The resulting CNFs solution was cooled to 0 °C followed by the addition of NaNO2 and 4-amino-2,6-dimethylpyridine (ADMP) drop wise. The mixture was maintained at 0 °C for 4 h and then stirred at room temperature for 20 h. The resulting product (CNFs-ADMP) was collected by centrifugation, washing with 2 M HCl, 2 M NaOH, water, and then dried at 80 °C under vacuum for 12 h.‡
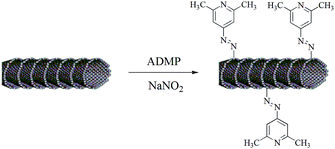 | ||
| Scheme 2 Synthesis of carbon nanofibres functionalized with 4-amino-2,6-dimethylpyridines (CNFs-ADMP). | ||
Molecular structural changes occurred during the chemical functionalization of CNFs with pyridyl moieties to give CNF-ADMP were investigated by FTIR spectroscopy (Fig. 1a). As shown in Fig. 1b, the peaks locating at 3300–3600 cm−1 related to the hydroxyl groups were remarkably decreased. In addition bands at 2900 cm−1 and 1585 cm−1 corresponding to the aromatic C–H and C–C ring stretching, respectively were found to be reduced or disappeared completely after the association with pyridine molecules. The successful loading of pyridyl moieties to the CNFs was further confirmed by the appearance of two characteristic bands at 1442 and 1080 cm−1, which are corresponding to the vibration –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and C–N respectively. In addition, bands at 870 and 625 cm−1 corresponds to the C–H out of plane deformation and in plane ring deformation of pyridyl moiety respectively.
N– and C–N respectively. In addition, bands at 870 and 625 cm−1 corresponds to the C–H out of plane deformation and in plane ring deformation of pyridyl moiety respectively.
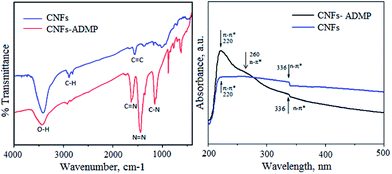 | ||
| Fig. 1 (a) FT-IR spectra; (b) UV/Vis spectra of carbon nanofibre CNFs and carbon nanofibres functionalized with 4-amino-2,6-dimethylpyridines, CNFs-ADMP. | ||
The UV-Vis spectrum of CNFs in water shows an intense peak at 220 nm due to π → π* transitions of small domains of sp2 hybrid carbons (Fig. 1b). However, in case of CNF-ADMP, the increased intensity of this peak can be attributed to the sp2 carbons of pyridine moiety (Fig. 1b). In addition, appearance of new shoulder at 260 nm is attributed to the n → π* transitions due to the lone pair electrons on the nitrogen of pyridine. Another peak at 336 nm in both the spectra is probably due to the presence of some C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups causing n → π* transitions. These studies further confirmed that the pyridyl moieties have been successfully grafted to the CNF support.
O groups causing n → π* transitions. These studies further confirmed that the pyridyl moieties have been successfully grafted to the CNF support.
The morphology of the synthesized CNFs-ADMP was determined by combined study of SEM and TEM analyses (Fig. 2). The individual carbon nanofibre has a hollow core surrounded by the cylindrical fibre, along a longitudinal axis, comprised of graphite basal planes stacked forming stacked cup shape (Fig. 2a and b).15 Although, longitudinal axis is clearly visible after the chemical functionalization but hollow core surrounded by the cylindrical fiber for the single carbon nano-fiber cannot be clearly seen, suggesting some change in the morphology of CNFs after the chemical functionalization in pyridyl units (Fig. 2b and d).
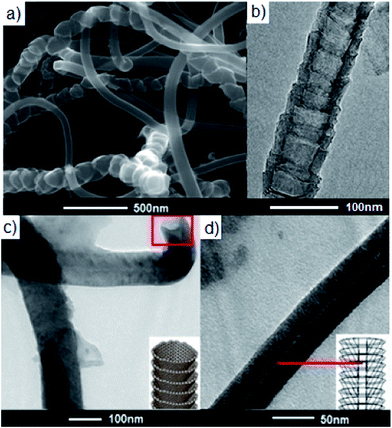 | ||
| Fig. 2 (a) SEM image; (b) HRTEM image of carbon nanofibres, CNFs; (c) and (d) HRTEM images of carbon nanofibres functionalized with 4-amino-2,6-dimethylpyridines, CNFs-ADMP. | ||
Raman spectra of CNFs and CNFs-ADMP were also recorded to study the degree of functionalization. As shown in Fig. 3a, two fundamental vibrations D band and G band are observed at 1570 and 1342 cm−1 respectively. D band is attributed to the presence of disorder or defects in the sp2 graphitic structure while G band corresponds to ordered graphitic structure. The intensity ratio of D to G band (ID/IG) of the carbon nanofibres was found to be significantly increased because of the lowering the symmetry of the quasi-infinite lattice due to the diazotization reaction, suggesting the high degree of covalent functionalization of carbon nanofibres with pyridyl moiety. For a visual examination, CNFs and CNFs-ADMP were dispersed in water by sonication for 5 min (Fig. 3b). The CNFs dispersed in water began to flocculate within 5 min after sonication (Fig. 3b(A)). In contrast, CNFs-ADMP dispersed in water did not show any flocculation even after 24 h of sonication (Fig. 3b), which further confirmed the successful functionalization of CNFs.
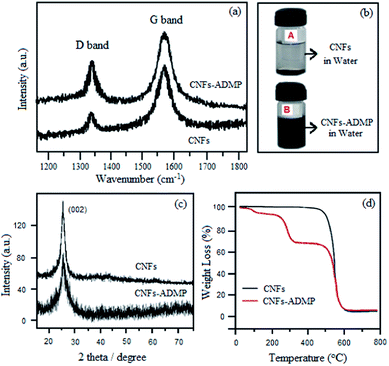 | ||
| Fig. 3 (a) Raman spectra; (b) digital photograph; (c) XRD pattern; (d) TGA graph of carbon nanofibre CNFs and carbon nanofibres functionalized with 4-amino-2,6-dimethylpyridines, CNFs-ADMP. | ||
The crystalline structure of synthesized CNFs and CNF-ADMP was determined by XRD analysis (Fig. 3c). The profile of (002) reflection at 2θ = 26° in the CNFs indicates that the material is semi crystalline in nature.16
The thermal stability of synthesized CNFs and CNF-ADMP was determined by TGA analysis (Fig. 3d). For CNFs, the degradation occurs at about 550 °C. Whereas a three step thermal degradation can be observed for CNF-ADMP. The initial weight loss at 100 °C is probably due to the evaporation of the water trapped in the material. The second weight loss at around 280 °C (30%) is attributed to the thermal decomposition of the organic moieties located on the surface of CNFs. Third stage weight loss occurs at 550 °C which is related to the decomposition of CNFs. The variation in thermal events proved the formation of a new material.
The content of ADMP moieties on CNFs was calculated from the value of nitrogen content as determined by elemental analysis. The loading of ADMP on CNFs based on the nitrogen content (8.7%) was found to be 2.1 mmol g−1. This value is also found to be in good agreement with the TGA results.
The synthesized carbocatalyst was tested for the synthesis of quinazoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzonitrile using water as solvent at 100 °C under 20 bar pressure of CO2.
In an effort to find the best catalytic system, various catalysts (2 mol%) such as CNF, CNF-ADMP and ADMP were screened using different solvents water, DMF and DMSO. The results of these experiments are summarized in Table 1. To our delight, CNF immobilized ADMP catalyst showed high efficiency for this reaction using water as the reaction media (Table 1, entry 3). The reaction was found to be very slow in the absence of any catalyst or using CNFs as catalyst (Table 1, entry 1 and 2). The use of ADMP and physical mixture of CNFs and ADMP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as catalyst provided lower yield of the product (Table 1, entry 6 and 7). These results suggested that the covalent grafting of ADMP moieties to CNFs had a significant effect on catalytic activity (Table 1, entry 3). Importantly, the reaction did not occur when other solvents such as DMF and DMSO were used instead of water (Table 1, entry 4 and 5).
1) as catalyst provided lower yield of the product (Table 1, entry 6 and 7). These results suggested that the covalent grafting of ADMP moieties to CNFs had a significant effect on catalytic activity (Table 1, entry 3). Importantly, the reaction did not occur when other solvents such as DMF and DMSO were used instead of water (Table 1, entry 4 and 5).
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 2-aminobenzonitrile (5 mmol), solvent (5 ml), catalyst (2 mol%, 0.05 g) at 100 °C and 20 bar pressure of CO2 for 10 h.b Determined by GC-MS. | |||
| 1 | — | H2O | 10 |
| 2 | CNFs | H2O | 30 |
| 3 | CNFs-ADMP | H2O | 91 |
| 4 | CNFs-ADMP | DMF | — |
| 5 | CNFs-ADMP | DMSO | — |
| 6 | ADMP | H2O | 39 |
| 7 | CNFs + ADMP | H2O | 39 |
The effect of other parameters, such as reaction temperature, CO2 pressure and reaction time was also investigated (Fig. 4).
At ambient temperature (25 °C), the reaction rate was found to be slow and depended on the CO2 pressure applied. The yield of the product was found to be increased with temperature at 2.0 MPa (20 bar) CO2 pressure. It was found that efficient and selective conversion of 2-amionobenzonitrile to quinazoline-2,4(1H,3H)-diones could be achieved at 100 °C and 2.0 MPa pressure of CO2 (Fig. 4a and b). Further increase in temperature and CO2 pressure did not affect the catalytic activity to any significant extent. Similarly, the reaction was found to increase with reaction time at 100 °C and 2.0 MPa pressure of CO2 and showed the highest yield in 10 h (Fig. 4c). Further increase in reaction time did not improve the results to any significant extent (Fig. 4c). Furthermore, the reusability of the CNF-ADMP catalyst was examined for subsequent six runs under described experimental conditions (Fig. 4d). The yield of quinazoline-2,4(1H,3H)-diones was found to be almost same in all cases, indicating that the catalyst is highly stable and can be recycled for several runs with consistent catalytic activity. These results clearly indicated the merits of the developed heterogeneous catalyst over the homogeneous organic base catalysts and use of water as reaction media make this method more attractive from green chemistry viewpoints.
Once the optimal conditions for this reaction were established, we looked at examining the utility of this methodology. Thus, the scope of the reaction was tested with respect to other substituted benzonitriles and the results of these experiments are summarized in Table 2. Among the various substituents, electron-donating substituents provided higher product yields (Table 2, entry 3 and 4). However, the reaction was found to be slow in substrates having electron withdrawing groups (Table 2, entry 5 and 6).
In order to establish the utility of the developed method in large scale synthesis, we have performed the reaction by using 50 mmol of 2-aminobenzonitrile using 2 mol% of catalyst under otherwise identical experimental conditions (Table 2, entry 2). The yield of the desired quinazoline-2,4(1H,3H)-dione was found to be comparable, indicating that the developed method could be equally applicable for the large scale synthesis of these compounds. All the experiments were carried out in a stainless steel (15 ml) autoclave equipped with a stirrer. After completion of the reaction, the reaction mixture was cooled to room temperature and the heterogeneous catalyst was easily recovered by centrifugation. The obtained aqueous layer was concentrated under reduced pressure to give crude product. The crude product was washed with methyl-tert-butyl-ether (MTBE) to give the desired quinazoline-2,4(1H,3H)-dione.
In summary, we have demonstrated for the time the use of chemically functionalized carbon nanofibers as efficient carbocatalyst for the reaction of carbon dioxide with 2-aminobenzonitrile to yield quinazoline-2,4(1H,3H)-diones. The main advantage of the developed catalyst is that there is no metal and high loading of pyridine moieties to CNFs via diazotization makes the developed system truly heterogeneous. Furthermore, facile recovery and efficient recycling of the catalyst make the developed methodology an ideal for utilization of carbon dioxide in a sustainable manner.
Acknowledgements
The authors, SK and SV thank to Director, CSIR-Indian Institute of Petroleum for granting permission to publish these results. We acknowledge CSIR, New Delhi for providing research fellowship. DST, New Delhi is kindly acknowledged for funding.Notes and references
- (a) A. Behr and G. Henze, Green Chem., 2011, 13, 25 RSC; (b) X. Han and M. Poliakoff, Chem. Soc. Rev., 2012, 41, 1428 RSC.
- (a) M. Aresta and A. Dibenedetto, Catal. Today, 2004, 98, 455 CrossRef CAS PubMed; (b) M. Aresta, Carbon Dioxide as Chemical Feedstock, Wiley-VCH, Weinheim, 2010 Search PubMed; (c) D. Chaturvedi, A. K. Chaturvedi and V. Mishra, Curr. Org. Chem., 2012, 16, 1609 CrossRef CAS.
- (a) S. Sun, C. Cheng, J. Yang, A. Taheri, D. Jiang, B. Zhang and Y. Gu, Org. Lett., 2014, 16, 4520 CrossRef CAS PubMed; (b) S. Sun, R. Bai and Y. Gu, Chem.–Eur. J., 2014, 20, 549 CrossRef CAS PubMed; (c) D. Wang and F. Gao, Chem. Cent. J., 2013, 7, 95 CrossRef PubMed.
- (a) M. B. Andrus, S. N. Mettath and C. Song, J. Org. Chem., 2002, 67, 8284 CrossRef CAS PubMed; (b) E. Mounetou, J. Legault, J. Lacroix and R. C. Gaudreault, J. Med. Chem., 2001, 44, 694 CrossRef CAS PubMed; (c) S. Goto, H. Tsuboi and K. Kagara, Chem. Express, 1993, 8, 761 CAS; (d) S. Mohri, J. Synth. Org. Chem., Jpn., 2001, 59, 514 CrossRef CAS.
- (a) M. C. Willis, R. H. Snell, A. J. Fletcher and R. L. Woodward, Org. Lett., 2006, 8, 5089 CrossRef CAS PubMed; (b) H. Vorbruggen and K. Krolikiewicz, Tetrahedron, 1994, 50, 6549 CrossRef CAS; (c) D. Q. Shi, G. L. Dou, Z. Y. Li, S. N. Ni, X. Y. Li, X. S. Wang, H. Wu and S. J. Ji, Tetrahedron, 2007, 63, 9764 CrossRef CAS PubMed; (d) F. Nikpour and T. Paibast, Chem. Lett., 2005, 34, 1438 CrossRef CAS.
- J. Ma, B. Han, J. Song, J. Hu, W. Lu, D. Yang, Z. Zhang, T. Jiang and M. Hou, Green Chem., 2013, 15, 1485 RSC.
- (a) Y.-N. Zhao, B. Yu, Z.-Z. Yang and L.-N. He, RSC Adv., 2014, 4, 28941 RSC; (b) T. Mizuno, T. Iwai and Y. Ishino, Tetrahedron Lett., 2004, 45, 7073 CrossRef CAS PubMed; (c) Y. P. Patil, P. J. Tambade, K. M. Deshmukh and B. M. Bhanage, Catal. Today, 2009, 148, 355 CrossRef CAS PubMed; (d) Y. P. Patil, P. J. Tambade, K. D. Parghi, R. V. Jayaram and B. M. Bhanage, Catal. Lett., 2009, 133, 201 CrossRef CAS PubMed; (e) J. Gao, L. N. He, C. X. Miao and S. Chanfreau, Tetrahedron, 2010, 66, 4063 CrossRef CAS PubMed.
- D. Nagai and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 653 CrossRef CAS.
- Y. Zhao, B. Yu, Z. Yang, H. Zhang, L. Hao, X. Gao and Z. Liu, Angew. Chem., Int. Ed., 2014, 53, 5922 CrossRef CAS PubMed.
- (a) S. J. Trans, M. H. Devoret, A. Thess, R. E. Smalley, L. J. Geerligs and C. Dekker, Nature, 1997, 386, 474 CrossRef; (b) M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes, Academic Press Inc., London, 1996, ch.19 Search PubMed; (c) G. Che, B. B. Lakshmi, C. R. Martin and E. R. Fisher, Chem. Mater., 1998, 10, 260 CrossRef CAS; (d) K. P. De Jong and J. W. Geus, Catal. Rev., 2000, 42, 481 CrossRef CAS PubMed.
- H. Guang-Ping, O. Martin, N. Winfried, A. Marion and K. Stefan, Curr. Org. Chem., 2014, 18, 1262 CrossRef.
- (a) G. G. D. Nessim, Nano Lett., 2009, 9, 3398 CrossRef CAS PubMed; (b) K. P. De Jong and J. W. Geus, Catal. Rev.: Sci. Eng., 2000, 42, 481 CrossRef CAS PubMed; (c) G. D. Nessim, M. Seita, K. P. O'Brien and S. A. Speakman, Carbon, 2010, 48, 4519 CrossRef CAS PubMed; (d) E. Bekyarova, Y. Ni, E. B. Malarkey, V. Montana, J. L. McWilliams, R. C. Haddon and V. Parpura, J. Biomed. Nanotechnol., 2005, 1, 3 CrossRef CAS PubMed; (e) H. He, L. A. Pham-Huy, P. Dramou, D. Xiao, P. Zuo and C. Pham-Huy, BioMed Res. Int., 2013, 2013, 578290 Search PubMed.
- E. Shawat, I. Perelshtein, A. Westover, C. L. Pint and G. D. Nessim, J. Mater. Chem. A, 2014, 2, 15118 CAS.
- R. Cao, R. Thapa, H. Kim, X. Xu, M. G. Kim, Q. Li, N. Park, M. Liu and J. Cho, Nat. Commun., 2013, 4, 2076 Search PubMed.
- I. Martin-Gullon, J. Vera, J. A. Conesa, J. L. Gonzalez and C. Merino, Carbon, 2006, 44, 1572 CrossRef CAS PubMed.
- A. G. El-Deen, N. A. M. Barakat, K. A. Khalil and H. Y. Kim, New J. Chem., 2014, 38, 198 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01900a |
| ‡ Detailed experimental procedure for the preparation of CNFs-ADMP: functionalization of CNFs with 4-amino-2,6-dimethylpyridine (4-ADMP) groups was carried out by using diazonium reaction. Briefly, 2.45 g of NaNO2 was dissolved in 5 ml H2O, and then cooled to 0 °C by ice bath. In all, 3.29 g of 4-amino-2,6-dimethylpyridine (4-ADMP) was dissolved in 4 ml 4 M HCl, and then cooled to 0 °C by ice bath. The solution of NaNO2 was added drop wise to 4-ADMP solution. The resulting yellow solution was maintained at 0 °C in ice bath and stirred for 30 min. CNFs as synthesized from CNF mats were purified before use by being immersed in concentrated HCl solution for 5 days and then refluxed for 5 h, followed by thoroughly washing with water and acetone. The purified CNFs were dried overnight at 80 °C in vacuum oven for stock. Purified CNFs (50 mg) were dispersed in 100 ml N,N-dimethylformamide using bath sonicator for 2 h and probe sonicator for 30 min. The CNFs solution was cooled to 0 °C by ice bath, and then the yellow solution of NaNO2 and 4-ADMP was added drop wise and maintained at 0 °C to prevent the decomposition of diazonium salts. The mixture was reacted at 0 °C for 3 h and then was stirred at room temperature for 15 h. The resulting product (CNFs-ADMP) was collected by centrifugation and then thoroughly washed with 2 M HCl, 2 M NaOH, water and acetone. The CNFs-ADMP was dried overnight at 80 °C in vacuum oven. The content of ADMP moieties on CNFs was calculated from the value of nitrogen content as determined by elemental analysis. The loading of ADMP on CNFs based on the nitrogen content (8.7%) was found to be 2.1 mmol g−1. Typical experimental procedure for synthesis of quinazoline-2,4(1H,3H)-diones: 2-aminobenzonitrile (5 mmol) and catalyst CNF-ADMP (2 mol%, 0.05 g) were charged in to the 15 ml high pressure stainless steel reactor using water (5 ml) as a solvent. The reactor was sealed and purged with carbon dioxide to replace all others gases. Reactor was then initially charged with CO2 upto 20 bar pressure. The reaction mixture was stirred for 10 h at 100 °C. After this, the reactor was placed into ice water and unreacted CO2 gas was released slowly passing through a cold trap containing ethanol. After depressurization, the reaction mixture in the reactor was transferred in a beaker and the catalyst was separated by filtration. The obtained aqueous layer was concentrated under reduced pressure to give crude product. The crude product was washed with methyl-tert-butyl-ether (MTBE) to give 0.53 g (91%) colourless crystals of quinazoline-2,4(1H,3H)-dione (mp 348–349 °C). |
| This journal is © The Royal Society of Chemistry 2015 |