Confined nanospace pyrolysis for synthesis of N-doped few-layer graphene-supported yolk–shell carbon hollow spheres for electrochemical sensing†
Sen Liua,
Yong Zhanga,
Ziying Wanga,
Bo Yua,
Shenguan Songa and
Tong Zhang*ab
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, P. R. China. E-mail: zhangtong@jlu.edu.cn; Fax: +86 431 85168270; Tel: +86 431 85168385
bState Key Laboratory of Transducer Technology, Chinese Academy of Sciences, P. R. China
First published on 20th April 2015
Abstract
N-doped few-layer graphene-supported yolk–shell carbon hollow spheres (CHSs/N-G) have been prepared by confined nanospace pyrolysis of GO–polypyrrole (GO–PPy) hybrids obtained by self-assembly of GO and PPy particles. More importantly, such CHSs/N-G exhibits electrochemical catalytic activity for oxidation of L-cysteine, leading to a high performance L-cysteine sensor with detection limit and linear range of 0.2 μM and 2 μM to 80 μM, respectively.
Introduction
During the past few years, yolk–shell materials, a new class of special core/shell structures with a distinctive core@void@shell configuration,1–3 have attracted much attention due to their unique properties, which ensure their wide applications in the fields of catalysis,4 adsorption,5 gas sensing,6 lithium-ion battery,7 drug delivery,8 and so on. It is well known that the structure and properties of the core materials and shell materials play an important role in their applications, and the regulating and controlling the structure of yolk–shell materials are generally considered to potentially realize the high performances for their applications. This situation has prompted a recent drive towards the exploration of highly effective methods for preparation of yolk–shell materials.Up to now, several methods have been successfully developed to prepare yolk–shell materials and the composites of the yolk–shell materials include metal oxides, noble metals, conducting polymers, metal–organic framework, etc. Among all the methods, SiO2-assisted hard-template method has been proven as an effective method to prepare various yolk–shell materials, where the yolk–shell structure is obtained after removal of SiO2 from the core/SiO2/shell hybrids. For example, Au@SiO2,9 Au@TiO2,10 Au@carbon,11 Pt@carbon,12 Fe3O4@poly(methacrylic acid) (PMAA),13 have been successfully prepared. In addition, some modified methods have also been used to prepare yolk–shell materials. For example, Zhao and co-workers reported hydrothermal etching assisted crystallization route to prepare F3O4@titanate microspheres;14 Yang and co-workers developed an organosilane-assisted selective etching method for preparation of basic core and acidic shell nanoreactor for cascade reactions;15 Tsung and co-workers prepared Pt@ZIF-8 using Cu2O as template instead of SiO2.16 Later, poly(acrylic acid),17 and PMAA,18 have also been used as templates for preparation of yolk–shell materials. However, all these above mentioned methods exhibit obvious shortcomings, such as multiple steps for preparation of hybrids, high-cost of the organosilane, and so on. As a result, some new methods have been developed. For example, Kang and co-workers developed a simple spray pyrolysis process for preparation of yolk–shell materials, such as LiMn2O4,19 Pd@SnO2,20 and ZnO@Mn3O4.21 Unfortunately, a spray pyrolysis equipment is required. Furthermore, the vesicle soft-template method,22,23 ship-in-bottle,24–26 and Kirkendall27 have also been used for preparation of yolk–shell materials. Although much progress has been achieved, the improvements are still far from overcoming the many limitations, such as complexion synthesis process, high cost, which still prevent the practical implementation of the yolk–shell materials.
More recently, Lu and co-workers have developed a confined nanospace pyrolysis method to prepare hollow structure, providing a new and effective method to construct hollow structures.28–30 To the best of our knowledge, however, using confined nanospace pyrolysis method for preparation of yolk–shell materials has been few reported so far.
On the other hand, graphene, a rapidly rising star on the horizon of materials science, has fascinated the scientific community in recent years due to its remarkable electronic conductivity, superior mechanical properties, large surface area, and high thermal stability.31–34 Construction of graphene-based materials has been proven as a promising method to improve performance. Thus, preparation of graphene and yolk–shell structured hybrids is generally considered to potentially realize enhancing properties of yolk–shell materials.
In this Communication, N-doped few-layer graphene-supported yolk–shell carbon hollow spheres (CHSs/N-G) hybrids have been successfully prepared by confined nanospace pyrolysis of GO–polypyrrole (GO–PPy) hybrids obtained by self-assembly of GO and PPy particles. More importantly, such CHSs/N-G exhibits electrochemical catalytic oxidation of L-cysteine, leading to a high performance cysteine sensor. The detection limit and linear range are estimated as 0.2 μM and 2 μM to 80 μM, respectively.
Experimental section
Materials
Pyrrole, FeCl2, methanol, KMnO4, H2O2 (30 wt%), NaNO3, and H2SO4 (98%) were purchased from Beijing Chemical Corp (Beijing, China). Graphite powder, and L-cysteine were purchased from Aladin Ltd. (Shanghai, China). All chemicals were used without any further purification. The water used throughout all experiments was purified through a Millipore system.Preparation of PPy particles
PPy particles were prepared by a modified method according to the previously reported publication.35 In a typical synthesis, 0.1 g of FeCl2 was added into 60 mL of H2O, followed by addition of 1 mL of pyrrole. After stirring for 10 min at room temperature, 5 mL of H2O2 was added into the mixture. Pyrrole polymerization was initiated and lasted for 24 h, leading to a deep dark precipitate. The PPy particles were collected by centrifugation at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 8 min, washed with water for twice and dried in vacuum. The PPy particles were redispered into water with concentration of 2 mg mL−1 for further use.
000 rpm for 8 min, washed with water for twice and dried in vacuum. The PPy particles were redispered into water with concentration of 2 mg mL−1 for further use.
Preparation of CHSs/N-G hybrids
GO was prepared by the modified Hummers method according to our previous report.36 In a typical synthesis of CHSs/N-G, 0.1 mL of GO (1 mg mL−1) was added into 15 mL of PPy dispersion (2 mg mL−1). After sonication for 10 min, the GO–PPy hybrids were collected by centrifugation at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min, followed by dryness in vacuum. The CHSs/rGO was obtained by pyrolysis of GO–PPy hybrids at 800 °C under a nitrogen atmosphere for 4 h.
000 rpm for 5 min, followed by dryness in vacuum. The CHSs/rGO was obtained by pyrolysis of GO–PPy hybrids at 800 °C under a nitrogen atmosphere for 4 h.
Characterizations
Raman spectra were obtained on J-YT64000 Raman spectrometer with 514.5 nm from a 20 mW air-cooled argon ion laser light (the laser power at the sample position was typically 400 μW with an average spot size of 1 μm in diameter). The samples for Raman spectra were prepared by depositing the CHSs/N-G dispersion on glass, followed by drying at room temperature. X-Ray photoelectron spectroscopy (XPS) analysis was measured on an ESCALABMK IIX-ray photoelectron spectrometer using Mg as the exciting source. The morphology of the samples was observed by field emission scanning electron microscopy (FE-SEM) on a JSM-6700F electron microscope (JEOL, Japan). Transmission electron microscopy (TEM) measurement was made on a HITACHI H-8100 electron microscopy (Hitachi, Tokyo, Japan) with an accelerating voltage of 200 kV. Electrochemical measurements were performed with a CHI 660D electrochemical analyzer (CH Instruments, Inc., Shanghai). A conventional three-electrode cell was used, including a glassy carbon electrode (GCE, geometric area = 0.07 cm2) as the working electrode, a Ag/AgCl (saturated KCl) electrode as the reference electrode, and platinum foil as the counter electrode. The potentials are measured with a Ag/AgCl electrode as the reference electrode. All the experiments were carried out at room temperature.Electrochemical detection of L-cysteine
The modified electrodes were prepared by a simple casting method. Prior to the surface coating, the GCE was polished with 1.0 and 0.3 μm alumina powder, respectively, and rinsed with water, followed by sonication in ethanol solution and water successively. Then, the electrode was allowed to dry in a stream of nitrogen. After that, 3 μL of CHSs/N-G dispersion (5 mg mL−1) were dropped on the clean surface of GCE, and dried at room temperature.Results and discussion
The PPy particles were prepared using FeCl2 and H2O2 as oxidants according to the previous report.35 Fig. S1† shows the SEM images of PPy particles thus obtained. It is seen that the samples are consisting of numerous particles separated from each other. The corresponding high magnification SEM image indicates that the size of PPy particles is about 300 nm. Then, PPy particles were used to construct PPy–GO hybrids by addition of PPy particles into GO dispersion, followed by sonication for 10 min, where TEM image of GO is shown in Fig. S2.† Due to the strong π–π interaction between GO and PPy, GO–PPy hybrids could be formed by self-assembly. Fig. 1a shows low magnification SEM image of GO–PPy hybrids, revealing that the hybrids are consisting of spheres and plate-like samples, which are attributed to the GO and PPy particles, respectively. The corresponding high magnification SEM image (Fig. 1b) further confirms the formation of GO–PPy hybrids, where the PPy particles are about 300 nm. To converse conducting polymer into carbon materials, a high temperature heat treatment process was performed for the GO–PPy hybrids. Fig. 1c and d show the SEM images of GO–PPy hybrids after heat treatment. It is seen that the morphology of the samples is similar to that of GO–PPy hybrids. Notably, the size of particles decreases to about 200 nm after heat treatment, which is attributed to the pyrolysis of PPy into carbon-based materials.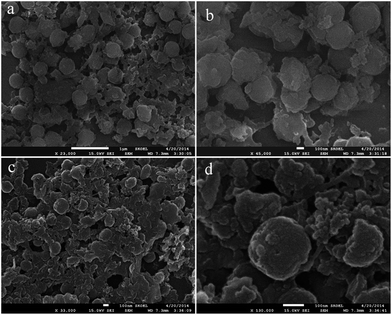 | ||
| Fig. 1 (a) Low and (b) high magnification SEM images of GO–PPy hybrids, and (c) low and (d) high magnification SEM images of CHSs/N-G hybrids. | ||
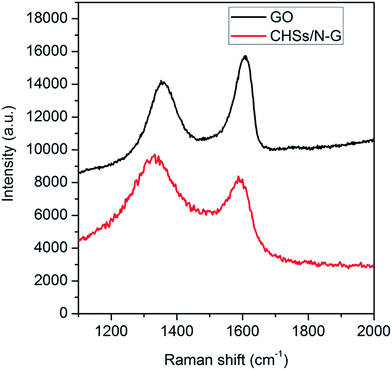
The structure conversion of hybrids was examined by the Raman spectrum. Fig. 2 shows the Raman spectra of GO and the hybrids after heat treatment. It is seen that GO exhibits a D band at 1356 cm−1 and a G band at 1608 cm−1, which are attributed to arising from a breathing mode of κ-point photons of A1g symmetry, and the first order scattering of the E2g phonon of sp2 C atoms, respectively.37 In contrast, the hybrids show a D band at 1335 cm−1, which is smaller than that of GO, and the shift of the D band is attributed to formation of N-doped carbon materials from PPy.38 Additionally, the hybrids also exhibit a G band at 1592 cm−1, and the shift of G band is attributed to N-doping of graphene.39 It is also found that the ratio of relative intensity (ID/IG) for the hybrids after pyrolysis is 1.17, which is much higher than that of GO (0.90), indicating the successful preparation of graphene by heat reduction of GO. All these observations indicate that CHSs/N-G hybrids have been successfully prepared by pyrolysis of GO–PPy hybrids, which is obtained by self-assembly of GO and PPy by the strong π–π interaction between them. Additionally, the structure of the CHSs/N-G hybrids was also examined by XPS technique. Fig. S3a† shows the C1s spectrum of CHSs/N-G hybrids, revealing the presence of C–C, C–N, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands, further indicating the formation of CHSs/N-G hybrids. The XPS data shows that the content of the nitrogen in the samples is 1.75%, indicating the formation of N-G samples.40 Furthermore, O1s spectrum of CHSs/N-G hybrids is also examined, as shown in Fig. S3b,† revealing that the C
O bands, further indicating the formation of CHSs/N-G hybrids. The XPS data shows that the content of the nitrogen in the samples is 1.75%, indicating the formation of N-G samples.40 Furthermore, O1s spectrum of CHSs/N-G hybrids is also examined, as shown in Fig. S3b,† revealing that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, epoxy and C–O bands are observed for CHSs/N-G hybrids.41 All these observations further confirm the formation of CHSs/N-G hybrids.
O, epoxy and C–O bands are observed for CHSs/N-G hybrids.41 All these observations further confirm the formation of CHSs/N-G hybrids.
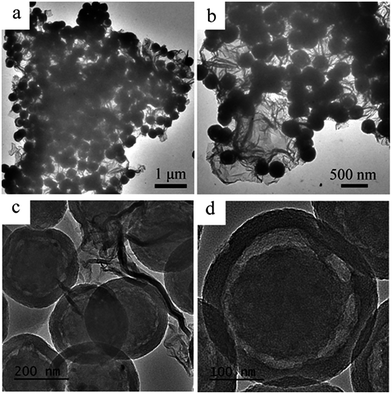
The structure of CHSs/N-G was further characterized by TEM images, as shown in Fig. 3. The low magnification TEM images (Fig. 3a and b) show that the samples exhibit typical plate-like structure about several micrometers, which is similar with the morphology of graphene-based materials, indicating the formation of graphene-based materials. Furthermore, there are numerous spheres decorated on the surface of graphene. The high magnification TEM image further confirms the formation of spheres-graphene hybrids and the spheres show typical yolk–shell structure, as shown in Fig. 3c. A high magnification TEM image of one single sphere on the graphene was showed in Fig. 3d. It is seen that the size of the sphere is about 200 nm, in agreement with the result of SEM image. Interestingly, the sphere exhibits an obvious yolk–shell structure with 10–20 nm void between the core and shell. According to the previously reported methods for preparation of yolk–shell structures,28–30 it is deduced that the formation of yolk–shell structure may be attributed to the confined nanospace pyrolysis of PPy particles using GO as protecting shell. Furthermore, the effect of the pyrolysis temperature on the structure of CHSs/N-G has been examined. Fig. S4† show the TEM image of CHSs/N-G pyrolysis at 700 °C for 4 h. It is seen that the sample also shows typical CHSs supported on N-G similar with the CHSs/N-G pyrolysis at 800 °C.
It is well known that L-cysteine is a typical amino acid, playing an important role in regulating the biological activity of protein and in the cellular antioxidant defense system.42,43 Therefore, it is very important to investigate the electrochemical behavior and sensitive detection of L-cysteine.44,45 To demonstrate the application of CHSs/N-G hybrids, electrochemical L-cysteine sensors were fabricated by dropping the CHSs/N-G dispersion on the surface of GCE. Fig. 4 shows the cyclic voltammetries (CVs) of bare GCE and CHSs/N-G modified GCE (CHSs/N-G/GCE) in 0.1 M PBS at pH 7.0 at various conditions. It is seen that bare GCE exhibits no oxidation peak in the absence of L-cysteine. After addition of 5 mM L-cysteine, no obvious oxidation peak is observed for bare GCE, indicating the poor electrocatalytic activity for oxidation of L-cysteine. Notably, a strong oxidation peak at +0.60 V with the intensity of 57.69 μA for CHSs/N-G/GCE in the presence of 5 mM L-cysteine is observed. In contrast, CHSs/N-G/GCE exhibits no obvious oxidation peak in the absence of L-cysteine. All these observations indicate that CHSs/N-G hybrids exhibit good electrocatalytic activity for oxidation of L-cysteine, which can be used as sensing materials for electrochemical L-cysteine sensors.
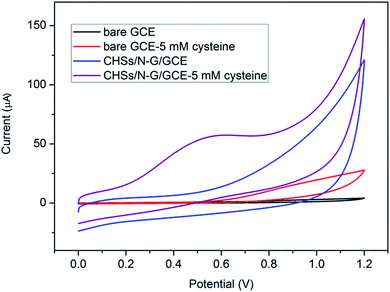 | ||
| Fig. 4 Cyclic voltammetries (CVs) of a bare GCE, and CHSs/N-G/GCE in 0.1 M PBS at pH 7.0 in the presence and absence of 5.0 mM L-cysteine (scan rate: 0.05 V s−1). | ||
The sensing performance of the sensor based on CHSs/N-G toward L-cysteine is also compared with rGO and PPy–GO samples. Fig. S5† shows the CVs of the CHSs/N-G/GCE, PPy–GO/GCE and rGO/GCE in the presence of 5 mM L-cysteine. Although all modified electrodes exhibit peak attributed to the oxidation of L-cysteine, the peak current of the sensor based on CHSs/N-G is much higher than the other ones. Additionally, the potential of the sensors based on CHSs/N-G is also much negative than the other ones. The improvement of the sensing performances may be attributed to the presence of CHSs in the matrix of N-G. Furthermore, the sensing performance of the sensor based on CHSs/N-G is also examined by the differential pulse voltammetry (DPV) method. Fig. S6a† shows the DPV of CHSs/N-G/GCE in the presence of various concentrations of L-cysteine. It is seen that the CHSs/N-G/GCE exhibits a strong oxidation peak at +0.58 V attributed to the oxidation of L-cysteine. Additionally, the oxidation peak currents increase with increasing the concentrations of L-cysteine from 0.2 mM to 3 mM, and the a good linear relationship between the peak current and concentrations of L-cysteine is obtained, as shown in Fig. S6b.†
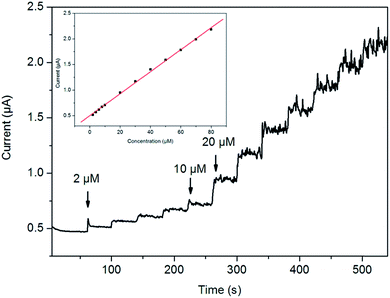
Fig. 5 shows the typical current–time plot of the CHSs/N-G/GCE in 0.1 M PBS 7.0 buffer on successive step change of L-cysteine concentrations. When an aliquot of L-cysteine was added into the stirring PBS solution, CHSs/N-G/GCE responded rapidly to the substrate and the current rose steeply to reach a stable value. At the applied potential of +0.60 V, the cathode current of the sensor increased dramatically and achieved 95% of the steady state current within 2 s, revealing a fast amperometric response behavior, where a higher applied potential could reduce noise and enhance the sensing performance. The inset in Fig. 5 shows the calibration curve of the sensor. The linear detection range is estimated to be from 2.0 μM to 80 μM (r = 0.9998). The detection limit is estimated to be 0.2 μM at a signal-to-noise ratio of 3, where the detection limit is calculated by the formula that the detection limit equals to 3 (standard deviation of the blank)/(slope of the calibration plot). It should be noted that our present sensing system gives a lower detection limit than those of the sensors for detection of L-cysteine, such as boron-doped carbon nanotube (0.26 μM),45 multi-walled carbon nanotubes-cobalt tetraaminophthalocyanine mixture (0.28 μM),46 multi-walled carbon nanotubes (5.4 μM),47 and cobalt(II) salophen (0.4 μM).48 Furthermore, the good sensing performances of CHSs/N-G hybrids may be attributed to the increasing peak currents and reducing the overpotentials for electrochemical oxidation of L-cysteine due to unique properties of CHSs/N-G such as the good conductivity, porous structure, N-doped graphene structure. Indeed, N-doped graphene materials exhibit good sensing performances for electrochemical detection of H2O2,49 and dopamine.50 However, few reports on this issue have been published so far, and thus further work need be done to exploit the synthesis and applications of N-G hybrids for electrochemical sensing.
Conclusions
In summary, CHSs/N-G hybrids have been successfully prepared by pyrolysis of GO–PPy hybrids obtained by self-assembly of GO and PPy particles, which exhibit good performance for electrochemical detection of L-cysteine. Our present study is important because it provides a new and effective method for preparation of carbon-based materials for electrochemical detection.Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (Grant no. 51202085) and the Open Project from State Key Laboratory of Transducer Technology (Grant no. SKT1402).Notes and references
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578 RSC.
- G. Li and Z. Tang, Nanoscale, 2014, 6, 3995 RSC.
- Z. Zhang, F. Xiao, J. Xi, T. Sun, S. Xiao, H. Wang and Y. Liu, Sci. Rep., 2014, 4, 4053 Search PubMed.
- P. M. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 45, 8224 CrossRef CAS PubMed.
- H. Wan, H. Qin, Z. Xiong, W. Zhang and H. Zou, Nanoscale, 2013, 5, 10936 RSC.
- P. Rai, J.-W. Yoon, H.-M. Jeong, S.-J. Hwang, C.-H. Kwak and J.-H. Lee, Nanoscale, 2014, 6, 8292 RSC.
- Y. J. Hong, M. Y. Son and Y. C. Kang, Adv. Mater., 2013, 25, 2279 CrossRef CAS PubMed.
- J. Yang, D. Shen, L. Zhou, W. Li, X. Li, C. Yao, R. Wang, S. M. El-Toni, F. Zhang and D. Zhao, Chem. Mater., 2013, 25, 3030 CrossRef CAS.
- J. Lee, J. C. Park and H. Song, Adv. Mater., 2008, 20, 1523 CrossRef CAS PubMed.
- I. Lee, J. B. Joo, Y. Yin and F. Zaera, Angew. Chem., Int. Ed., 2011, 50, 10208 CrossRef CAS PubMed.
- R. Liu, F. Qu, Y. Guo, N. Yao and R. D. Priestley, Chem. Commun., 2014, 50, 478 RSC.
- C. Galeano, C. Baldizzone, H. Bongard, B. Spliethoff, C. Weidenthaler, J. C. Meier, K. J. J. Mayrhofer and F. Schüth, Adv. Funct. Mater., 2014, 24, 220 CrossRef CAS PubMed.
- L. Zhao, H. Liu, F. Wang and L. Zeng, J. Mater. Chem. A, 2014, 2, 7065 CAS.
- W. Li, Y. Deng, Z. Wu, X. Qian, J. Yang, Y. Wang, D. Gu, F. Zhang, B. Tu and D. Zhao, J. Am. Chem. Soc., 2011, 133, 15830 CrossRef CAS PubMed.
- Y. Yang, X. Liu, X. Li, J. Zhao, S. Bai, J. Liu and Q. Yang, Angew. Chem., Int. Ed., 2012, 51, 9164 CrossRef CAS PubMed.
- C.-H. Kuo, Y. Tang, L.-Y. Chou, B. T. Sneed, C. N. Brodsky, Z. Zhao and C.-K. Tsung, J. Am. Chem. Soc., 2012, 134, 14345 CrossRef CAS PubMed.
- L. Chen, L. Li, T. Wang, L. Zhang, S. Xing, C. Wang and Z. Su, Nanoscale, 2014, 6, 6603 RSC.
- W.-F. Ma, C. Zhang, Y.-T. Zhang, M. Yu, J. Guo, Y. Zhang, H.-J. Lu and C.-C. Wang, Langmuir, 2014, 30, 6602 CrossRef CAS PubMed.
- C. M. Sim, S. H. Choi and Y. C. Kang, Chem. Commun., 2013, 49, 5978 RSC.
- Y. J. Hong, J.-W. Yoon, J.-H. Lee and Y. C. Kang, Chem.–Eur. J., 2014, 20, 2737 CrossRef CAS PubMed.
- S. H. Choi and Y. C. Kang, Chem.–Eur. J., 2014, 20, 3014 CrossRef CAS PubMed.
- J. Liu, H. Q. Yang, F. Kleitz, Z. G. Chen, T. Yang, E. Strounina, G. Q. Lu and S. Z. Qiao, Adv. Funct. Mater., 2012, 22, 591 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, S. B. Hartono and G. Q. Lu, Angew. Chem., Int. Ed., 2010, 49, 4981 CrossRef CAS PubMed.
- C. Dai, A. Zhang, J. Li, K. Hou, M. Liu, C. Song and X. Guo, Chem. Commun., 2014, 50, 4846 RSC.
- Z.-A. Qiao, Q. Huo, M. Chi, G. M. Veith, A. J. Binder and S. Dai, Adv. Mater., 2012, 24, 6017 CrossRef CAS PubMed.
- S. N. Shmakov, Y. Jia and E. Pinkhassik, Chem. Mater., 2014, 26, 1126 CrossRef CAS.
- J. Wang, X. Li, X. Li, J. Zhu and H. Li, Nanoscale, 2013, 5, 1876 RSC.
- A.-H. Lu, T. Sun, W.-C. Li, Q. Sun, F. Han, D.-H. Liu and Y. Guo, Angew. Chem., Int. Ed., 2011, 50, 11765 CrossRef CAS PubMed.
- C. Lei, F. Han, Q. Sun, W.-C. Li and A.-H. Lu, Chem.–Eur. J., 2014, 20, 139 CrossRef CAS PubMed.
- Q. Sun, W.-C. Li and A.-H. Lu, Small, 2013, 9, 2086 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666 RSC.
- S. Yin, Y. Zhang, J. Kong, C. Zou, C. Li, X. Lu, J. Ma, F. Y. C. Chiang and X. Chen, ACS Nano, 2011, 5, 3831 CrossRef CAS PubMed.
- S. Liu, J. Tian, L. Wang and X. Sun, Carbon, 2011, 49, 3158 CrossRef CAS PubMed.
- Z. Liu, Y. Liu, S. Poyraz and X. Zhang, Chem. Commun., 2011, 47, 4421 RSC.
- S. Liu, J. Tian, L. Wang, H. Li, Y. Zhang and X. Sun, Macromolecules, 2010, 43, 10078 CrossRef CAS.
- S. Yin, Y. Zhang, J. Kong, C. Zou, C. M. Li, X. Lu, J. Ma, F. Y. C. Boey and X. Chen, ACS Nano, 2011, 5, 3831 CrossRef CAS PubMed.
- X. Ning, W. Zhong, S. Li, Y. Wang and W. Yang, J. Mater. Chem. A, 2014, 2, 8859 CAS.
- X. Li, H. Wang, J. T. Robinson, H. Sanchez, G. Diankov and H. Dai, J. Am. Chem. Soc., 2009, 131, 15939 CrossRef CAS PubMed.
- S. Kundu, T. C. Nagaiah, W. Xia, Y. Wang, S. Van Dommele, J. H. Bitter, M. Santa, G. Grundmeier, M. Bron, W. Schuhmann and M. Muhler, J. Phys. Chem. C, 2009, 113, 14302 CAS.
- Z. Sun, N. Dong, K. Xie, W. Xia, D. König, T. C. Nagaiah, M. D. Sánchez, P. Ebbinghaus, A. Erbe, X. Zhang, A. Ludwig, W. Schuhmann, J. Wang and M. Muhler, J. Phys. Chem. C, 2013, 117, 11811 CAS.
- M. Zhou, J. Ding, L. Guo and Q. Shang, Anal. Chem., 2007, 79, 5328 CrossRef CAS PubMed.
- H. Hosseini, H. Ahmar, A. Dehghani, A. Bagheri, A. Tadjarodi and A. R. Fakhari, Biosens. Bioelectron., 2013, 42, 426 CrossRef CAS PubMed.
- X. Tang, Y. Liu, H. Hou and T. You, Talanta, 2010, 80, 2182 CrossRef CAS PubMed.
- C. Deng, J. Chen, X. Chen, M. Wang, Z. Nie and S. Yao, Electrochim. Acta, 2009, 54, 3298 CrossRef CAS PubMed.
- S. Nyoni, T. Mugadza and T. Nyokong, Electrochim. Acta, 2014, 128, 32 CrossRef CAS PubMed.
- A. Salimi and R. Hallaj, Talanta, 2005, 66, 967 CrossRef CAS PubMed.
- M. K. Amini, J. H. Khorasani, S. S. Khaloo and S. Tangestaninejad, Anal. Biochem., 2003, 320, 32 CrossRef CAS.
- Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790 CrossRef CAS PubMed.
- A. Pandikumar, G. T. S. How, T. P. See, F. S. Omar, S. Jayabal, K. Z. Kamali, N. Yusoff, A. Jamil, R. Ramaraj, S. A. John, H. N. Lim and N. M. Huang, RSC Adv., 2014, 4, 63296 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of PPy particles. See DOI: 10.1039/c5ra04961j |
| This journal is © The Royal Society of Chemistry 2015 |