Nature of reactant and influence of water on the supramolecular patterns and luminescent properties of organic salts comprising (1,1′-biphenyl)-4,4′-disulfonate and triphenylmethanaminium†
Ya-Nan Li,
Li-Hua Huo,
Zhao-Peng Deng*,
Xin-Yu Xie,
Zhi-Biao Zhu and
Shan Gao*
Key Laboratory of Functional Inorganic Material Chemistry, Ministry of Education, School of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, People's Republic of China. E-mail: dengzhaopeng@hlju.edu.cn; shangao67@yahoo.com; Fax: +86-0451-86608040; Tel: +86-0451-86608426
First published on 23rd April 2015
Abstract
The solvent reaction of 1,1′-biphenyl-4,4′-disulfonic acid (H2BPDSA) and triphenylmethylamine (TPMA) gives rise to fourteen supramolecular compounds, namely, 2(HTPMA)+·(BPDSA)2−·(H2O) (1), 2(HTPMA)+·(BPDSA)2−·(MeOH) (2), 2(HTPMA)+·(BPDSA)2−·(EtOH) (3), 2(HTPMA)+·(BPDSA)2−·3(n-PrOH) (4), 2(HTPMA)+·(BPDSA)2−·2(n-BuOH) (5), 2(HTPMA)+·(BPDSA)2−·2(n-PeOH) (6), 2(HTPMA)+·(BPDSA)2−·4(DMF) (7), 2(HTPMA)+·(BPDSA)2−·4(DMSO) (8), 2(HTPMA)+·(BPDSA)2−·(DO) (9), 2(HTPMA)+·(BPDSA)2−·2(H2O)·2(MeOH) (10), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PrOH) (11), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-BuOH) (12), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PeOH) (13) and 2(HTPMA)+·(BPDSA)2−·4(H2O)·2(DMF) (14) (DO = 1,4-dioxane), which have been characterized by elemental analysis, IR, TG, PL, and powder and single-crystal X-ray diffraction. Structural analyses indicate that salts 1–14 show seven types of packing diagram. Moreover, salts 1–9 present regular supramolecular structural variations with the change of the nature of the solvent molecules. Salts 1–3 exhibit double chain motifs with small molecules of H2O, MeOH and EtOH. Salts 4–7 present single chain structures with larger n-PrOH, n-BuOH, n-PeOH and DMF molecules. Salt 8 only exhibits a simple discrete motif owing to the triangular pyramid configuration of the DMSO molecule. In contrast, the DO molecules in salt 9 have two opposite acceptor O atoms, which extend adjacent HTPMA+ cations and BPDSA2− dianions into a (4,4) layer motif. Furthermore, the involvement of water molecules could modulate the dimension level of supramolecular patterns according to the different hydrogen bonding modes of H2O molecules. Salts 10 and 14 present different double and single chain structures from the single chains in salts 2 and 7 owing to the HB′3 and HB′2 modes of H2O molecules. Similarly, salts 11–13 show different (4,4) layer motifs from the chain structures in salts 4–6 with the assistance of the H2O molecules in HB4 mode. Luminescence investigations reveal that the emission maximum of salts 1–9 varies from 343 to 358 nm. In comparison, salts 10 and 14 show stronger emission intensity, whereas salts 12 and 13 show weaker emission intensity than their corresponding anhydrous salts owing to the structure transformations induced by the involvements of water molecules.
Introduction
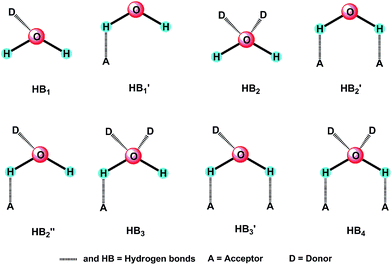
Supramolecular complexes have drawn public attention during recent decades owing to their potential applications in the field of photochemistry, biomedicine and pharmaceutics and intriguing supramolecular patterns determined by the nature of synthons and solvents.1–5 The solvents, such as alcohol, DMF, DMSO, and DO, with their own hydrogen bonding features (the number of donor and acceptor), can lead to different supramolecular structures in a given system.6 In particular, water, as the most abundant molecule on the earth's surface, is an ubiquitous reactant in terrestrial chemical processes.7 On one hand, it has some particular merits such as clean, low-priced, and environment-friendliness. On the other hand, from the viewpoints of coordination chemistry and crystal engineering, a series of infinite arrays of water structures such as discrete clusters (H2O)n,8 chains or tapes,9 2D layers,10 and 3D networks11 can be created and play crucial structure directing roles during the assembly of coordination complexes or polymers.12 Moreover, water molecules involved in the formation of supramolecular complexes as hydrogen bond donors and acceptors have more evident effects on the supramolecular structures and properties.13 Importantly, it has unique structural features: small size and the presence of two lone pairs on the oxygen that exhibit the ability to form two directional hydrogen bonds forming a tetrahedral arrangement of four oxygen atoms around each water oxygen.10–13 According to these characteristics, water, as an excellent hydrogen bonding solvent, can influence the crystal structures of organic salts.9–13 However, compared to scattered reports on this topic,9 systematic and intentional research on the influence of water on supramolecular patterns in view of the structures of organic hosts and hydrogen bonding features of water molecules (Scheme 1) are less apparent. Therefore, further exploring of the variations of supramolecular structures and luminescent properties of organic salts by a careful selection of solvents, especially, water/solvent inclusion within a crystalline lattice will be an interesting work.Sulfonate anions (–SO3−) and –NH3+ cations are good candidates for constructing attractive supramolecular patterns because of their same ‘AB3’ form and C3v symmetry. The three acceptor oxygen atoms on the –SO3− themselves have a closely related tetrahedral geometry to the three donor H atoms on the –NH3+ cations,14 which has been demonstrated by a series of outstanding works from Tohnai,15 our group6 and other research groups16 using diverse aliphatic or aromatic sulfonates and ammoniums. However, these present studies are mainly focused on the influence of synthons, e.g. sulfonic acids and amines, on the supramolecular patterns and properties of target organic salts. In contrast, the roles of solvent molecules are less focused upon. A representative work finished by Tohnai and co-workers presents the systematic research on the supramolecular structures and luminescent properties of anthraces-1,8-disulfonic acid and triphenylmethylamine in a variety of organic solvents.15e Our recent research6b also shows the effect of series of common solvent molecules on the structure variations and luminescent properties, especially, the chair-type (H2O)6 clusters induce the formation of a high symmetrical 3D supramolecular network. In order to extend our research on such system and further explore the role of water molecule during the assemble process, 1,1′-biphenyl-4,4′-disulfonic acid (H2BPDSA) was employed in the present work based on the following considerations: (i) H2BPDSA keeps the same structural feature of our previously reported 4,4′-dihydroxybiphenyl-3,3′-disulfonic acid so that the longer spacer of the biphenyl ring could afford the formation of a large hydrophobic cavity with a large primary amine such as triphenylmethylamine (TPMA) to capture the solvent molecules. (ii) The absence of two hydroxyl groups make the –SO3 groups have more clear directivity when they from hydrogen bonds with diverse solvent molecules, especially to the water molecules, which is beneficial to the structure regulation. Accordingly, a variety of solvents (MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH, DMF, DMSO, DO) and their combination with H2O are employed to study their effects on the supramolecular patterns and luminescent properties of organic salts comprising H2BPDSA and TPMA. Then, fourteen supramolecular compounds, namely, 2(HTPMA)+·(BPDSA)2−·(H2O) (1), 2(HTPMA)+·(BPDSA)2−·(MeOH) (2), 2(HTPMA)+·(BPDSA)2−·(EtOH) (3), 2(HTPMA)+·(BPDSA)2−·3(n-PrOH) (4), 2(HTPMA)+·(BPDSA)2−·2(n-BuOH) (5), 2(HTPMA)+·(BPDSA)2−·2(n-PeOH) (6), 2(HTPMA)+·(BPDSA)2−·4(DMF) (7), 2(HTPMA)+·(BPDSA)2−·4(DMSO) (8), 2(HTPMA)+·(BPDSA)2−·(DO) (9), 2(HTPMA)+·(BPDSA)2−·2(H2O)·2(MeOH) (10), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PrOH) (11), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-BuOH) (12), 2(HTPMA)+·(BPDSA)2−·(H2O)·2(n-PeOH) (13) and 2(HTPMA)+·(BPDSA)2−·4(H2O)·2(DMF) (14), have been obtained and structurally characterized. The different carbochain-length, configuration and volume of the solvent molecules can effectively influence the supramolecular patterns and cause the formation of seven types of packing diagram in salts 1–14. Interestingly, the involvement of H2O molecules could induce the structure variations from double chain in salt 2 to single chain in salt 10 and single chains in salts 4–6 to layer motifs in salts 11–13. Luminescence investigations reveal that the emission intensities of salts 10 and 12–14 are evidently stronger that those in salts 1–9 owing to the involvements of water molecules in the supramolecular skeletons, which increases the crystal packing and decreases the rotational degree of freedom. Moreover, the elemental analysis, IR, TG and powder X-ray diffraction for salts 1–14 are also carefully studied.
Experimental
Materials and methods
All chemicals were of A. R. grade and used without further purification in the syntheses. All organic solvents were of A. R. grade and underwent drying process before they were used. Elemental analyses were carried out with a Vario MICRO from Elementar Analysensysteme GmbH, and the infrared spectra (IR) were recorded from KBr pellets in the range of 4000–400 cm−1 using a Bruker Equinox 55 FT-IR spectrometer. Powder X-ray diffraction (PXRD) patterns for the three complexes were measured at 293 K using a Bruker D8 diffractometer (Cu Kα, λ = 1.54059 Å). The TG analyses were carried out using a Perkin Elmer TG/DTA 6300 thermal analyzer under a flowing N2 atmosphere, with a heating rate of 10 °C min−1. Luminescence spectra were measured using a Perkin Elmer LS 55 luminescence spectrometer.Synthesis of salts 1–9
The nine salts were synthesized by adding H2BPDSA (0.5 mmol, 360 mg) and TPMA (1 mmol, 521 mg) into H2O and diverse solvents (MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH, DO, DMF, DMSO). The mixture was stirred for 10 min at 343 K in a water bath and then filtered after cooling to room temperature. Colorless crystals of 1–9 suitable for X-ray diffraction were isolated from the filtrate in a desiccator filled by silica gel after several days.Salt 1: yield: 82%. Elemental analysis calcd (%) for C50H46N2O7S2: C 70.57, H 5.45, N 3.29; found: C 70.55, H 5.49, N 3.26. IR (ν/cm−1): 3444 m, 3066–2861 br, s, 1554 m, 1492 m, 1446 m, 1211 s, 1134 s, 1033 s, 1007 s.
Salt 2: yield: 81%. Elemental analysis calcd (%) for C51H48N2O7S2: C 70.81, H 5.59, N 3.24; found: C 70.84, H 5.63, N 3.28. IR (ν/cm−1): 3442 m, 3058–2897 br, s, 1558 m, 1494 m, 1447 m, 1207 s, 1128 s, 1035 s, 1001 s.
Salt 3: yield: 78%. Elemental analysis calcd (%) for C52H50N2O7S2: C 71.05, H 5.73, N 3.19; found: C 71.09, H 5.78, N 3.17. IR (ν/cm−1): 3441 m, 3066–2847 br, s, 1552 m, 1496 m, 1446 s, 1218 s, 1186 s, 1134 s, 1037 s, 1000 s.
Salt 4: yield: 80%. Elemental analysis calcd (%) for C59H68N2O9S2: C 69.93, H 6.76, N 2.76; found: C 69.95, H 6.72, N 2.74. IR (ν/cm−1): 3435 m, 3062–2878 br, s, 1585 m, 1535 m, 1496 m, 1448 m, 1206 s, 1128 s, 1035 s, 998 s.
Salt 5: yield: 83%. Elemental analysis calcd (%) for C58H64N2O8S2: C 70.99, H 6.57, N 2.85; found: C 70.96, H 6.53, N 2.88. IR (ν/cm−1): 3342 m, 3054–2867 br, s, 1554 m, 1494 m, 1448 m, 1198 s, 1134 s, 1036 s, 1001 s.
Salt 6: yield: 79%. Elemental analysis calcd (%) for C60H68N2O8S2: C 71.40, H 6.79, N 2.78; found: C 71.43, H 6.75, N 2.81. IR (ν/cm−1): 3345 m, 3052–2863 br, s, 1556 m, 1492 m, 1448 m, 1196 s, 1128 s, 1035 s, 1000 s.
Salt 7: yield: 79%. Elemental analysis calcd (%) for C62H72N6O10S2: C 66.17, H 6.45, N 7.47; found: C 66.13, H 6.49, N 7.51. IR (ν/cm−1): 3054–2859 br, m, 1677 s, 1555 m, 1496 m, 1446 m, 1235 m, 1178 s, 1126 s, 1029 s, 1001.
Salt 8: yield: 84%. Elemental analysis calcd (%) for C58H68N2O10S6: C 60.81, H 5.98, N 2.45; found: C 60.85, H 6.03, N 2.48. IR (ν/cm−1): 3058–2834 br, s, 1598 m, 1535 m, 1492 m, 1446 m, 1215 s, 1132 s, 1034 s, 1009 s.
Salt 9: yield: 77%. Elemental analysis calcd (%) for C54H52N2O8S2: C 70.41, H 5.69, N 3.04; found: C 70.45, H 5.75, N 3.01. IR (ν/cm−1): 3068–2809 br, s, 1602 m, 1538 m, 1492 m, 1449 m, 1197 s, 1134 s, 1031 s, 1000 s.
Synthesis of salts 10–14
During the processes for synthesizing salts 2–9, different amounts of H2O (Table 3) were introduced into the reaction system. The mixture was also stirred for 10 min at 343 K in a water bath and then filtered after cooling to room temperature. Colorless crystals of 10–14 suitable for X-ray diffraction were isolated from the filtrate after several days. It should be noted that salt 11 was only obtained in a very low yield due to its sensitivity to the water molecules. Hence, only the structure analysis was performed.Salt 10: yield: 78%. Elemental analysis calcd (%) for C52H56N2O10S2: C 66.93, H 6.05, N 3.00; found: C 66.97, H 6.09, N 3.02. IR (ν/cm−1): 3374 m, 3062–2836 br, s, 1558 m, 1494 m, 1448 m, 1223 s, 1167 s, 1138 s, 1031 s, 1003 s.
Salt 12: yield: 86%. Elemental analysis calcd (%) for C58H66N2O9S2: C 69.71, H 6.66, N 2.80; found: C 69.73, H 6.61, N 2.77. IR (ν/cm−1): 3374 m, 3062–2847 br, s, 1599 m, 1538 m, 1494 m, 1448 m, 1208 s, 1134 s, 1031 s, 999 s.
Salt 13: yield: 80%. Elemental analysis calcd (%) for C60H70N2O9S2: C 70.15, H 6.87, N 2.73; found: C 70.12, H 6.83, N 2.71. IR (ν/cm−1): 3355 m, 3074–2859 br, s, 1601 m, 1537 m, 1496 m, 1447 m, 1196 s, 1128 s, 1035 s, 1000 s.
Salt 14: yield: 82%. Elemental analysis calcd (%) for C56H64N4O11S2: C 65.10, H 6.24, N 5.42; found: C 65.13, H 6.19, N 5.39. IR (ν/cm−1): 3487 m, 3066–2862 br, s, 1667 s, 1600 m, 1537 m, 1496 m, 1448 m, 1216 s, 1186 s, 1134 s, 1031 s, 1002 s.
X-Ray crystallographic measurements
Table 1 provides a summary of the crystal data, data collection and refinement parameters for the salts 1–14. All diffraction data were collected at 295 K using a Xcalibur Eos diffractometer with graphite monochromatized Mo-Kα (λ = 0.71073 Å) radiation in ω scan mode. All structures were solved by direct method and difference Fourier syntheses. All non-hydrogen atoms were refined by full-matrix least-squares techniques on F2 with anisotropic thermal parameters. The hydrogen atoms attached to carbons in these fourteen salts, O9 in salt 4 and O2W in salt 14 were placed in calculated positions with C–H = 0.93 Å (aromatic H atoms), C–H = 0.97 Å (methylene H atoms), C–H = 0.96 Å (methyl H atoms), O–H = 0.82 or 0.85 Å, and U(H) = 1.2Ueq(C) and 1.5Ueq(O) in the riding model approximation. The hydrogen atoms of nitrogen atoms and the other hydroxyl groups and water molecules in 1–14 were located in difference Fourier maps and were also refined in the riding model approximation, with N–H and O–H distance restraint (0.86(1) or 0.85(1) Å) and U(H) = 1.5Ueq(N, O). EtOH, n-PrOH, n-BuOH, n-PeOH, and DMSO molecules in salts 3–6, 8, 11–13 and phenyl rings of BPDSA2− dianions in salt 12 are disordered over two positions with the ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. All calculations were carried out with the SHELXTL97 program.17† Selected hydrogen bond parameters for salts 1–14 are presented in Table 2.
0.5. All calculations were carried out with the SHELXTL97 program.17† Selected hydrogen bond parameters for salts 1–14 are presented in Table 2.
| Salts | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C50H46N2O7S2 | C51H48N2O7S2 | C52H50N2O7S2 | C59H68N2O9S2 |
| Formula weight | 851.01 | 865.03 | 879.06 | 1013.27 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n | P21/n | P21/n |
| a/Å | 13.412(4) | 15.2630(4) | 15.3836(5) | 15.4604(5) |
| b/Å | 13.544(3) | 15.5332(3) | 15.5242(3) | 19.5093(7) |
| c/Å | 14.951(4) | 19.3594(7) | 19.5258(6) | 18.6701(6) |
| α/° | 113.82(2) | 90.00 | 90.00 | 90.00 |
| β/° | 90.15(2) | 100.221(3) | 100.466(3) | 96.156(3) |
| γ/° | 111.63(2) | 90.00 | 90.00 | 90.00 |
| V/Å−3 | 2272.8(10) | 4517.0(2) | 4585.5(2) | 5598.8(3) |
| Z | 2 | 4 | 4 | 4 |
| Dc/g cm−3 | 1.244 | 1.272 | 1.273 | 1.202 |
| μ (Mo Kα)/mm−1 | 0.170 | 0.172 | 0.171 | 0.151 |
| F(000) | 896 | 1824 | 1856 | 2160 |
| Reflections collected | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 314 314 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 228 228 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165 165 |
| Unique reflections | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 303 303 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 476 476 |
9838 |
| Parameters | 574 | 581 | 595 | 704 |
| R (int) | 0.0275 | 0.0238 | 0.0232 | 0.0262 |
| GOF on F2 | 1.036 | 1.020 | 1.033 | 1.047 |
| Final R indices | R1 = 0.0540 | R1 = 0.0536 | R1 = 0.0569 | R1 = 0.0718 |
| [I ≥ 2σ(I)] | wR2 = 0.1165 | wR2 = 0.1157 | wR2 = 0.1194, wR2 = 0.0596 | wR2 = 0.1816 |
| Salts | 5 | 6 | 7 | 8 |
|---|---|---|---|---|
| Empirical formula | C58H64N2O8S2 | C60H68N2O8S2 | C62H72N6O10S2 | C58H68N2O10S6 |
| Formula weight | 981.23 | 1009.28 | 1125.38 | 1145.50 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 10.2879(11) | 10.4668(7) | 9.6402(6) | 8.8152(6) |
| b/Å | 11.4520(10) | 11.3787(7) | 11.3384(8) | 9.3074(9) |
| c/Å | 11.8164(8) | 11.8377(8) | 13.9981(12) | 19.3273(11) |
| α/° | 103.006(7) | 103.561(6) | 83.054(6) | 87.102(6) |
| β/° | 93.516(7) | 92.449(6) | 83.325(6) | 89.767(5) |
| γ/° | 91.882(8) | 92.814(5) | 86.826(5) | 63.850(8) |
| V/Å−3 | 1352.3(2) | 1366.76(16) | 1507.20(19) | 1421.31(18) |
| Z | 1 | 1 | 1 | 1 |
| Dc/g m−3 | 1.205 | 1.226 | 1.240 | 1.338 |
| μ (Mo Kα)/mm−1 | 0.153 | 0.153 | 0.150 | 0.300 |
| F(000) | 522 | 538 | 598 | 606 |
| Reflections collected | 7296 | 6686 | 8976 | 5696 |
| Unique reflections | 4738 | 4805 | 6887 | 4950 |
| Parameters | 334 | 352 | 365 | 355 |
| R (int) | 0.0238 | 0.0241 | 0.0165 | 0.0224 |
| GOF on F2 | 1.074 | 1.015 | 1.033 | 1.060 |
| Final R indices | R1 = 0.0684 | R1 = 0.0639 | R1 = 0.0679 | R1 = 0.0669 |
| [I ≥ 2σ(I)] | wR2 = 0.1897 | wR2 = 0.1417 | wR2 = 0.1753, wR2=0.0596 | wR2 = 0.1480, wR2=0.0596 |
| Salts | 9 | 10 | 11 | 12 |
|---|---|---|---|---|
| Empirical formula | C54H52N2O8S2 | C52H56N2O10S2 | C56H62N2O9S2 | C58H66N2O9S2 |
| Formula weight | 921.10 | 933.11 | 971.22 | 999.25 |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21212 | P21212 |
| a/Å | 10.2797(10) | 10.8382(11) | 15.2948(7) | 18.878(3) |
| b/Å | 10.6209(14) | 13.0040(10) | 18.2388(13) | 15.3625(17) |
| c/Å | 12.2253(9) | 17.5401(16) | 9.5627(6) | 9.4488(10) |
| α/° | 81.195(9) | 96.672(7) | 90.00 | 90.00 |
| β/° | 74.204(8) | 91.670(8) | 90.00 | 90.00 |
| γ/° | 69.673(11) | 94.678(8) | 90.00 | 90.00 |
| V/Å−3 | 1201.8(2) | 2445.3(4) | 2667.6(3) | 2740.3(6) |
| Z | 1 | 2 | 2 | 2 |
| Dc/g m−3 | 1.273 | 1.267 | 1.209 | 1.211 |
| μ (Mo Kα)/mm−1 | 0.168 | 0.169 | 0.156 | 0.154 |
| F(000) | 486 | 988 | 1032 | 1064 |
| Reflections collected | 7157 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
5017 | 6756 |
| Unique reflections | 5493 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
3811 | 5342 |
| Parameters | 307 | 633 | 330 | 378 |
| R (int) | 0.0170 | 0.0238 | 0.0365 | 0.0234 |
| GOF on F2 | 1.050 | 1.012 | 1.012 | 1.064 |
| Final R indices | R1 = 0.0652 | R1 = 0.0624 | R1 = 0.0644 | R1 = 0.0686 |
| [I ≥ 2σ(I)] | wR2 = 0.1549 | wR2 = 0.1348 | wR2 = 0.0884 | wR2 = 0.1349 |
| Salts | 13 | 14 |
|---|---|---|
| Empirical formula | C60H70N2O9S2 | C56H64N4O11S2 |
| Formula weight | 1027.30 | 1033.25 |
| Space group | P21212 | P21/n |
| a/Å | 19.8998(9) | 13.8413(12) |
| b/Å | 15.4214(6) | 12.9378(8) |
| c/Å | 9.2206(3) | 18.2777(17) |
| α/° | 90.00 | 90.00 |
| β/° | 90.00 | 125.265(6) |
| γ/° | 90.00 | 90.00 |
| V/Å−3 | 2829.6(2) | 2672.5(4) |
| Z | 2 | 2 |
| Dc/g m−3 | 1.206 | 1.284 |
| μ (Mo Kα)/mm−1 | 0.151 | 0.163 |
| F(000) | 1096 | 1096 |
| Reflections collected | 7066 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 086 086 |
| Unique reflections | 5740 | 6111 |
| Parameters | 360 | 346 |
| R (int) | 0.0259 | 0.0262 |
| GOF on F2 | 1.046 | 1.027 |
| Final R indices | R1 = 0.0651 | R1 = 0.0601 |
| [I ≥ 2σ(I)] | wR2 = 0.1210 | wR2 = 0.1398 |
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | <(DHA) |
|---|---|---|---|---|
| a Symmetry operations: for 1, (i) x,y − 1,z − 1; (ii) x,y + 1,z + 1; (iii) −x,−y + 1,−z. For 2, (i) −x + 1,−y + 1,−z; (ii) −x + 1,−y,−z; (iii) x,y − 1,z. For 3, (i) x,y + 1,z; (ii) x,y − 1,z; (iii) −x + 1,−y,−z. For 4, (i) x − 1,y,z; (ii) x + 1,y,z. For 5, (ii) −x,−y + 2,−z + 1. For 6, (ii) −x,−y + 1,−z + 2. For 7, (ii) −x + 1,−y + 1,−z + 1. For 9, (iii) −x,−y + 2,−z + 2. For 10, (i) −x + 1,−y + 1,−z; (ii) −x − 1,−y + 2,−z + 1. For 11, (ii) −x + 1/2,y + 1/2,−z + 2; (iii) x − 1/2,−y + 3/2,−z + 1; (iv) −x + 1/2,y + 1/2,−z + 1. For 12, (ii) x,y,z − 1; (iii) −x,−y,z. For 13, (ii) x − 1/2,−y + 3/2,−z + 3; (iii) −x + 1,−y + 1,z; (iv) −x + 1/2,y − 1/2,−z + 2. For 14, (ii) −x,−y + 1,−z; (iii) −x,y + 1/2,−z + 1/2. | ||||
| 1 | ||||
| O(1W)–H(1W1)⋯O(6) | 0.85(2) | 1.941(12) | 2.767(2) | 165(3) |
| O(1W)–H(1W2)⋯O(3)i | 0.85(2) | 2.062(14) | 2.882(2) | 161(3) |
| N(1)–H(1N1)⋯O(1) | 0.86(2) | 1.928(12) | 2.743(2) | 157.7(18) |
| N(1)–H(1N2)⋯O(5)ii | 0.86(2) | 1.917(10) | 2.783(2) | 171(2) |
| N(1)–H(1N3)⋯O(1W)ii | 0.88(2) | 2.018(13) | 2.857(3) | 158.9(19) |
| N(2)–H(2N1)⋯O(3)i | 0.86(2) | 2.085(9) | 2.948(2) | 171.2(17) |
| N(2)–H(2N2)⋯O(2)iii | 0.88(2) | 1.923(10) | 2.801(2) | 172.0(17) |
| N(2)–H(2N3)⋯O(4) | 0.88(2) | 1.904(10) | 2.774(2) | 168.1(19) |
| 2 | ||||
| O(7)–H(7O)⋯O(4) | 0.85(3) | 1.977(15) | 2.806(3) | 165(4) |
| N(1)–H(1N1)⋯O(2)i | 0.86(2) | 1.856(10) | 2.717(2) | 170(2) |
| N(1)–H(1N2)⋯O(3) | 0.86(2) | 2.024(9) | 2.870(2) | 167.6(19) |
| N(1)–H(1N3)⋯O(5)ii | 0.86(2) | 2.111(15) | 2.878(3) | 147.1(18) |
| N(2)–H(2N1)⋯O(6) | 0.86(2) | 1.940(11) | 2.792(2) | 166(2) |
| N(2)–H(2N2)⋯O(7) | 0.86(2) | 2.001(10) | 2.850(3) | 165.9(17) |
| N(2)–H(2N3)⋯O(1)iii | 0.86(2) | 1.958(10) | 2.817(2) | 174(2) |
| 3 | ||||
| O(7)–H(7O)⋯O(3)i | 0.85(3) | 1.967(17) | 2.786(3) | 160(4) |
| N(1)–H(1N1)⋯O(1)i | 0.86(2) | 1.961(12) | 2.801(2) | 162(2) |
| N(1)–H(1N2)⋯O(7) | 0.86(2) | 1.913(10) | 2.775(3) | 169.1(19) |
| N(1)–H(1N3)⋯O(4) | 0.86(2) | 1.962(10) | 2.824(2) | 174(2) |
| N(2)–H(2N1)⋯O(6)ii | 0.86(2) | 1.856(10) | 2.721(2) | 173(2) |
| N(2)–H(2N2)⋯O(5)iii | 0.86(2) | 1.990(9) | 2.846(2) | 168(2) |
| N(2)–H(2N3)⋯O(2) | 0.86(2) | 2.174(17) | 2.911(3) | 143(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 4 | ||||
| O(7)–H(7O)⋯O(5) | 0.85(4) | 1.892(17) | 2.731(4) | 165(5) |
| O(8)–H(8O)⋯O(7)i | 0.85(4) | 2.20(2) | 2.996(4) | 156(5) |
| O(8)–H(8O)⋯O(3) | 0.85(4) | 2.55(5) | 3.004(4) | 115(4) |
| O(9′)–H(9′O)⋯O(1) | 0.82 | 2.58 | 3.40(2) | 177.6 |
| N(1)–H(1N1)⋯O(1) | 0.86(3) | 2.019(14) | 2.862(3) | 165(3) |
| N(1)–H(1N2)⋯O(6)i | 0.86(3) | 2.006(11) | 2.854(4) | 169(2) |
| N(1)–H(1N3)⋯O(8) | 0.86(3) | 1.980(11) | 2.842(4) | 174(3) |
| N(2)–H(2N2)⋯O(2)ii | 0.86(3) | 2.047(12) | 2.892(4) | 169(3) |
| N(2)–H(2N2)⋯O(3)ii | 0.86(3) | 2.59(3) | 3.116(4) | 120(2) |
| N(2)–H(2N3)⋯O(7) | 0.86(3) | 2.035(12) | 2.886(4) | 169(3) |
| N(2)–H(2N1)⋯O(4) | 0.86(3) | 1.942(14) | 2.786(3) | 164(3) |
| 5 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(4) | 1.916(14) | 2.762(4) | 172(6) |
| N(1)–H(1N1)⋯O(3)ii | 0.86(4) | 1.918(12) | 2.781(4) | 171(3) |
| N(1)–H(1N2)⋯O(1) | 0.86(4) | 1.914(15) | 2.765(3) | 165(3) |
| N(1)–H(1N3)⋯O(4) | 0.86(4) | 1.934(13) | 2.793(4) | 168(3) |
| 6 | ||||
| O(4)–H(4O)⋯O(2) | 0.85(3) | 1.947(14) | 2.788(3) | 170(5) |
| N(1)–H(1N1)⋯O(3)ii | 0.86(3) | 1.930(12) | 2.785(3) | 168(3) |
| N(1)–H(1N2)⋯O(1) | 0.87(3) | 1.896(12) | 2.755(3) | 167(3) |
| N(1)–H(1N3)⋯O(4) | 0.86(3) | 1.926(12) | 2.791(3) | 169(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 7 | ||||
| N(1)–H(1N1)⋯O(3)ii | 0.86(3) | 1.982(17) | 2.767(3) | 151(2) |
| N(1)–H(1N2)⋯O(1) | 0.86(3) | 1.994(10) | 2.858(3) | 176(2) |
| N(1)–H(1N3)⋯O(4) | 0.86(3) | 1.926(9) | 2.791(3) | 176(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 8 | ||||
| N(1)–H(1N1)⋯O(4) | 0.86(4) | 1.910(11) | 2.767(4) | 172(3) |
| N(1)–H(1N2)⋯O(1) | 0.86(4) | 2.076(11) | 2.940(4) | 174(3) |
| N(1)–H(1N3)⋯O(5) | 0.86(4) | 1.913(15) | 2.754(4) | 162(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 9 | ||||
| N(1)–H(1N1)⋯O(2) | 0.86(3) | 1.923(11) | 2.783(3) | 172(3) |
| N(1)–H(1N2)⋯O(4) | 0.86(3) | 2.044(12) | 2.891(4) | 168(2) |
| N(1)–H(1N3)⋯O(3)iii | 0.86(3) | 2.320(15) | 3.112(4) | 153(2) |
| N(1)–H(1N3)⋯O(1)iii | 0.86(3) | 2.435(16) | 3.190(4) | 147(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 10 | ||||
| O(1W)–H(1W1)⋯O(2)i | 0.85(2) | 1.97(10) | 2.82(2) | 173.00 |
| O(1W)–H(2W1)⋯O(1) | 0.85(2) | 1.95(11) | 2.80(2) | 170.00 |
| O(2W)–H(2W2)⋯O(6)ii | 0.85(2) | 1.93(11) | 2.78(3) | 172(33) |
| O(2W)–H(1W2)⋯O(5) | 0.85(2) | 2.03(14) | 2.86(3) | 163.00 |
| O(7)–H(7O)⋯O(1)i | 0.85(3) | 1.96(18) | 2.78(3) | 162(47) |
| O(8)–H(8O)⋯O(5)ii | 0.85(3) | 1.90(15) | 2.74(3) | 161.00 |
| N(1)–H(1N1)⋯O(7) | 0.86(3) | 1.90(11) | 2.76(3) | 169.00 |
| N(1)–H(1N3)⋯O(1W) | 0.86(3) | 1.95(14) | 2.79(3) | 161(24) |
| N(1)–H(1N2)⋯O(3) | 0.86(3) | 1.92(13) | 2.77(3) | 163(23) |
| N(2)–H(2N1)⋯O(8) | 0.86(3) | 1.94(11) | 2.79(3) | 169.00 |
| N(2)–H(2N2)⋯O(4) | 0.86(3) | 1.93(13) | 2.76(3) | 161.00 |
| N(2)–H(2N3)⋯O(2W) | 0.86(3) | 1.99(11) | 2.85(3) | 169.00 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 11 | ||||
| N(1)–H(1N1)⋯O(4)ii | 0.868(10) | 1.986(12) | 2.847(5) | 171(5) |
| N(1)–H(1N2)⋯O(2)iii | 0.871(10) | 1.921(13) | 2.787(5) | 172(4) |
| N(1)–H(1N3)⋯O(1)iv | 0.867(10) | 1.948(17) | 2.800(5) | 167(5) |
| O(1W)–H(1W)⋯O(3) | 0.842(10) | 1.926(17) | 2.755(5) | 168(6) |
| O(4)–H(4O)⋯O(1W) | 0.854(11) | 2.36(12) | 2.771(6) | 110(9) |
| 12 | ||||
| O(1W)–H(1W)⋯O(2) | 0.85(4) | 1.934(16) | 2.769(4) | 167(5) |
| O(4)–H(4O)⋯O(1W) | 0.85(4) | 2.03(3) | 2.793(4) | 150(6) |
| N(1)–H(1N2)⋯O(4)ii | 0.86(4) | 1.965(10) | 2.829(4) | 177(4) |
| N(1)–H(1N1)⋯O(1)iii | 0.86(4) | 1.955(11) | 2.823(4) | 173(3) |
| N(1)–H(1N3)⋯O(3) | 0.86(4) | 1.939(14) | 2.799(4) | 168(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 13 | ||||
| O(1W)–H(1W)⋯O(2) | 0.85(3) | 1.940(13) | 2.781(3) | 168(4) |
| O(4)–H(4O)⋯O(1W)ii | 0.85(3) | 1.955(16) | 2.786(4) | 168(5) |
| N(1)–H(1N1)⋯O(3) | 0.86(3) | 1.932(11) | 2.803(4) | 173(3) |
| N(1)–H(1N2)⋯O(1)iii | 0.86(3) | 1.967(10) | 2.839(4) | 176(3) |
| N(1)–H(1N3)⋯O(4)iv | 0.86(3) | 1.941(10) | 2.807(4) | 174(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| 14 | ||||
| O(1W)–H(1W1)⋯O(3)ii | 0.85(2) | 2.08(2) | 2.874(3) | 156(5) |
| O(1W)–H(1W2)⋯O(4) | 0.85(2) | 2.19(3) | 2.949(4) | 148(5) |
| O(2W)–H(2W1)⋯O(2) | 0.85 | 2.35 | 3.167(8) | 162.2 |
| O(2W)–H(2W2)⋯O(1W)iii | 0.85 | 1.99 | 2.812(9) | 161.3 |
| N(1)–H(1N3)⋯O(2) | 0.86(2) | 1.963(10) | 2.823(2) | 174(2) |
| N(1)–H(1N2)⋯O(1)ii | 0.86(2) | 1.975(10) | 2.836(2) | 173(2) |
| N(1)–H(1N1)⋯O(4) | 0.86(2) | 2.013(9) | 2.861(3) | 169.6(19) |
Results and discussion
Experimental strategy
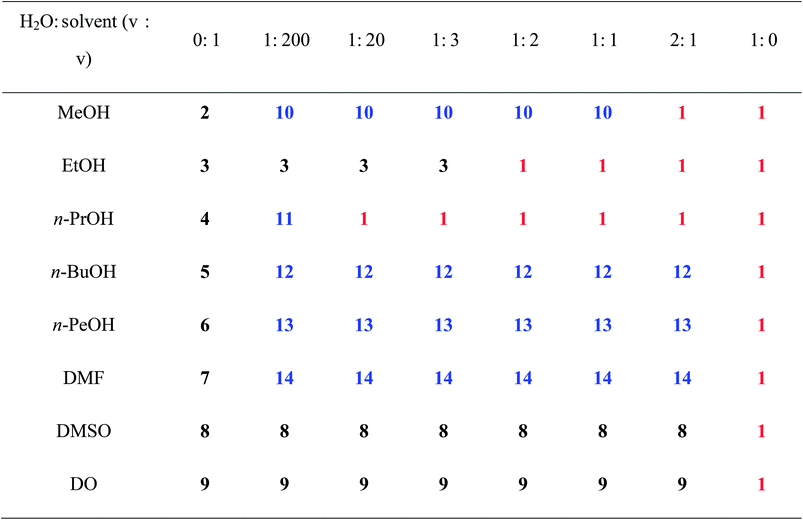
Except for the synthons, solvent molecules also have important effects on the structures and properties of organic salts. Our previous works presented that different solvent molecules have large effects on the supramolecular patterns and luminescent properties of organic salts assembled by arenesulfontate and arylamine.6b,c Hence, in this text, H2BPDSA and TPMA are first employed to construct organic salts in different solvents with different volume, carbochain length, and diverse hydrogen bonding donors and acceptors, that is, H2O, MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH, DMF, DMSO, and DO. As anticipated, diverse discrete, single chain, double chain and layer motifs are obtained in salts 1–9 owing to the different carbochain-length, configuration and volume in the nine types of solvents. It is interesting to note that none of salts 2–9 contains H2O molecules in its crystal structure, which is evidently different from our previously reported two series.6b,c In combined previous and present works, we ask whether the involvement of the most common H2O molecules during the assemble process could generate novel supramolecular structures? Bearing this idea in mind, water is introduced to the reaction system for synthesizing salts 2–9. Owing to the distinct property of organic solvents, four types of results are observed, as shown in Table 3. The first type covers MeOH and n-PrOH, which have clear boundary between the salts crystallized with pure or mixed solvents. When the ratio between H2O and MeOH, n-PrOH is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (v/v) or more, the salts crystallized with mixed solvents, 10 and 11, would be replaced by salt 1 that contains pure H2O. It should be indicated that salt 11 is very sensitive to H2O and can only be obtained with the ratio of 1
20 (v/v) or more, the salts crystallized with mixed solvents, 10 and 11, would be replaced by salt 1 that contains pure H2O. It should be indicated that salt 11 is very sensitive to H2O and can only be obtained with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (v/v). Therefore, only a few crystals 11 were separated from the reaction system. The second type, including n-BuOH, n-PeOH and DMF, can sustain the mixed solvents in their crystal structures in a wide ratio range. When the ratio between H2O and the three solvents becomes 1
200 (v/v). Therefore, only a few crystals 11 were separated from the reaction system. The second type, including n-BuOH, n-PeOH and DMF, can sustain the mixed solvents in their crystal structures in a wide ratio range. When the ratio between H2O and the three solvents becomes 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, that is, only a small amount of H2O is being introduced to the reaction system, the salts crystallized with mixed solvents, 12–14, would be obtained and never transfer into salt 1 in any ratio except for 1
200, that is, only a small amount of H2O is being introduced to the reaction system, the salts crystallized with mixed solvents, 12–14, would be obtained and never transfer into salt 1 in any ratio except for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (H2O
0 (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent). The third type contains EtOH, in which only the salts crystallized with pure H2O or EtOH molecules are obtained around the ratio of 1
solvent). The third type contains EtOH, in which only the salts crystallized with pure H2O or EtOH molecules are obtained around the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The fourth type, involving DMSO and DO, is not sensitive to H2O. No matter what the ratio is, only the salts (8 and 9) crystallized with pure solvents are obtained. These results show that solvent molecules with short carbochain tend to result in double chain structures, whereas solvent molecules with long carbochain tend to result in single chain structures. Moreover, the involvement of H2O molecules lead to the structural transformation from double chain to single chain and single chain to layer motif. Further crystal structure analyses indicate that such influence is achieved by its abundant hydrogen bonding modes (Scheme 1) as stated above.
2. The fourth type, involving DMSO and DO, is not sensitive to H2O. No matter what the ratio is, only the salts (8 and 9) crystallized with pure solvents are obtained. These results show that solvent molecules with short carbochain tend to result in double chain structures, whereas solvent molecules with long carbochain tend to result in single chain structures. Moreover, the involvement of H2O molecules lead to the structural transformation from double chain to single chain and single chain to layer motif. Further crystal structure analyses indicate that such influence is achieved by its abundant hydrogen bonding modes (Scheme 1) as stated above.
a With these ratios (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 to 2 200 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the two solvents are layered. After several days, salts 12 and 13 are obtained at the junction. 1), the two solvents are layered. After several days, salts 12 and 13 are obtained at the junction. |
|---|
 |
Structure analyses of anhydrous salts (2–9)
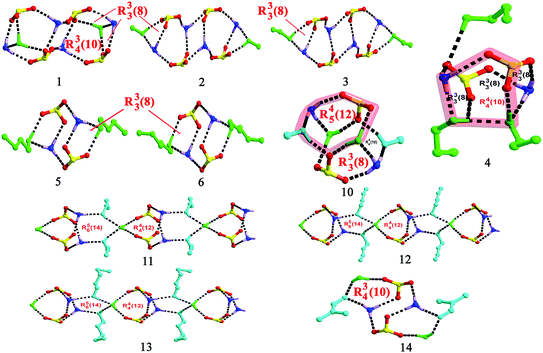
Single-crystal X-ray analyses indicate that crystal structures for salts 2–9 (Fig. S1†) contain one 2(HTPMA+)–BPDSA2− ion pair and several organic solvent molecules, that is, one for salts 2, 3 and 7, two for salts 5 and 6, three for salt 4, and four for salts 8 and 9. The modulation of solvent molecules with different volume and ability for building hydrogen bonds results in multiple supramolecular patterns in these eight salts.For salts 2 and 3, although they crystallize from different solvents, similar supramolecular patterns are observed. As shown in Fig. 1, four –SO3 groups and four –NH3 groups interact with each other through N–H⋯O hydrogen bonding interactions to form a finite [(SO3)4(NH3)4] cluster, which contains three interconnecting R44(12) rings. Then, two solvent molecules decorate the two sides of the cluster with different hydrogen bonds, thus generating a [(SO3)4(NH3)4(solvent)2] (solvent = MeOH and EtOH) cluster with one additional R33(8) ring (Scheme 2). Adjacent clusters are further bridged by biphenyl rings of BPDSA2− dianions to generate double chain along a certain direction (Fig. 1).
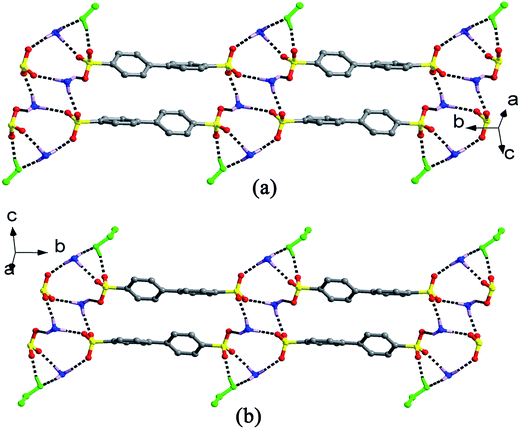 | ||
| Fig. 1 Double chain structures of salts 2 and 3 extended by the biphenyl rings of the BPDSA2− dianions bridging [(SO3)4(NH3)4(solvent)2] (solvent = MeOH and EtOH: green ball-and-stick) clusters. | ||
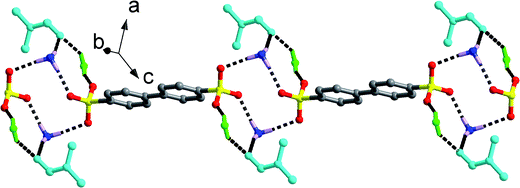
Salts 4–7, crystallized from n-PrOH, n-BuOH, n-PeOH and DMF solution, also exhibit similar supramolecular patterns, as shown in Fig. 2. Two –SO3 groups and two –NH3 groups interact with each other through N–H⋯O hydrogen bonds to form a classic [2 + 2] ring described by the graph set R44(12). Then, different amounts of solvent molecules attach to the [2 + 2] ring in different hydrogen bonding modes and generate distinct clusters. In salt 4, three n-PrOH molecules decorate the [2 + 2] ring with A0D1 (O9), A1D2 (O8) and A2D1 (O7) modes (A = Acceptor, D = Donor; superscript representing the number of A or D), leading to [(SO3)2(NH3)2(n-PrOH)3] cluster formation with one additional R44(10) ring and three additional R33(8) rings (Scheme 2). In comparison, only two n-BuOH, n-PeOH and DMF molecules in salts 5–7 attach to the [2 + 2] ring with simple A1D1 (5 and 6) and A1D0 (7) modes. Such connection results in the formation of a [(SO3)2(NH3)2(solvent)2] (solvent = n-BuOH, n-PeOH and DMF) cluster with two additional R33(8) rings in 5 and 6 (Scheme 2). Adjacent clusters are further bridged by biphenyl rings of BPDSA2− dianions to generate single chain along a certain direction (Fig. 2).
Compared with the single and double chain structures in salts 2–7, only a discrete [(SO3)(NH3)(DMSO)2] motif for salt 8 is observed with each H atom of the –NH3 group forming hydrogen bonds with one sulfonate O atom and two dimethylsulfoxide O atoms (Fig. S1†). Hence, the hydrogen bonding modes for –SO3 and –NH3 groups are A1A0A0 and A1A1A1. The structure difference of this salt could be mainly ascribed to the triangular pyramid configuration of DMSO molecule arising from the lone pair electrons of the S atom. The electrostatic attraction between the lone pair electrons of the S atom and the sulfonate O3 atom leads to a short S⋯O distance of 3.351(6) Å, which then keeps additional –NH3 group away from the –SO3 group.
The chairform DO molecules in salt 9 have two opposite oxygen atoms that can serve as acceptors to form a different supramolecular pattern and packing diagram from the above-mentioned solvent molecules. As shown in Fig. 3, two symmetry-related –SO3 groups and two symmetry-related –NH3 groups interact with each other through N–H⋯O hydrogen bonding interactions to form a classic [2 + 2] ring described by the graph set R44(12). The DO molecules form hydrogen bonds with adjacent [2 + 2] rings at the points of H1N2, which extend these discrete rings into a [(SO3)2(NH3)2(DO)]n chain structure along b axis. Then, the biphenyl rings of the BPDSA2− anions join these chains to generate a (4,4) layer motif by considering the [2 + 2] rings as four-connected nodes.
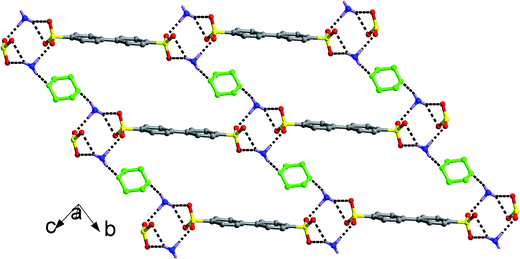 | ||
| Fig. 3 (4,4) Layer structure of salt 9 formed by the linkage of [(SO3)2(NH3)2(DO)]n chains through BPDSA2− dianions. The DO molecules were denoted as green balls-and-sticks. | ||
From the above description, one can see that solvent molecules with both hydrogen bonding donors and acceptors (MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH) tend to result in single or double chain structures. In comparison, the solvents without hydrogen bonding donors (DMF, DMSO, DO) lead to diverse structures according to the number of acceptors in solvent molecules. Solvent with single acceptor generates discrete or chain structure, whereas solvent with double acceptors generate a layer structure. It can be concluded that solvent molecules with different carbochain, volume and ability for building hydrogen bonds have an important effect on the supramolecular patterns formed by –SO3 and –NH3 groups, which then certainly result in five types of packing diagram.
Structure analyses of hydrous salts (1, 10–14)
Reaction of H2BPDSA and TPMA in pure water and mixed water/solvents (MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH, DMF, DMSO and DO) leads to the formation of another six new salts. As shown in Fig. S1,† crystal structures for salts 1 and 10–14 contain one 2(HTPMA+)–BPDSA2− ion pair, two solvent molecules (MeOH in salt 10, n-PrOH in salt 11, n-BuOH in salt 12, n-PeOH in salt 13 and DMF in salt 14) and several water molecules, that is, one for salts 1, 11–13, two for salt 10, and four for salt 14. In view of the diverse hydrogen bonding modes of water molecules (Scheme 1), these six salts present different supramolecular patterns.For salt 1, although it crystallizes from different solvents from that of salts 2 and 3, it exhibits a similar supramolecular pattern as observed in salts 2 and 3. As shown in Fig. 4, four –SO3 groups and four –NH3 groups interact with each other through N–H⋯O hydrogen bonding interactions to form a finite [(SO3)4(NH3)4] cluster, which contains three interconnecting R44(12) rings. Then, two H2O molecules decorate the two sides of the cluster with different hydrogen bonds, thus generating a [(SO3)4(NH3)4(H2O)2] cluster with one additional R34(10) ring and two additional R33(8) rings (Scheme 2). The H2O molecules exhibit HB′3 mode as illustrated in Scheme 1. Adjacent clusters are further bridged by biphenyl rings of BPDSA2− dianions to generate double chains along a certain direction (Fig. 4).
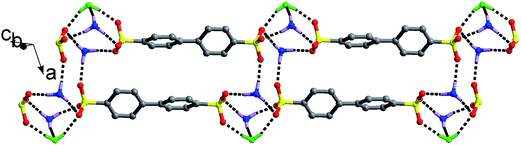 | ||
| Fig. 4 Double chain structure of salt 1 extended by the biphenyl rings of the BPDSA2− dianions bridging [(SO3)4(NH3)4(H2O)2] clusters with the H2O molecules being denoted as green balls-and-sticks. | ||
The –SO3 groups and –NH3 groups in salt 10 form the smallest [(SO3)(NH3)] units, which are linked by pairs of H2O molecules and MeOH molecules through N/O–H⋯O hydrogen bonds to form “cage” motifs, [(SO3)2(NH3)2(H2O)2(MeOH)2] with two R33(8), two R44(10) and two R45(12) rings (Fig. 5, Scheme 2). The H2O molecules exhibit HB′3 mode (Scheme 1) as seen in salt 1, which break down the direct connection between adjacent [(SO3)(NH3)] units. Adjacent cage motifs are further bridged by the biphenyl rings of the BPDSA2− anions to form single chains.
Salts 11–13 exhibit similar supramolecular patterns, as shown in Fig. 6. Two –SO3 groups and two –NH3 groups interact with each other through N–H⋯O hydrogen bonds to form a classic R44(12) ring. Then, two solvent molecules (n-PrOH, n-BuOH and n-PeOH) attach to this ring on the same side at the points of two H atoms of –NH3 groups. In addition, one water molecule attaches to the 12-membered ring on the other side at the points of two sulfonate O atoms. The left O atoms of H2O molecules form O–H⋯O hydrogen bonds with H atoms of solvent molecules, which then extend adjacent rings into infinite [(SO3)2(NH3)2(solvent)2(H2O)]n (solvent = n-PrOH, n-BuOH and n-PeOH) chains along the c-axis with two R44(12) and two R56(14) rings (Scheme 2). In this case, the solvent molecules exhibit A1D1 mode and H2O molecules exhibit HB4 mode, as illustrated in Scheme 1. Adjacent chains are further bridged by biphenyl rings of BPDSA2− dianions to generate a (4,4) layer motif (Fig. 6). It is interesting to note that these (4,4) layers contain quadrangular windows with the diagonal distances (two opposite S atoms) in the three salts being 14.25 × 14.25 Å2 (11), 14.18 × 14.18 Å2 (12) and 14.03 × 14.03 Å2 (13). Such windows are too large so that they can accommodate a pair of n-PrOH, n-BuOH and n-PeOH molecules.
As shown in Fig. 7, two additional H2O molecules in salt 14 attach to the [(SO3)2(NH3)2(DMF)2] cluster in HB′2 mode (Scheme 1), which generate two new R34(10) rings (Scheme 2). In a word, the obtainment of the abovementioned six salts clearly exhibits the influence of hydrogen bonding modes of H2O molecules on the supramolecular patterns and packing diagram.
Role of water molecules on structure modulation
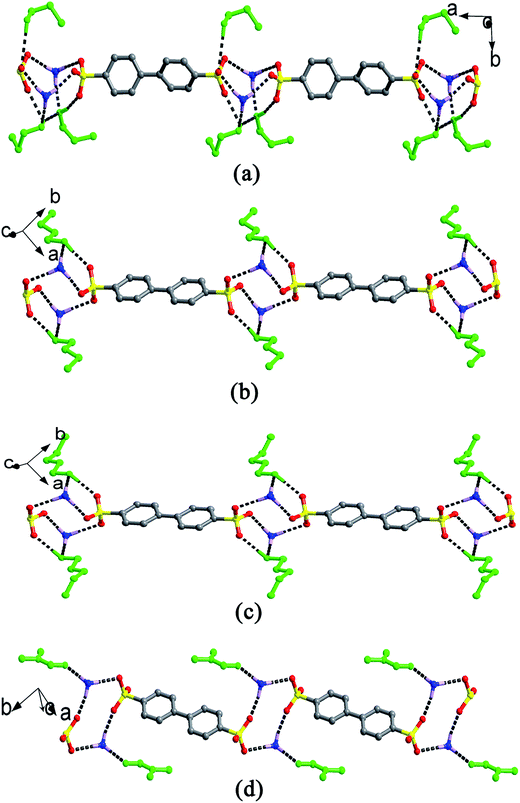
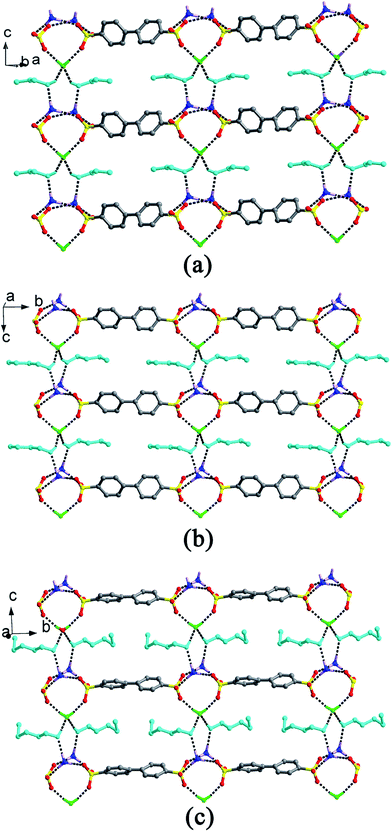
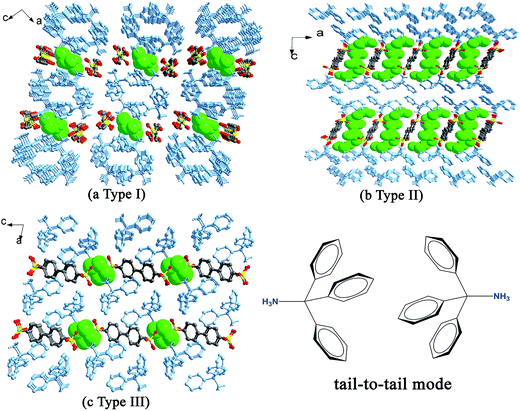
When H2BPDSA and TPMA were added to mixed water/solvents (MeOH, EtOH, n-PrOH, n-BuOH, n-PeOH, DMF, DMSO and DO), two types of results were obtained. The first type covers EtOH, DMF and DO, which exhibit no effect on the final structures. That is, salts 3, 8 and 9 could be obtained in pure or mixed solvents. Owing to the difference of solvent molecules in the three salts, three different types of packing diagram are observed. In salt 3, pairs of HTPMA+ cations arrange in tail-to-tail mode to form column motifs along the b-axis, which extend the layers of BPDSA2− dianions into a pillared layered supramolecular network (Type I). The EtOH molecules fill in the channels formed by adjacent column pillars and BPDSA2− dianions (Fig. 8a). As shown in Fig. 8b, the HTPMA+ cations in salt 8 arrange in tail-to-tail mode to form layer motifs which are then pillared by BPDSA2− dianions to generate a pillared layered supramolecular network (Type II). The large separation between adjacent opposite biphenyl rings of BPDSA2− dianions allows the residence of four DMSO molecules. Salt 9 shows a new Type III packing diagram, in which the HTPMA+ cations arrange along the b-axis in tail-to-tail mode to form column motifs, which are then sandwiched between BPDSA2− dianions. The interlayer spaces are filled by DO molecules (Fig. 8c). Moreover, salt 1 presents the similar packing diagram as that of salt 3 due to their similar chain structure (Fig. S2†).On the contrary, the second type involving the other five organic solvents presents different supramolecular patterns and packing diagrams before and after addition of water. For clarity, pairs of salts containing the same organic solvent molecule are described together.
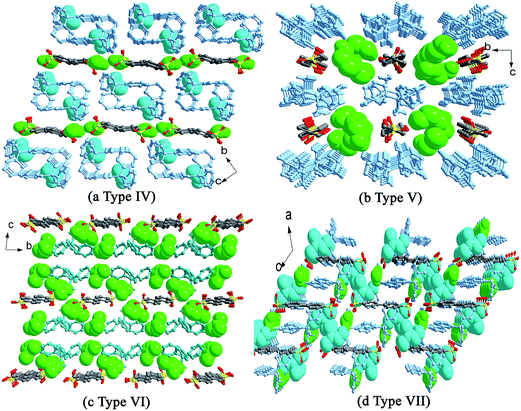
For salts 2 and 10, the participation of H2O molecules makes the double chain structure in 2 change into the single chain in 10. In addition, their packing diagrams are also different. Salt 2 presents the similar packing diagram as that of salt 3 due to their similar chain structure (Fig. S2†). Salt 10 exhibits a new type of packing diagram. As shown in Fig. 9a, the HTPMA+ cations arrange along the a-axis in tail-to-tail mode to form column motifs, which are then sandwiched between BPDSA2− dianions, thus generating layer structure. The packing of adjacent layers affords the formation of Type IV packing diagram. The H2O molecules locate in the sulfonate layers, whereas the MeOH molecules locate in the triphenylmethanaminium layers.
For salts 4–6 and 11–13, the participation of H2O molecules makes the single chain structures in 4–6 change into (4,4) layer motifs in 11–13. The packing diagrams of 5 and 6 are in accord with that of salt 9 (Fig. S3†). In contrast, salt 4 presents a different packing diagram. As shown in Fig. 9b, pairs of HTPMA+ cations arrange in tail-to-tail mode to form tabular motifs along the a-axis, which are further extended by BPDSA2− dianions into layers. Subsequently, the packing of adjacent layers affords the formation of Type V packing diagram. The horseshoe n-PrOH molecules fill in the interlayer space. Interestingly, salts 11–13 exhibit similar Type V packing diagram with that of salt 4 owing to the modulation of H2O molecules (Fig. S4†).
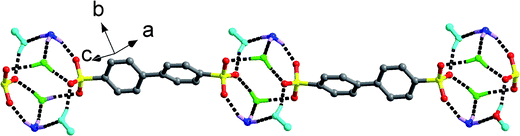
Different from the significant effects of H2O molecules in the abovementioned two series, salt 14 only exhibits changes in its packing diagram with the participation of H2O molecules. The DMF molecule possesses a similar structure with isobutanol by neglecting the hydrogen atom of hydroxyl group. Therefore, it should lead to the similar packing diagram with that crystallized from n-BuOH solvent. However, the absence of the hydroxyl H atom and the rigidity of DMF molecule bring about a new type of packing diagram, Type VI (Fig. 9c). Different from Type III, the HTPMA+ columns in salt 7 are not completely sandwiched between BPDSA2− dianions, which then cause the formation of larger intra- and inter-layer spaces to accommodate more DMF molecules. In salt 14, the HTPMA+ cations arrange along the b-axis in tail-to-tail mode and form layer motifs with large channels, which are inserted by BPDSA2− dianions. The packing of adjacent layers affords the formation of Type VII packing diagram with the H2O and DMF molecules filled in the intra- and inter-layer space (Fig. 9d).
IR spectroscopy
The stretching bands around 3444–3342 cm−1 in the IR spectra of salts 1–10 and 12–14, as well as the broad and strong stretching bands appearing in the range of 3074–2809 cm−1, can be attributed to the existence of the hydroxyl groups, primary ammonium cations, water molecules, as well as the formation of extensive hydrogen bonding interactions. The bands appearing in the range of 1602–1446 cm−1 in salts 1–10 and 12–14 are ascribed to the skeleton stretching vibrations of the BPDSA2− dianions and HTPMA+ cations. Moreover, the peak at 1677 and 1667 cm−1 in salts 7 and 14 can be attributed to the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the DMF molecule. The IR spectra of these crystals clearly exhibit the characteristic vibrations of νas(SO3−), which are present at the range of 1235–1167 cm−1 and 1138–1126 cm−1, whereas the νs(SO3−) absorptions are at the range of 1037–1029 cm−1 and 1009–998 cm−1.6 The variety of characteristic νas(SO3−) vibrations indicate the abundant hydrogen bonding modes of the sulfonate groups in the thirteen salts.
O stretching vibration of the DMF molecule. The IR spectra of these crystals clearly exhibit the characteristic vibrations of νas(SO3−), which are present at the range of 1235–1167 cm−1 and 1138–1126 cm−1, whereas the νs(SO3−) absorptions are at the range of 1037–1029 cm−1 and 1009–998 cm−1.6 The variety of characteristic νas(SO3−) vibrations indicate the abundant hydrogen bonding modes of the sulfonate groups in the thirteen salts.
Luminescent properties
As stated above, salt 11 is very sensitive to H2O and is difficult to obtain in high quantities. Hence, its luminescent properties are not performed in this study. As shown in Fig. 10, the luminescent properties of salts 1–10 and 12–14 and the reactants in the solid state at room temperature were investigated, in which the emission spectrum for TPMA molecules (λex = 345 nm, λem = 423 nm) has been reported in our previous work.6 In comparison with the weak emission of TPMA, H2BPDSA molecule exhibits stronger emission maximum at 361 nm (λex = 310 nm). Both the emissions of the two molecules could be attributed to the π–π* transitions.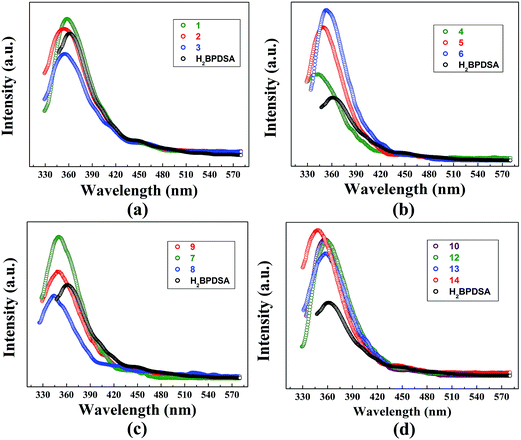 | ||
| Fig. 10 Emission spectra of H2BPDSA salts 1–3 (a), 4–6 (b), 7–9 (c) and 10, 12–14 (d) in the solid-state at room temperature. | ||
In view of the aforementioned supramolecular structures of these thirteen organic salts, the luminescent properties of salts 1–10 and 12–14 are also discussed in four groups: salts 1–3, salts 4–6, salts 7–9, and salts 10, 12–14. For each group, the properties of the solvent molecules play crucial roles in the emission intensity. Usually, high polar solvents cause red-shift and decreasing of emission intensity.6,15e However, in these four groups, this rule is not strictly obeyed. As shown in Fig. 10a, upon the same excitation at 309 nm, the emission spectra of salts 1–3 in the first group exhibit similar shapes and maxima at 358, 356, and 355 nm, respectively. With the decrease of the solvent polarity, blue-shift and decreasing of emission intensity are observed with the sequence of 1 to 3. The inverse order of emission intensity is mainly attributed to the increase of carbochain, which intensifies the thermal vibrations and decreases the emission intensity. In contrast, the emission intensity of salts 4–6 in the second group obeys the rule that high polar solvents cause the decreasing of emission intensity. Subsequently, the emission intensity follows the order 6 > 5 > 4 (Fig. 10b). Moreover, they also exhibit similar shape and maximum at 343 nm (λex = 310 nm), 349 nm (λex = 309 nm), and 353 nm (λex = 314 nm) upon similar excitations. In the third group, the emission maximum for salts 7–9 appears at 350, 344, and 348 nm upon the same excitation at 309 nm (Fig. 10c). Owing to the high polarity of DMSO molecules, salt 8 exhibits the weakest emission intensity among the three salts. Salt 7 has the similar supramolecular patterns with those of salts 4–6, which makes salt 7 showing the strongest emission intensity. It can be concluded from the abovementioned results that emission intensity of salts is determined by the synergistic effect of the polarity and carbochain of solvent molecules. For the solvent molecules with short carbochain, thermal vibrations of carbochain play crucial role, whereas the solvent polarity play the leading role for the solvent molecules with long carbochain.
Fig. 10d presents the emission spectra of the fourth group, in which salts 10 and 12–14 exhibit similar shape and maximum at 357 nm (λex = 314 nm), 359 nm (λex = 309 nm), 358 nm (λex = 311 nm), and 348 nm (λex = 310 nm) upon similar excitation. In comparison with the pure solvent containing salts 1, 2 and 5–7, these four salts present stronger emission intensity. For salts 10, 12 and 13, their layer supramolecular patterns intensify the rigidity of the target structures, which further increases the emission intensity. In addition, with the increase of the carbochain, the thermal vibrations of solvent carbochains gradually intensify from MeOH in salt 10 to n-PeOH in salt 13. Therefore, partially excited electrons are quenched through the non-radioactive transition, which then leads to the decline of emission intensity.13b,c In this sense, the emission intensity of the three salts follows the order 10 > 12 > 13. An exception in this group is the stronger emission of 14, which can be attributed to the side-to-plane π⋯π interactions among adjacent phenyl rings of HTPMA+ cations and BPDSA2− dianions (Fig. S5†). Such π⋯π interactions could increase the mobility of the electrons and further enhance the emission intensity.6b,c,18 For all the four groups, their emission intensities are roughly accordance with their photographs under UV source (Fig. S8†).
Moreover, from the comparison of the emission spectra in Fig. 10a, b and d, in contrast to the pure solvent containing salts 2, 5, 6, and 7, the involvement of H2O molecules in salts 10 and 12–14 causes different changes in the emission intensity, which could be caused by the transformation of supramolecular structures after the small water molecules being involved in the supramolecular skeletons. For salts 2 and 10, the distribution of water molecules in the sulfonate layer of 10 can effectively increase the mobility of the electrons than that of salts 2, which then increases the emission intensity of 10. n-BuOH and n-PeOH involved salts present the same supramolecular structures before (5 and 6) and after (12 and 13) the involvement of H2O molecules. Different from the former case, the anhydrous salts 5 and 6 present stronger emission intensity than that of hydrous salts 12 and 13. This is mainly ascribed to the fact that the involvements of water molecules increases the distance between adjacent chains (Fig. 6), which then amplifies the activity space of n-BuOH and n-PeOH molecules. Therefore, the thermal vibrations of carbochain intensify and decreases the emission intensity in hydrous salts 12 and 13. The emission intensity for salts 7 and 14 follows the change of salts 2 and 10, which is also caused by the fact that the small water molecules involved in the supramolecular skeletons increases the crystal packing through diverse hydrogen bonding modes and thus decreases the rotational degrees of freedom of the synthons.
Conclusions
In summary, fourteen salts have been synthesized by the solvent reaction of 1,1′-biphenyl-4,4′-disulfonic acid and triphenylmethylamine in nine types of solvents. For the pure solvent containing salts 1–9, solvent molecules with short carbochain tend to result in double chain structures, whereas solvent molecules with long carbochain tend to result in single chain structures. In addition, different configuration of solvent molecules such as the planar DMF, triangular pyramidal DMSO, chair-type DO lead to diverse single chain, discrete and layer motifs. These different supramolecular patterns then lead to five types of packing diagrams. In contrast, H2O molecules in salts 10–14 cause the structural transformation from double and single chains to single chains and layer motifs with diverse HB′2, HB′3, HB4 modes and results in two new types of packing diagrams. Moreover, the involvement of water molecules makes salts 10 and 14 show stronger emission intensity, whereas salts 12 and 13 show weaker emission intensity than their corresponding anhydrous salts. The present study provides a good example that the nature of reactant and hydrogen bonding modes of H2O molecules could effectively influence the structures of target supramolecules, which then further regulate the luminescent properties.Acknowledgements
This work is financial supported by the National Natural Science Foundation of China (51302067), Specialized Research Fund for the Doctoral Program of Higher Education of China (20132301120002), Key Project of Education bureau of Heilongjiang Province (no. 12511z023, no. 2011CJHB006), and the Innovation team of Education bureau of Heilongjiang Province (2013td002). We thank the University of Heilongjiang for supporting this study.Notes and references
- (a) D. Yan, A. Delori, G. O. Lloyd, T. Friščić, G. M. Day, W. Jones, J. Lu, M. Wei, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2011, 50, 12483 CrossRef CAS PubMed; (b) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493 RSC.
- (a) L. Fábían, N. Hamill, K. S. Eccles, H. A. Moynihan, A. R. Maguire, L. McCausland and S. E. Lawrence, Cryst. Growth Des., 2011, 11, 3522 CrossRef; (b) J. I. Arenas-García, D. Herrera-Ruiz, K. Mondragón-Vásquez, H. Morales-Rojas and H. Höpfl, Cryst. Growth Des., 2012, 12, 811 CrossRef.
- (a) K. T. Holman, A. M. Pivovar and M. D. Ward, Science, 2001, 294, 1907 CrossRef CAS PubMed; (b) K. T. Holman, A. M. Pivovar, J. A. Swift and M. D. Ward, Acc. Chem. Res., 2001, 34, 107 CrossRef CAS PubMed; (c) R. Custelcean and M. D. Ward, Angew. Chem., Int. Ed., 2002, 41, 1724 CrossRef CAS; (d) N. J. Burke, A. D. Burrows, M. F. Mahon and J. E. Warren, CrystEngComm, 2006, 8, 931 RSC.
- (a) P. C. Leverd, P. Berthault, M. Lance and M. Nierlich, Eur. J. Inorg. Chem., 2000, 133 CrossRef CAS; (b) F. Perret, V. Bonnard, O. Danylyuk, K. Suwinska and A. W. Coleman, New J. Chem., 2006, 30, 987 RSC; (c) X. Su, D.-S. Guo and Y. Liu, CrystEngComm, 2010, 12, 947 RSC; (d) G. Bolla, S. Mittapalli and A. Nangia, CrystEngComm, 2014, 16, 24 RSC.
- (a) P. H. Stahl and C. G. Wermuth, Handbook of Pharmaceutical Salts: Properties, Selection and Use, Wiley-VCH, Chichester, 2002 Search PubMed; (b) S. L. Childs, G. P. Stahly and A. Park, Mol. Pharmaceutics, 2007, 4, 323 CrossRef CAS PubMed; (c) C. B. Aakeröy, M. E. Fasulo and J. Desper, Mol. Pharmaceutics, 2007, 4, 317 CrossRef PubMed.
- (a) Z.-P. Deng, L.-H. Huo, H. Zhao and S. Gao, Cryst. Growth Des., 2012, 12, 3342 CrossRef CAS; (b) Y.-N. Li, L.-H. Huo, Z.-P. Deng, X. Zou, Z.-B. Zhu, H. Zhao and S. Gao, Cryst. Growth Des., 2014, 14, 2381 CrossRef CAS; (c) Y.-N. Li, L.-H. Huo, Y.-Z. Yu, F.-Y. Ge, Z.-P. Deng, Z.-B. Zhu and S. Gao, RSC Adv., 2014, 4, 64802 RSC.
- R. G. Bryant, M. A. Johnson and P. J. Rossky, Acc. Chem. Res., 2012, 45, 1 CrossRef CAS PubMed.
- For example, see: (a) M. H. Mir and J. J. Vittal, Angew. Chem., Int. Ed., 2007, 46, 5925 CrossRef CAS PubMed; (b) J. C. Jin, Y. Y. Wang, P. Liu, R. T. Liu, C. Ren and Q. Z. Shi, Cryst. Growth Des., 2010, 10, 2029 CrossRef CAS; (c) R. Vaidhyanathan, C. A. Bridges, D. Bradshaw and M. J. Rosseinsky, Cryst. Growth Des., 2010, 10, 4348 CrossRef CAS; (d) G.-G. Luo, H.-B. Xiong, D. Sun, D.-L. Wu, R.-B. Huang and J.-C. Dai, Cryst. Growth Des., 2011, 11, 1948 CrossRef CAS; (e) D. Sun, D.-F. Wang, X.-G. Han, N. Zhang, R.-B. Huang and L.-S. Zheng, Chem. Commun., 2011, 47, 746 RSC; (f) X. Li, X. Xu, D. Yuan and X. Weng, Chem. Commun., 2011, 48, 9014 RSC.
- For example, see: (a) L. Cheng, J. B. Lin, J. Z. Gong, A. P. Sun and B. H. Ye, Cryst. Growth Des., 2006, 6, 2739 CrossRef CAS; (b) S. R. Choudhury, A. D. Jana, E. Colacio, H. M. Lee, G. Mostafa and S. Mukhopadhyay, Cryst. Growth Des., 2007, 7, 212 CrossRef CAS; (c) X. F. Shi and W. Q. Zhang, Cryst. Growth Des., 2007, 7, 595 CrossRef CAS; (d) Z.-P. Deng, L.-H. Huo, S. Gao and H. Zhao, Z. Anorg. Allg. Chem., 2010, 636, 835 CrossRef CAS PubMed; (e) H. B. Xiong, D. Sun, G. G. Luo, R. B. Huang and L. S. Zheng, J. Mol. Struct., 2011, 990, 164 CrossRef CAS PubMed.
- For example, see: (a) X. J. Luan, Y. C. Chu, Y. Y. Wang, D. S. Li, P. Liu and Q. Z. Shi, Cryst. Growth Des., 2006, 6, 812 CrossRef CAS; (b) R. Carballo, B. Covelo, N. Fernández-Hermida, E. García-Martínez, A. B. Lago, M. Vázquez and E. M. Vázquez-López, Cryst. Growth Des., 2006, 6, 629 CrossRef CAS; (c) S. Upreti, A. Datta and A. Ramanan, Cryst. Growth Des., 2007, 7, 966 CrossRef CAS.
- For example, see: (a) R. Carballo, B. Covelo, C. Lodeiro and E. M. Vázquez-López, CrystEngComm, 2005, 7, 294 RSC; (b) C. M. Jin, Z. Zhu, Z. F. Chen, Y. J. Hu and X. G. Meng, Cryst. Growth Des., 2010, 10, 2054 CrossRef CAS.
- (a) J. Canivet, A. Fateeva, Y. Guo, B. Coasne and D. Farrusseng, Chem. Soc. Rev., 2014, 43, 5594 RSC; (b) K. Tan, S. Zuluaga, Q. Gong, P. Canepa, H. Wang, J. Li, Y. J. Chabal and T. Thonhauser, Chem. Mater., 2014, 26, 6895 CrossRef; (c) S. Hameury, P. d. Frémont, P.-A. R. Breuil, H. Olivier-Bourbigou and P. Braunstein, Organometallics, 2015 DOI:10.1021/om5008506.
- (a) S. Kohmoto, S. Okuyama, N. Yokota, M. Takahashi, K. Kishikawa, H. Masu and I. Azumaya, Cryst. Growth Des., 2011, 11, 3698 CrossRef CAS; (b) V. N. Yadav and C. H. Görbitz, CrystEngComm, 2013, 15, 439 RSC; (c) K. Ohno, T. Sugaya, M. Kato, N. Matsumoto, R. Fukano, Y. Ogino, S. Kaizaki, T. Fujuhara and A. Nagasawa, Cryst. Growth Des., 2014, 14, 3675 CrossRef CAS; (d) P. Li, O. Alduhaish, H. D. Arman, H. Wang, K. Alfooty and B. Chen, Cryst. Growth Des., 2014, 14, 3634 CrossRef CAS.
- V. Videnova-Adrabinska, Coord. Chem. Rev., 2007, 251, 1987 CrossRef CAS PubMed.
- (a) Y. Mizobe, M. Miyata, I. Hisaki, Y. Hasegawa and N. Tohnai, Org. Lett., 2006, 8, 4295 CrossRef CAS PubMed; (b) Y. Mizobe, H. Ito, I. Hisaki, M. Miyata, Y. Hasegawa and N. Tohnai, Chem. Commun., 2006, 2126 RSC; (c) N. Tohnai, Y. Mizobe, M. Doi, S. Sukata, T. Hinoue, T. Yuge, I. Hisaki, Y. Matsukawa and M. Miyata, Angew. Chem., Int. Ed. Engl., 2007, 46, 2220 CrossRef CAS PubMed; (d) Y. Mizobe, T. Hinoue, A. Yamamoto, I. Hisaki, M. Miyata, Y. Hasegawa and N. Tohnai, Chem.–Eur. J., 2009, 15, 8175 CrossRef CAS PubMed; (e) T. Hinoue, M. Miyata, I. Hisaki and N. Tohnai, Angew. Chem., Int. Ed. Engl., 2012, 51, 155 CrossRef CAS PubMed.
- (a) D. S. Reddy, S. Duncan and G. K. H. Shimizu, Angew. Chem., Int. Ed., 2003, 42, 1360 CrossRef CAS PubMed; (b) V. Chandrasekhar and P. Singh, Cryst. Growth Des., 2010, 10, 3077 CrossRef CAS.
- G. M. Sheldrick, SHELXTL-97, Program for Crystal Structure Solution and Refinement, University of Gottingen, Germany, 1997 Search PubMed.
- D. Rendell, Fluorescence and Phosphorescence, Wiley, New York, 1987 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures, PXRD patterns, and TG curves for all salts. CCDC 1044769–1044782. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra04497a |
| This journal is © The Royal Society of Chemistry 2015 |