A high efficiency biological system for treatment of coal gasification wastewater – a key in-depth technological research†
Qinhong Jia,
Salma Tabassum*a,
Guangxin Yub,
Chunfeng Chua and
Zhenjia Zhang*a
aSchool of Environmental Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: ustb456@sjtu.edu.cn; salmazenith@gmail.com; zjzhang@sjtu.edu.cn; Fax: +86-021-54740836; Tel: +86-021-54747368 Tel: +86-152-21195745
bNew Energy Research Center, China National Offshore Oil Corporation Research Institute, Beijing 100027, China
First published on 7th April 2015
Abstract
Coal is the main energy resource in China, hence pollution caused by coal gasification wastewater has been severe for decades. A three stage system was adopted to treat coal gasification wastewater; anaerobic hydrolysis acidification (333 days), aerobic oxidation (300 days), and ozonation–aerobic fluidized bed process (220 days) with the lowest HRT of 45 h. After more than a year of trials, high efficiency and stability of the treatment process has been achieved and the results showed that with an average influent (COD 4400 mg L−1, total phenol 950 mg L−1, volatile phenol 530 mg L−1, NH4+–N 300 mg L−1, volatile acids 120 mg L−1 and high chromaticity color blow 1000 times), the effluent COD could decrease to <60 mg L−1, and total phenol, volatile phenol, NH4+–N, and volatile acids were not detected, and for the chromaticity, the higher color removal reached to 10 times showed an average removal efficiency of COD, total phenol, volatile phenol, NH4+–N and volatile acids of 96%, 99.9%, 99.9%, 99.9% and 99.9%, respectively. The pollutants removed were converted to biogas; organic transformations in the system were analysed by GC/MS equipment. The power consumption and the amount of sewage sludge were reduced by 30%. The wastewater treatment cost is 0.135 $ per m3. This study can be used to build a test to simulate future engineering applications of small scale technology platforms as it is a short, simple processing unit, with low energy consumption, low sludge production and easy management and maintenance.
1. Introduction
The stresses of environmental pollution, fossil-fuel depletion, water and other resource shortages are driving intensive efforts towards more sustainable treatment and utilization of wastewater. Indeed, while abating contamination continues to be an important task of wastewater treatment, sustainability is gradually becoming a pivotal criterion and is being driven to further its advancement.1 One of the most difficult wastewater pollution control tasks for coal gasification industries around the world is coal gasification wastewater (CGW) treatment.2CGW is discharged mainly from the gas washing and condensing operations of the coal gasifier, which contains high concentrations of organic pollutants.3 Basically, treatment of CGW mainly includes a series of biological treatments after a physico-chemical pre-treatment4,5 in order to reduce the concentration of phenols, ammonium6,7 and refractory organics; most of them have been reported to be carcinogenic and mutative.8,9 But still this dual process is confronted with several problems, like unsatisfactory effluent, complicated technology, high handling costs, and a large occupation of area.10 As well as absolute degradation of volatile phenol, degradation of total phenol and ammonia nitrogen is challenging. Even the reduction of effluent COD to a level below 200 mg L−1 remains difficult.11 Furthermore, one of the particular concerns is an increasing amount of sewage sludge generated by biological wastewater treatment plants, which would result in a serious problem for the environment in cases of inappropriate disposal.12
For the treatment of CGW certain traditional biochemical treatment processes have been deeply investigated, such as conventional activated sludge (CAS), sequencing batch reactor (SBR), anoxic–aerobic (A–O) and anaerobic–anoxic–aerobic (A–A–O) processes.13–16 The growth of nitrifying bacteria and specialized microbes in the aeration basin is restricted due to the presence of toxic and refractory compounds.17 Hence, the discharge standards of the effluent concentrations of COD, nitrogen compounds and so on are difficult to meet.18–21 Therefore, it is of great importance to enhance the CGW treatment process to pursue clean production.
It is very difficult to treat CGW by conventional biochemical treatment as the existing treatment processes are long, involve a complex processing unit, high energy treatment system, production of sludge, and maintenance of the processing system. It is a challenge to treat CGW around the globe.22 Nonetheless, coal gasification wastewater treatment does have the potential to become a sustainable process if suitable technologies can be applied that are operated using less energy, low operating costs and low investment.
In recent years, the use of aerobic granular sludge (AGS) technology, i.e. a special kind of biofilm structure composed of self immobilized cells, has been used as an aerobic system.23,24 But it has some drawbacks that have restricted the development of AGS technology from lab scale to pilot scale.13,25,26 The present study overcomes the weaknesses of AGS.
Anaerobic reactors sold worldwide during the last decade were Upflow Anaerobic Sludge Blanket (UASB) reactors (34%) and Expanded Granular Sludge Blanket (EGSB) reactors (52%).27–29 Both have some advantages over each other.30 The present study combines the advantages of UASB and EGSB technologies.
Anaerobic and aerobic biological treatment technologies have their advantages and disadvantages, and anaerobic–aerobic treatment processes can provide the advantage of separate processing technology, especially in refractory wastewater treatment.
In the current study we combine and extend the recent work of our lab;31,32 we build a high efficiency coal gasification wastewater treatment process in our laboratory that utilizes two patented water treatment technologies of our group AnaEG (a state-of-the-art advanced anaerobic expanded granular sludge bed)31,33 and BioAX (a novel environmental biotechnological aerobic process with internal circulation)32,34 along with an ozonation–aerobic fluidized bed (ABO + AF) system. A more elaborate efficiency of the whole system is employed that efficiently meets final effluent standards18,21 in spite of great fluctuation of water quality and the presence of bio-refractory organic pollutants. COD, phenols and ammonia removal were monitored at each stage of the treatment. An estimation of energy consumption and processing cost was also evaluated. The current study will become competitive in future industrial applications in terms of a technically and economically feasible method for the treatment of CGW.
2. Materials and methods
2.1 Characteristics of coal gasification wastewater
As seen in Table 1, CGW is highly contaminated which in turn has an inhibitory effect on the treatment. So, the treatment should be carried out in depth. The coal gasification wastewater was received from the Coal Long Hua Harbin Coal Chemical Industry Co. Ltd, Harbin, China, with COD conc. 3800–4400 mg L−1, BOD conc. 500–700 mg L−1, total phenol 850–950 mg L−1, ammonia 230–300 mg L−1, and a BOD/COD ratio between 0.13–0.16; so the wastewater has poor biodegradability and a strong inhibitory effect.| Test items | Concentration | Analytical method |
|---|---|---|
| a Unit: mg L−1, except pH and chromaticity. | ||
| COD | 3800–4400 | Potassium dichromate |
| BOD | 500–700 | Dilution inoculation |
| pH | 8.5–9 | pH meter method |
| Total phenol | 850–950 | Bromide titration |
| Volatile phenol | 450–530 | Pre-distillation – bromide titration |
| Total nitrogen | 240–320 | Persulfate oxidation – UV spectrophotometry |
| Ammonia nitrogen | 230–300 | Salicylic acid – hypochlorite spectrophotometry |
| Volatile acids | 80–120 | Pre-distillation – titration |
| Total phosphorus | 0.238 | Persulfate digestion – molybdenum, antimony anti-spectrophotometric |
| Suspended solids | 300–400 | Gravimetric method |
| Chromaticity color (times)39 | 1000, deep brownish red | Dilution ratio method |
| Turbidity | 5.82 | Turbidity meter method |
| Total alkalinity | 580 | Potentiometric titration |
| Petroleum | 24.2 | Infrared spectrophotometry |
| Fluoride | 50.6 | Ion selective electrode |
| Sulphide | 1.01 | Methylene blue spectrophotometry |
| Chloride | 113 | Silver nitrate titration |
| Total copper | 0.005 | Atomic absorption spectrophotometry |
| Total zinc | 0.035 | Atomic absorption spectrophotometry |
| Total manganese | 0.021 | Flame atomic absorption spectrophotometry |
| Total iron | 0.426 | Flame atomic absorption spectrophotometry |
| Total selenium | 0.091 | Atomic fluorescence spectrometry |
For microbial growth certain trace elements were added in the anaerobic influent like K2HPO4 (20 mg L−1), KH2PO4 (10 mg L−1), CaCl2·2H2O (20 mg L−1), FeSO4·7H2O (15 mg L−1), MgSO4·7H2O (50 mg L−1), MnCl2·4H2O (0.5 mg L−1), ZnCl2 (0.5 mg L−1), CuCl2 (0.5 mg L−1), (NH4)2MoO4·4H2O (0.5 mg L−1), AlCl3 (0.5 mg L−1), CoCl2·2H2O (0.5 mg L−1), and NiCl2·2H2O (0.5 mg L−1).
2.2 Coal gasification wastewater treatment process system setup
The setup of the anaerobic–aerobic–ozone oxidation and aerobic fluidized bed combined process flow diagram is shown in Fig. 1. Coal gasification wastewater after phenol and ammonia recovery was pumped into an anaerobic reactor AnaEG (Plexiglas, cylindrical, effective volume (EV) 13.4 L, inner diameter 100 mm, height 1500 mm, operated under mesophilic conditions at 35 °C). It undergoes hydrolysis acidification. The purpose of this unit is to remove volatile phenol and some total phenol; refractory organic pollutants are decomposed to smaller organic pollutants by the microorganisms in the AnaEG. Now, the effluent enters into an aerobic biological treatment reactor BioAX (8.0 L, inside diameter 0.15 m, height 1 m, operating temperature 15–20 °C). It was designed on the principle of a central tube air lift reactor. It added internal recycling in the bio-contact oxidation. It is an improvement over the original biological contact oxidation process. The aerobic metabolism treatment of the anaerobic effluent takes place in the aerobic reactor; most of volatile phenol (VP) and total phenol (TP) will be removed by aerobic heterotrophic bacteria.The aerobic effluent then enters into the ozonisation reactor. In this process the remaining TP will be removed, and biodegradability will be further enhanced. Then the ozonized effluent enters into the subsequent aerobic fluidized bed (EV 18 L, Plexiglas, inside diameter 0.15 m, height 1 m, operating temperature 15–20 °C). The circulating fluidized bed is designed according to the principles of a centre pipe airlift reactor. The reactor structure and flow channel enhanced the carrier in a fluidized state in a fluidized bed, i.e. solid phase (biofilm), liquid (wastewater), gas (air). Violent collisions occurred constantly between particles, and the biofilm surface gets renewed constantly. Wastewater in the reactor continuously circulating in a cycle has full contact with the microorganisms (sludge), where further decomposition of the organic matter by aerobic heterotrophic bacteria takes place. After this process the final discharged effluent meets national emission standards.19,20,35
2.3 Seed sludge and inoculum
2.4 Operational strategies
By shaker test36 and chromatographic analysis it was concluded that the appropriate time for anaerobic hydrolysis acidification was 45 h. For the anaerobic (AnaEG) and aerobic (BioAX) reactors the operational strategies were the same as those described in our recent studies.31,32The anaerobic reactor start-up stage operation was run for 330 days; the whole process takes place in three stages as start-up (run for 87 days, HRT 96 h), loading or stability (run for 110 days, HRT was reduced from 96 h to 48 h) and second start-up (anaerobic reactor was stopped for 10 days, followed by the start-up again and being operated for 133 days with HRT 48 h). As shown in Fig. 2a, when HRT was high the loading rate was low and vice versa based on the effluent COD and phenol concentration. HRT was controlled (loading rate max. 806 mg COD/L per day and min. 338 mg COD/L per day).
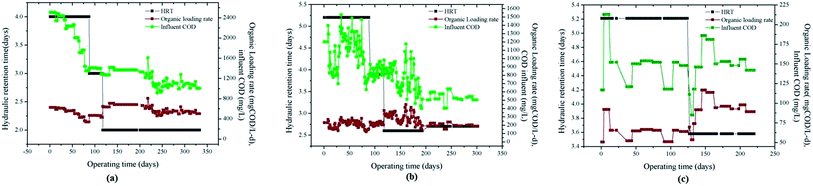 | ||
| Fig. 2 Start-up and operational strategy for the (a) anaerobic reactor, (b) aerobic reactor, and (c) aerobic fluidized bed. | ||
Considering the effluent quality (volatile) from the anaerobic reactor in the start-up stage, the aerobic reactor HRT was set at 125 h (Fig. 2b). After the successful completion of the start-up phase in the anaerobic reactor, the loading of the aerobic reactor was increased with a reduction in HRT from 94 h to 63 h. The reactor was intentionally stopped on the 200th day, a second start-up was given, and HRT was maintained at 64 h (loading rate max. 447.62 mg COD/L per day and min. 120.58 mg COD/L per day).
2.5 Advanced treatment process
The aerobic effluent needs advanced treatment in order to reach the national standard requirement (COD < 60 mg L−1), so a technically and economically feasible treatment process is required. The feasibility of two methods such as PAC (Poly Aluminum Chloride) coagulation and sedimentation, or ozonation and fluidized bed is discussed.PAC is a group of highly effective coagulants.37 Generally, the coagulation method is used to remove colloids, subtle suspended substances and refractory organic matter. In this study, coagulation is adopted initially to treat the aerobic effluent to study the removal effects of coagulation on refractory organic compounds in the aerobic effluent, thus learning whether the coagulation process should be adopted in practical engineering applications.
2.6 Analytical methods
The following parameters were analysed: COD, BOD, alkalinity, ammonia nitrogen, and Kjeldahl nitrogen. These were determined according to the standard procedure.38 Elemental composition was analysed with inductively coupled plasma (ICP) emission spectrophotometry (Thermo Electron, USA) using standard operating conditions and parameters.39 Volatile acid was analysed with an HP5890 series II gas chromatograph (HP, USA). To further understand the nature of the wastewater, GC/MS 2.0 (Shimadzu, Japan) was used. The ozone generator was type OZWAVE ND-OZS30.Biogas production was measured daily with a wet glass flow meter making corrections for atmospheric pressure and temperature. The methane concentration was determined by GC2010A gas chromatography (Shimadzu, Japan) with a stainless steel column (300 cm × 0.3 cm) packed with active carbon (30–60 mesh) using thermal conductivity detection (TCD).
GC/MS analytical conditions were as follows: a capillary column made of quartz with an inner diameter of 0.25 mm and length of 50 m was packed with OV-101; the temperature for the gasification compartment was maintained at 280 °C; the temperature control program was followed by retaining it at 70 °C for 3 min and then increasing it to 280 °C with an increment of 3 °C min−1; the temperature for the MS ion source was 200 °C and the electron energy was 70 eV.
(1) Sampling and fixation: take 2 to 3 mL of samples, fix in 2.5% glutaraldehyde for 12 h at pH 6.8;
(2) Dehydration: dehydration was done in steps of 50%, 70%, 80%, 90%, 95% and 100% ethanol concentration for 30 min each, and dehydration was done twice for each concentration. Then the samples were soaked in 100% ethanol for 12 h;
(3) Freeze drying: a freeze-dryer machine was used to dry the samples at −50 °C, until the ethanol volatilized completely from the samples;
(4) Gold sputter coating: with an ion sputtering coating machine, the surface of the sample is coated with a layer of metal film;
(5) Observation: samples are observed with SEM and the desired photographs were taken.
3. Results and discussion
3.1 Degradation performance in anaerobic and aerobic reactors
As can be seen from Fig. 3a–c in start-up stage I (day 1–87; water flow rate 3.4 L per day) in the anaerobic reactor, after successful completion of stage I the effluent COD conc. is 680 mg L−1 and the removal efficiency is 50%, the total phenol conc. is 170 mg L−1 and the removal efficiency is 44%, the volatile phenol conc. is 61 mg L−1 and the removal efficiency is 57%. In stage II (day 88–200), the TP conc. was maintained at 170–200 mg L−1, and the VP conc. at 80–130 mg L−1; in the latter half the TP conc. was maintained at 150 mg L−1, the VP was at 20–40 mg L−1, and the removal rates were 50% (TP) and 70% (VP). In stage III for the second start-up (day 201–222), the anaerobic treatment system uses a hydrolysis acidification process, therefore in the second start-up, the load was not reduced, but the original load was maintained, and HRT was still 48 h. After a successful and stable operation of the second start-up, we carried out a stable operation of the anaerobic reactor for 110 days (day 223–333), with COD effluent remaining at 500 mg L−1 with a removal rate of 50%. TP was maintained at 100 mg L−1, the removal rate of TP was over 50%, the effluent VP remained at 20 mg L−1 or less, with a removal rate above 80%. With the lowest HRT of 48 h, the average COD removal rate was 276.74 mg COD/L per day. | ||
| Fig. 3 Anaerobic reactor degradation trends and removal efficiencies; (a) COD, (b) total phenol, and (c) volatile phenol. | ||
Wang et al.41 investigated CGW treated by the mesophilic UASB reactor, with methanol addition and a hydraulic retention time of 24 h. During the study, the maximum COD and phenol removal rates were 71% and 75%, respectively. But it is important to note that the start-up period of the UASB reactor was as long as 227 days, and throughout the whole experiment period (359 days) the UASB requires the addition of methanol.
In another study, Wang, et al.42 investigated that the CGW was respectively treated by the mesophilic UASB and thermophilic UASB reactors, with a hydraulic retention time of 24 h. After the start-up period, the removal of COD and total phenols by the thermophilic reactor could reach 50–55% and 50–60% respectively. But the COD and phenol removal rates of the mesophilic UASB reactor were both only 20–30%. And it is important to note that the start-up period of the thermophilic and mesophilic reactors were both 120 days.
In the present study, after the first start-up period, when the HRT was 48 h, without methanol or glucose addition, COD, TP and VP removal rates could reach up to 50%, above 50%, and above 80%, respectively. The first and second start-up periods of the AnaEG reactor were only 90 days and 20 days, respectively. Compared with the other studies,41,42 the AnaEG reactor showed advantages with respect to shorter start-up periods and less methanol or glucose, which is important for the implementation of large-scale coal gasification wastewater treatment.
In the aerobic reactor the treatment efficiency of the aerobic influent depends on the effluent of the anaerobic reactor; when the anaerobic reactor runs in a stable operational phase, the aerobic reactor shows a stable and efficient treatment effect. The total operation of the aerobic reactor takes place over 302 days. In phase I (day 1–88, HRT 125 h), the amount of sludge in the system was higher due to new sludge dosing, therefore a higher removal of COD of about 70% was achieved (Fig. 4a and b). The aerobic reactor showed a fairly stable removal due to anaerobic hydrolysis acidification; its biodegradability increases, and so aerobic organisms exhibit high activity through a short acclimation period. In the second and third operational phases, COD removal is basically stable at around 70–80%, and the effluent COD concentration stabilized at 200 mg L−1. Aerobic microbial removal of phenolic compounds was also superb as shown in Fig. 4b, basically maintained at above 80%; the aerobic effluent had undetectable amounts of VP (removal rate 100%). During the stable secondary start-up of the aerobic reactor, the aerobic influent NH3–N concentration was 80–100 mg L−1, the effluent NH3–N concentration was 15–30 mg L−1, and the removal efficiency was 70–80%. The removal rate appears to follow a linear relationship with the organic loading rate in the aerobic reactor (R2 = 0.82795) (ESI Fig. S1†). A high loading rate shows high removal of COD and supports a 355.64 mg COD/L per day removal of COD with a load of 412.57 mg COD/L per day. With the lowest HRT of 64 h, the average COD removal rate was 24.43 mg COD/L per day. A detailed description regarding the working of anaerobic (AnaEG) and aerobic (BioAX) reactors can be seen in our recent study.31,32
3.2 Advanced treatment process results
| (a) | |||||||
|---|---|---|---|---|---|---|---|
| pH | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| COD (mg L−1) | 284 | 284 | 282 | 273 | 282 | 284 | 289 |
| COD removal efficiency (%) | 4.1 | 4.1 | 4.7 | 7.8 | 4.7 | 4.1 | 2.4 |
| (b) | |||||
|---|---|---|---|---|---|
| Coagulant dosage (mg L−1) | 100 | 200 | 300 | 400 | 500 |
| COD (mg L−1) | 275 | 269 | 257 | 251 | 261 |
| COD removal efficiency (%) | 0 | 2.5 | 6.9 | 9.1 | 5.4 |
| (c) | |||||||
|---|---|---|---|---|---|---|---|
| Ozonation time (min) | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
| COD conc. (mg L−1) | 271 | 167 | 173 | 90 | 76 | 92 | 57 |
| COD removal efficiency (%) | — | 38 | 36 | 67 | 72 | 66 | 79 |
| Total phenol conc. (mg L−1) | 90.6 | 0.2 | 0 | 0 | 0 | 0 | 0 |
However, the coagulation process does not have a removal effect on chromaticity, which is similar to those results of other research.43 Therefore, the coagulating sedimentation process does not show a good treatment effect on SNG biochemical effluent. The final effluent still can’t meet the standard.
As it is reported in the literature that pH affects the double action of ozone on the organic matter, there may be a direct or an indirect (free radical) ozonation pathway.44,45 At low pH, ozone solely reacts with compounds with specific functional groups through selective reactions such as electrophilic, nucleophilic or dipolar addition reactions (i.e. direct pathway).46 However, at basic conditions, ozone decomposes yielding hydroxyl radicals, which are highly oxidizing species47 that react in a non-selective way with a wide range of organic and inorganic compounds in water (i.e. indirect ozonation).48 Normally, under acidic conditions (pH < 4) the direct ozonation prevails, in the range of pH 4–9 both are present, and above pH > 9 the indirect pathway prevails.
It is also reported that the degradation of chlorophenols is favored at high pH.49 Phenolic compounds are the main pollutants in the effluent of the BioAX reactor. The pH of the BioAX reactor effluent is usually 7–8. In light of the above considerations, pH 9 was chosen for the BioAX reactor effluent for performing ozone oxidation experiments. The purpose is to improve the wastewater treatment effect by ozone oxidation.
Ozonation is technically feasible but expensive in treating an aerobic effluent. Therefore, in this test, ozonation is followed by aerobic biological treatment to furthest reduce operational cost.
3.3 Degradation performance in the advanced treatment process
The aerobic effluent enters the aerobic fluidized bed after the intermittent ozonation process. The ozonation–aerobic fluidized bed (O3 + AFB) reaction was run for 220 days, divided into three stages as shown in Fig. 5: phase I, the ozonisation process is carried out under an ozone flow rate of 2 L min−1, with a reaction time of 30 min, and the effluent enters into the AFB, operating for 100 days (HRT 125 h); phase II, the ozone flow is 1 L min−1, the reaction time is 30 min, and the effluent enters into the AFB running for 20 days (HRT 125 h); phase III, the ozone flow is 1.5 L min−1, the reaction time is 30 min, and the effluent enters the AFB, and is run for 100 days (HRT 86 h), as shown in Table 3(a).| (a) | ||||||
|---|---|---|---|---|---|---|
| Running stage | Operating time (days) | Ozonation | Aerobic fluidized bed | |||
| Ozone flow rate (L min−1) | COD conc. (mg L−1) | Total phenol conc. (mg L−1) | HRT (h) | COD conc. (mg L−1) | ||
| 1 | 1–103 | 2 | 200–300 | 20–30 | 125 | 150–160 |
| 2 | 104–124 | 1 | 180–250 | 20–30 | 125 | 150–160 |
| 3 | 125–220 | 1.5 | 180–250 | 20–30 | 86 | 150–160 |
| (b) | |||||
|---|---|---|---|---|---|
| Time (days) | Influent COD (mg L−1) | Effluent COD (mg L−1) | COD removal (%) | Influent total phenol (mg L−1) | Effluent total phenol (mg L−1) |
| 1 | 204 | 117 | 42.6 | 27.1 | Undetected |
| 4 | 308 | 213 | 30.8 | 29.7 | Undetected |
| 12 | 256 | 152 | 40.6 | 29.8 | Undetected |
| 37 | 204 | 121 | 40.7 | 29.9 | Undetected |
| 45 | 256 | 150 | 41.4 | 31.6 | Undetected |
| 58 | 268 | 154 | 42.5 | 26.3 | Undetected |
| 74 | 247 | 152 | 38.5 | 27.4 | Undetected |
| 91 | 198 | 118 | 40.4 | 30.1 | Undetected |
| 104 | 221 | 152 | 31.2 | 31.1 | Undetected |
| 114 | 212 | 148 | 30.2 | 33.6 | Undetected |
| 127 | 190 | 111 | 41.6 | 30.3 | Undetected |
| 130 | 163 | 85 | 47.9 | 36.4 | Undetected |
| 133 | 205 | 118 | 42.4 | 31.2 | Undetected |
| 136 | 255 | 146 | 42.7 | 25.1 | Undetected |
| 145 | 266 | 186 | 30.1 | 26.6 | Undetected |
| 152 | 263 | 181 | 31.2 | 27.2 | Undetected |
| 164 | 265 | 150 | 43.4 | 34.2 | Undetected |
| 173 | 213 | 153 | 28.2 | 32.9 | Undetected |
| 187 | 223 | 148 | 33.6 | 17.6 | Undetected |
| 200 | 240 | 156 | 35.0 | 24.8 | Undetected |
| 209 | 192 | 142 | 26.0 | 19.5 | Undetected |
| (c) | |||
|---|---|---|---|
| Operating time (days) | Influent NH3–N (mg L−1) | Effluent NH3–N (mg L−1) | NH3–N removal rate (%) |
| 152 | 22.7 | 0.36 | 98.4 |
| 164 | 28.7 | 0.27 | 99.1 |
| 173 | 26.4 | 0.45 | 98.3 |
| 187 | 17.6 | 0.13 | 99.3 |
| 200 | 27.8 | 0.21 | 99.2 |
Since the aerobic effluent concentration was 200 mg L−1, this cannot meet the effluent standard (less than 60 mg L−1). It was necessary to treat the effluent deeply. Ozonation-aerobic fludized bed was used for 220 days of operation; the use of the ozone oxidation unit operates intermittently (batch), and continuous operation was done in the AFB reactor. As can be seen from Table 3(b), when the ozone flow rate was 2 L min−1, COD removal efficiency was 40%; at an ozone flow rate of 1 L min−1, COD removal was 30%; at an ozone flow rate of 1.5 L min−1, COD removal efficiency was 30–40%; and the effluent COD was stable at 150 mg L−1. TP concentration was 30 mg L−1 or less, and after 30 min of ozonation, TP was undetected in the effluent. As can be seen from Fig. 5 in the first 10 days, the microbes exhibit inadaptability, and the COD removal rate dropped to about 30%. But after the 27th day, domestication of the microbes takes place, and the COD removal ratio gradually increases. The COD removal gradually picked up in the first 37 days and its removal efficiency reached higher than 60%, and the effluent COD dropped to 60 mg L−1 in the AFB; it’s probable that after ozonation, wastewater directly enters into the fluidized bed. The water still contains some ozone after 30 min.
If ozone is brought into the fluidized bed after aerobic decomposition, it would produce toxic effects on aerobic microorganisms and inhibit degradation performance.50,51 In this study, the ozone oxidation reactor was operated in an intermittent mode. The effluent of the ozone oxidation reactor was sent into the storage tank (‘12’ in Fig. 1), and the hydraulic retention time is 1 h (as we know the half-life period of ozone is very short, usually less than 1 h). This is to ensure that the residual ozone in the wastewater is mostly broken down. Therefore, it would not produce toxic effects on aerobic microorganisms in the fluidized bed reactor. Therefore, after ozonation, wastewater should be held for some time, and then enter into the fluidized bed.
In stage II, the COD concentration (effluent) was very unstable, and cannot be decreased to 60 mg L−1 after 20 days of running. Hence, in stage III, the ozone flow rate was adjusted to 1.5 L min−1, as the anaerobic–aerobic effluent shows a good effect; the COD concentration (effluent of the AFB) was basically stable below 60 mg L−1, with a removal rate higher than 60%.
During stable running of the Ozonation-aerobic fluidized bed, the NH3–N concentration was determined, and the results are shown in Table 3(c). The concentration of NH3–N was less than 30 mg L−1, in the effluent the NH3–N concentration was below 1 mg L−1, and the NH3–N level meets the national effluent standards.18,21 After the parameter optimization of anaerobic and aerobic treatment processes, the wastewater treatment by the ozonation–aerobic fluidized bed experiment can provide a key in-depth technological research in microalgae breeding. Fig. 2(c) illustrates the relationship between HRT, COD loading rate and COD influent. With the lowest HRT 86 h the average COD removal rate was 62.80 (mg COD/L per day) and the maximum organic loading rate was 116.79 mg COD/L per day. The minimum loading rate was observed at HRT 125 h (50.54 mg COD/L per day).
After the ozonation process, the phenol was oxidized into hydroquinone and catechol which were degraded into acids and carbon dioxide without high molecular weight byproducts.52 The ozone oxidation intermediates are generally more biodegradable than the original molecules.53 The water quality in each stage is shown in Table 4 and the water quality of the final effluent and related standards are shown in Table 5.
| Parameters | Raw wastewater | Diluted raw wastewater (3 times) | Anaerobic effluent | Aerobic effluent | Final effluent | National emission standards |
|---|---|---|---|---|---|---|
| a N.D. not detected; unit: mg L−1, except pH. | ||||||
| COD | 3800–4400 | 1000–1400 | 500–800 | 200–300 | <60 | 60 |
| NH3+–N | 230–300 | 80–100 | 80–100 | 15–30 | <1 | 1 |
| Total phenols | 850–950 | 250–320 | 150–200 | ∼50 | N.D. | — |
| Volatile phenol | 450–530 | 120–150 | 20–40 | N.D. | N.D. | 0.5 |
| pH | 8.5–9 | 7.5–8.5 | 7–8 | 7–8 | 7–8 | 7–8 |
| Parameters | Raw wastewater | Diluted raw wastewater (3 times) | Final effluent | Integrated wastewater discharge standards [GB8978-1996] | Standard for industrial circulating cooling water [HG/T 3923-2007] |
|---|---|---|---|---|---|
| a N.M. not measured; N.D. not detected. | |||||
| COD (mg L−1) | 3800–4400 | 1200–1500 | <60 | 60 | 80 |
| BOD (mg L−1) | 500–700 | 160–240 | <5 | 20 | 5 |
| pH | 8.5–9 | 8–9 | 7–8 | 6–9 | 6–9 |
| Total phenols (mg L−1) | 850–950 | 280–320 | N.D. | ||
| Volatile phenol (VP) (mg L−1) | 450–530 | 150–180 | N.D. | 0.5 | |
| Total nitrogen (TN) (mg L−1) | 240–320 | 80–110 | 70–110 | ||
| NH4+–N (mg L−1) | 230–300 | 80–100 | <1 | 15 | 15 |
| Volatile acids (VA) (mg L−1) | 80–120 | 30–40 | N.D. | ||
| Phosphate (mg-P per L) | 0.2–0.3 | 0.07–0.1 | 0.056 | 0.5 | |
| Suspended Solids (SS) (mg L−1) | 300–400 | 100–130 | 10 | 70 | 20 |
| Chromaticity (times) | 1000 | N.M. | 10 | 50 | |
| Turbidity | 5.82 | N.M. | 8.2 | 10 | |
| Total alkalinity (mg-CaCO3 per L) | 580 | 190 | 20 | 700 | |
| Total hardness (mg-CaCO3 per L) | N.M. | N.M. | 279 | ||
| Petroleum (mg L−1) | 24.2 | 8 | 0.18 | 5 | 0.5 |
| Fluoride (mg L−1) | 50.6 | 17 | 9.5 | 10 | |
| Sulphide (mg L−1) | 1.01 | 0.34 | 0.014 | 1 | 0.1 |
| Chloride (mg L−1) | 113–124 | 38–42 | 40–41 | 500 | |
| Total copper (mg L−1) | 0.005 | 0.002 | 0.001 | 0.5 | |
| Total zinc (mg L−1) | 0.035 | 0.012 | 0.011 | 2.0 | |
| Manganese (mg L−1) | 0.021 | 0.007 | 0.006 | 2.0 | |
| Total iron (mg L−1) | 0.426 | 0.142 | 0.133 | 0.3 | |
| Total selenium (mg L−1) | 0.091 | 0.031 | 0.030 | 0.1 | |
3.4 Characterization of anaerobic sludge
By SEM analysis, it is revealed in Fig. 6 that different types of microorganisms are intertwined randomly throughout the cross section. A large population of filamentous long-chain microorganisms were observed. In addition, many colonies consisting of cocci and Bacillus were also observed, representing the typical shape of acid-producing bacteria, and they were linked together. The methanogenic bacteria were dominant. SEM illustrates that the granules had porous and multiple cracks on the surface. These pores were likely to facilitate the passage of nutrients and substrates.543.5 Estimation of energy consumption and processing cost
Evaluation of energy consumption during ABO + AF treatment (excluding ozone oxidation) is done| E = nP/q |
If the water flow rate is 400 m3 h−1, the TP concentration is 300 mg L−1, and COD is 1500 mg L−1. For the anaerobic reactor influent pump P1 (Fig. 1): the pump selection CHD 545-250B, flow 420 m3 h−1, head 22 m, power 45 kW, 1 unit of energy consumption will be:
| E1 = n × P/q = 1 × 45 kW/400 m3 h−1 = 0.1125 kW h m−3 |
For the blowers in the aerobic system P3 and P6 (Fig. 1): the energy consumption with a fan selection of FSR 3OO Roots blower, flow 110 m3 min−1, power 160 kW, 2 units, is:
| E2 = n × P/q = 2 × 160 kW/400 m3 h−1 = 0.8 kW h m−3 |
Then the total cost of electricity consumption = price × (E1 + E2) = 0. 13 $ per kW per h × (0.1125 + 0.8) kW h m−3 = 0.119 $ per m3.
In the anaerobic process we add trace elements, which cost about 0.016 $ per m3 of wastewater. Therefore, the cost of the wastewater treatment process is
| 0.119 $ per m3 + 0.016 $ per m3 = 0.135 $ per m3 |
The cost estimate for treating CGW is closely related to the wastewater quality, effluent quality, wastewater treatment process and so on. The literature reported55,56 that the industrial scale coal gasification wastewater treatment process costs roughly $0.4–$0.7 per m3. In this study a lab scale attempt has been made to obtain the total treatment cost of CGW of 0.135 $ per m3 (without ozonation).
3.6 Sludge volume
There is no discharge of surplus sludge during 1 year of running. In each reactor unit the change is compelled between the pre-treatment and final treatment. During 1 year of running, the amount of sludge in each reactor does not significantly change, excluding the need of anaerobic–aerobic batch experiments when a certain volume of domesticated sludge from respective reactors are extracted.As such, sludge production is closely related to the processes involved. It is well known that the sludge production of an anaerobic process is less than an aerobic process, and the sludge production of the aerobic biofilm contact oxidation process is less than the activated sludge process. In this study the advanced anaerobic expanded granular sludge bed (AnaEG) and the advanced bio-membrane technology aerobic reactor (BioAX) were used to treat the CGW, which is the foundation of the lower sludge production of the ABO + AF system.
Secondly, the lower sludge production for the ABO + AF system could be explained from the perspective of carbon balance. The conversion pathways of the COD in the CGW includes sludge growth, biogas, VOCs (aeration blowing off) and residual COD in CGW. In this study, the total COD removal rate of AnaEG was 50%, and part of the removed COD was transformed into biogas.31
The COD removal by BioAX only accounts for 30–35% of the total COD removal in the ABO + AF system. Due to the long and complex biological chain of the BioAX biofilm, and also because the BioAX reactor was run in sections with dominant bacteria,32 the sludge production of BioAX was less than the conventional bio-contact oxidation process.
Moreover, it should be noted that during the aerobic aeration, part of the COD, such as VOCs, directly escaped from CGW into the air, which was not degraded by microorganisms. Furthermore, about 10% of the total COD removal was degraded by ozonation. Therefore during this study, sludge production is much less and we can ignore it, which is a big advantage of this technology. This process will become competitive in future industrial applications.
4. Conclusions
A laboratory scale AnaEG-BioAX-Ozonation + Aerobic Fluidized bed (ABO + AF) system was used to treat coal gasification wastewater. The biggest characteristic of the treatment process is that it exhibits high capability for treatment of highly contaminated and toxic coal gasification wastewater. Although the removal of ammonia along with the other pollutants from coal gasification wastewater is a complicated process, our system treated it efficiently, effectively and simultaneously with excellent removal efficiency of COD (96%), ammonia (99%), total phenol (100%), volatile phenol (100%) and volatile acids (100%). This process has obvious technical advantages: in the whole treatment process there is no reflux of effluent, short residence time, low energy consumption, low sludge production, and easy management and maintenance. Both the opportunities and the limitations of the ABO + AF technology meet the multiple criteria of water sustainability, so it should have a negligible environmental and ecological impact.A simplified processing unit AnaEG reactor was used in this study that focused on enhancing the wastewater biodegradability. The anaerobic reactor primarily converted refractory and inhibitory compounds into biodegradable organic substances, hence wastewater toxicity is reduced.31 The BioAX reactor used in this study has its own advantages as maintenance is negligible, shortcut flow does not exist, air distribution is uniform, there is plug flow with overturning, growth of the biofilm is not disturbed by aeration, it has a higher oxygen transfer efficiency, faster start-up, shorter microorganism cultivation time, lower power consumption, lower blower capacity, and no need for replenishment of microorganisms.32
This paper presents a laboratory attempt to explore the possibility of applying ABO + AF as a sustainable technology for wastewater treatment and to guide its future development on an industrial scale. This technology will be competitive in future industrial wastewater treatment.
Acknowledgements
The authors express their gratitude to the School of Environmental Science and Engineering, Shanghai Jiao Tong University for providing the research facilities.References
- W. W. Li, H. Q. Yu and Z. He, Energy Environ. Sci., 2014, 7, 911–924 CAS.
- H. Gai, Y. Jiang, Y. Qian and A. Kraslawski, Chem. Eng. J., 2008, 138, 84–94 CrossRef CAS PubMed.
- Z. J. Yu, Y. Chen, D. C. Feng and Y. Qian, Ind. Eng. Chem. Res., 2010, 49, 2874–2881 CrossRef CAS.
- H. Sun, Z. Zhang and L. M. Song, Environ. Prog. Sustainable Energy, 2010, 494–498 CrossRef CAS PubMed.
- X. Zhou, Y. X. Li, Y. Zhao and X. P. Yue, J. Chem. Technol. Biotechnol., 2013, 305–310 CrossRef PubMed.
- D. Park, D. S. Lee, Y. M. Kim and J. M. Park, Bioresour. Technol., 2008, 99, 2092–2096 CrossRef CAS PubMed.
- M. K. Ghose, Water Res., 2002, 36, 1127–1134 CrossRef CAS.
- Y. Qian, Y. Wen and H. Zhan, Water Res., 1994, 28, 701–710 CrossRef CAS.
- R. F. Yu, Polluted Environment and Human Health Protection, Fudan University Press, Shanghai, China, 1989 Search PubMed.
- Y. Wang, S. Wang, Y. Guo, D. Xu, Y. Gong, X. Tang and J. Zhang, Environ. Prog. Sustainable Energy, 2014, 33, 1258–1265 CAS.
- W. Wang, H. Han, M. Yuan, H. Li, F. Fang and K. Wang, Bioresour. Technol., 2011, 102, 5454–5460 CrossRef CAS PubMed.
- H. Zhuang, H. Han, B. Hou, S. Jia and Q. Zhao, Bioresour. Technol., 2014, 166, 178–186 CrossRef CAS PubMed.
- E. Maranon, I. Vazquez, J. Rodriguez, L. Castrillon, Y. Fernandez and H. Lopez, Bioresour. Technol., 2008, 99, 4192–4198 CrossRef CAS PubMed.
- Y. M. Li, G. W. Gu, J. F. Zhao, H. Q. Yu, Y. L. Qiu and Y. Z. Peng, Chemosphere, 2003, 52, 997–1005 CrossRef CAS.
- M. Zhang, J. H. Tay, Y. Qian and X. S. Gu, Water Res., 1998, 32, 519–527 CrossRef CAS.
- I. Vázquez, J. Rodríguez, E. Marañón, L. Castrillón and Y. Fernández, J. Hazard. Mater., 2006, 137, 1773–1780 CrossRef PubMed.
- Z. Wang, X. Xu, J. Chen and F. Yang, J. Environ. Chem. Eng., 2013, 1, 899–905 CrossRef CAS PubMed.
- Integrated wastewater discharge standard National standard of the Republic of China, China, GB8978-1996.
- Standard of Water Pollutants for Iron and Steel Industry, China, GB13456-92.
- Pollutants discharge plant in China, State Environmental Protection Administration of China, GB 18918-2002.
- Reusing wastewater quality standard for industrial Circulating cooling water, National standard of the Republic of China, HG/T 3923-2007.
- C. Yang, Prospects for Coal Gasification in china, Sino-Global Energy, 2010, 15, 35–40 CAS.
- S. S. Adav, D. J. Lee, K. Y. Show and J. H. Tay, Biotechnol. Adv., 2008, 26, 411–423 CrossRef CAS PubMed.
- T. Seviour, Z. Yuan, M. C. van Loosdrecht and Y. Lin, Water Res., 2012, 46, 4803–4813 CrossRef CAS PubMed.
- Y. M. Kim, D. Park, C. O. Jeon, D. S. Lee and J. M. Park, Bioresour. Technol., 2008, 99, 8824–8832 CrossRef CAS PubMed.
- W. Jianlong, Q. Xiangchun, W. Libo, Q. Yi and W. Hegemann, Process Biochem., 2002, 38, 777–781 CrossRef.
- R. J. Franklin, Anaerobic digestion for sustainable development, 2001, vol. 1, pp. 2–8 Search PubMed.
- J. B. V. Lier, Water Sci. Technol., 2008, 57, 1137–1148 CrossRef PubMed.
- N. Musee and L. Lorenzen, Water SA, 2012, 31, 131–142 Search PubMed.
- D. Jeison and R. Chamy, Water Sci. Technol., 1999, 40, 91–97 CrossRef CAS.
- C. Li, S. Tabassum and Z. Zhang, RSC Adv., 2014, 4, 57580–57586 RSC.
- C. Li, S. Tabassum and Z. Zhang, RSC Adv., 2014, 4, 35156–35162 RSC.
- Z. Zhang, Anaerobic treatment method for organic wastewater, China Pat., ZL 2009 1 0050787.1, 7 May 2009–20 April 2011.
- Z. Zhang, Aerobic biofilm device for wastewater treatment, China Pat., ZL 02157780.3, 26 December 2002–9 November 2005.
- Ministry Standards for Sludge Management in China, Quality of sludge from municipal wastewater treatment plant, Ministry of Housing and Urban-Rural Development, CJ 247-2007, 01 October 2007 Search PubMed.
- Q. Ji, S. Tabassum, C. Chu, C. Li and Z. Zhang, Adv. Mater. Res., 2014, 1049–1050, 39–43 CrossRef CAS.
- A. I. Zouboulis and N. Tzoupanos, Desalination, 2010, 250, 339–344 CrossRef CAS PubMed.
- D. E. Andrew and H. F. M. Ann, Standard Methods for the, Examination of Water and Wastewater, APHA (American Public Health Association), AWWA (American Water Works Association), WEF (Water Environment Federation), 21st edn, 2005 Search PubMed.
- F. S. Wei, W. Q. Qi, Z. G. Sun, Y. R. Huang and Y. W. Shen, Water and Wastewater Monitoring and Analysis Method, China Environmental Science Press, Beijing, 4th edn, 2002 Search PubMed.
- M. Zhang, T. J. Hwa, Q. Yi and G. X. Sheng, Water Res., 1998, 32, 519–527 CrossRef CAS.
- W. Wang, H. Han, M. Yuan and H. Li, J. Environ. Sci., 2010, 22, 1868–1874 CrossRef CAS.
- W. Wang, W. Ma, H. Han, H. Li and M. Yuan, Bioresour. Technol., 2011, 102, 2441–2447 CrossRef CAS PubMed.
- Z. An and X. Xu, J. Chem. Pharm. Res., 2014, 6, 282–287 CAS.
- F. J. Beltrán, J. F. González and P. Álvarez, Ing. Quim., 1997, 331, 161–168 Search PubMed.
- J. Hoigné, Chemistry of aqueous ozone and transformation of pollutants by ozonation and advanced oxidation processes, ed. J. Hrubee, Springer, Berlin, 1998 Search PubMed.
- B. Langlais, D. A. Reckhow and D. R. Brink, Ozone in Water Treatment, Application and Engineering, Lewis Publishers, Chelsea, MI, 1991 Search PubMed.
- O. Legrini, E. Oliveros and A. M. Braun, Chem. Rev., 1993, 93, 671–698 CrossRef CAS.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS PubMed.
- M. P. Titus, V. G. Molina, M. A. Baños, J. Giméneza and S. Esplugas, Appl. Catal., B, 2004, 47, 219–256 CrossRef PubMed.
- S. Wang, J. Maa, B. Liu, Y. Jiang and H. Zhang, J. Hazard. Mater., 2008, 150, 109–114 CrossRef CAS PubMed.
- S. Zhang, J. Zheng and Z. Chen, Sep. Purif. Technol., 2014, 132, 610–615 CrossRef CAS PubMed.
- C. R. Huang and H. Y. Shu, J. Hazard. Mater., 1995, 41, 47–64 CrossRef CAS.
- M. C. Valsania, F. Fasano, S. D. Richardson and M. Vincenti, Water Res., 2012, 46, 2795–2804 CrossRef CAS PubMed.
- H. Liu and H. H. P. Fang, Water Sci. Technol., 2003, 47, 153–158 CAS.
- H. J. Han, H. Q. Li, M. A. Du, W. C. Ma, C. Y. Xu and W. Wang, China Water Wastewater, 2010, 26, 75–77 CAS.
- H. J. Han, P. Xu, S. Y. Jia, B. L. Hou and H. F. Zhuang, China Water Wastewater, 2013, 29, 65–67 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra04215a |
| This journal is © The Royal Society of Chemistry 2015 |




