Highly sensitive and humidity-independent ethanol sensors based on In2O3 nanoflower/SnO2 nanoparticle composites†
Yang Liu,
Shiting Yao,
Qiuyue Yang,
Peng Sun,
Yuan Gao*,
Xishuang Liang,
Fengmin Liu* and
Geyu Lu
State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun 130012, People's Republic of China. E-mail: 18946580425@163.com; liufm@jlu.edu.cn; Fax: +86-431-85167808; Tel: +86-431-85168384
First published on 8th June 2015
Abstract
As an ethanol sensing material, the composites of In2O3–SnO2 were composed of In2O3 microflowers and SnO2 nanoparticles. Both In2O3 microflowers and SnO2 nanoparticles were synthesized by hydrothermal method and then mixed in an ultrasonic environment. The morphology and phase composition of the as-synthesized samples were characterized by X-ray powder diffraction (XRD) and scanning electron microscopy (SEM). The results on gas sensing properties showed that when the mass ratio of In2O3 and SnO2 was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the sensor based on the as-prepared In2O3–SnO2 composite exhibited high response and good selectivity to ethanol at 250 °C. The response to 100 ppm ethanol gas was 53.2. UV illumination stabilized the responses of the sensors while the relative humidity increased. The gas sensing mechanism proposed was that the addition of SnO2 to In2O3 enhanced the catalytic activity for the ethanol reaction, which changed the electrical resistance of the materials. Besides, the morphology was helpful to the gas reaction on the surface of the sensing materials.
1, the sensor based on the as-prepared In2O3–SnO2 composite exhibited high response and good selectivity to ethanol at 250 °C. The response to 100 ppm ethanol gas was 53.2. UV illumination stabilized the responses of the sensors while the relative humidity increased. The gas sensing mechanism proposed was that the addition of SnO2 to In2O3 enhanced the catalytic activity for the ethanol reaction, which changed the electrical resistance of the materials. Besides, the morphology was helpful to the gas reaction on the surface of the sensing materials.
1. Introduction
Ethanol detection of breath is an effective way to aid police in apprehending drink driving offenders.1 In addition, as a kind of volatile organic compound (VOC), ethanol detection can potentially be utilized as a breath marker for specific diseases.2 So far, several types of ethanol sensors have been reported for breath analysis.3–5 In recent years, the semiconductor gas sensors have attracted the widespread attention of scientific researchers because of their high sensitivity, low cost and simplicity.6–8 It is well known that the performances of sensors are significantly influenced by the components, crystallite size and morphology of the sensing materials.9–11Generally, a smaller crystal size owns a larger surface area, which provides more active sites for the target gas reacting with the sensing materials. However, agglomeration of fine particles greatly reduces the valid surface area. Fortunately, hierarchical nanostructure oxides have emerged due to their high active surface area and loose microstructure favourable for gas adsorption and rapid gas diffusion, and have dominated the research in enhancing the sensitivity of the metal oxide gas sensors. Another important sensing parameter is selectivity that can be improved through the use of composite metal oxides.12–14 The physical interface between two dissimilar materials is often referred to as a heterojunction. In Akbar et al.15 review, the authors detailed the dominant electronic and chemical mechanisms that influence the performance of the metal oxide heterojunction as resistive-type gas sensors.
Up to date, many composites such as In2O3–CeO2, ZnO–SnO2, TiO2–SnO2, SnO2–Fe2O316–19 have been reported to be promising sensing materials to obtain the sensitive and selective gas sensors. As two important kinds of fundamental materials, In2O3 and SnO2 have been widely studied due to their responses towards different gases.20–23 In2O3–SnO2 composites24,25 have also been proved to improve the properties of sensors. However, to the best of our knowledge, there are few reports on the morphology of In2O3–SnO2 composites and no reports have shown that In2O3–SnO2 composites have an excellent response and selectivity for ethanol.
Commonly, the water vapour strongly interacts with the oxide semiconductor surface, which leads to a significant deterioration of the sensor performance. There is high humidity in breath, so it is very important to reduce the influence of humidity on the performance of the sensors.
In this work, In2O3 microflowers and SnO2 nanoparticles were both synthesized by hydrothermal method. Then the two kinds of materials were mixed in the ultrasound environment. The testing on gas sensing properties showed that while the mass ratio of In2O3 and SnO2 was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the sensors based on In2O3–SnO2 composite oxides showed the highest response to ethanol, the response to 100 ppm ethanol gas was 53.2 at 250 °C. UV illumination was adopted to reduce the influence of relative humidity on the performances of the sensors.
1, the sensors based on In2O3–SnO2 composite oxides showed the highest response to ethanol, the response to 100 ppm ethanol gas was 53.2 at 250 °C. UV illumination was adopted to reduce the influence of relative humidity on the performances of the sensors.
2. Experimental
2.1 Synthesis and characterization of the samples
In2O3 was synthesized according to the literatures.26 In a typical synthesis process, 0.381 g of In(NO3)3·4.5H2O and 0.15 g urea were dissolved in 36 mL deionized water with stirring until the solution was clear. Then, the mixture solution was transferred into a Teflon-lined stainless steel autoclave, heated at 160 °C for 4 h. After the autoclave was cooled to room temperature naturally, the result product was washed with deionized water and ethanol for six times alternately by centrifuge, and then dried at 80 °C for 3 h. The products were sintered to 500 °C at a rate of 2 °C min−1 and then kept at 500 °C for 2 h. Then In2O3 microflowers were synthesized.SnO2 was synthesized according to the literatures.27 In a typical synthesis process,0.526 g of SnCl4·5H2O, 0.6 g of cetyltrimethyl ammonium bromide (CTAB) and 0.2 g of hexamethylenetetramine (HMT) were added to 40 mL mixed solvent of ethanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with stirring until the solution was clear. Then, the mixture solution was transferred into a Teflon-lined stainless steel autoclave, heated at 200 °C for 4 h. The processes after this were same with the process used to synthesize In2O3.
1, v/v) with stirring until the solution was clear. Then, the mixture solution was transferred into a Teflon-lined stainless steel autoclave, heated at 200 °C for 4 h. The processes after this were same with the process used to synthesize In2O3.
Then In2O3 microflowers and SnO2 nanoparticles were mixed physically in certain mass ratio under ultrasound environment. Four In2O3–SnO2 composites samples with mass concentration of 0% SnO2, 33.3% SnO2, 70% SnO2 and 100% SnO2 were obtained, represented by S0, S1, S2 and S3, respectively. Until this step, In2O3–SnO2 composites were obtained.
2.2 Fabrication and measurement of sensors
Gas sensors were fabricated as follows: the prepared materials (S0, S1, S2 and S3) were mixed with deionized water to form something like slurry, and then spread on an alumina tube (4 mm in length, 1.2 mm in external diameter, and 0.8 mm in internal diameter, attached with a pair of gold electrodes, each electrode was connected with two Pt wire) by a small brush until forming a film thick enough to cover the gold electrode entirely. A Ni–Cr heating wire went through the tube to support heat for the sensors. The resistances of the sensors were measured by multimeter (fluke 8846A) under constant temperature and humidity. The measurement was processed by a static test system: Ra and Rg were the resistances of the sensors in air and tested gas, respectively. A certain amount of the tested gas was injected into a closed chamber, and the sensor was put into the chamber for the measurement of the sensitive performance. When the response reached a constant value, the sensor was removed to air to recover. The response of the sensor was defined as S = Ra/Rg for reducing gases or Rg/Ra for oxidizing gases. The response and recovery times are defined as the times taken by the resistance change of sensor achieving 90% of the total in the case of adsorption and desorption, respectively.UV illumination was processed according our previous works.28,29 UV-LED (peak wavelength = 380 nm; operating voltage = 3 V) was chosen as the light source, and the distance between the UV-LED and gas sensors was 2 mm. The measured power of the UV-LED at this distance was 0.7 Cd/m2 (PR650, California, USA). Relative humidity (%RH) was obtained by using Humidity generator (Shanghai ESPEC environmental equipment corp. SETH-Z-022L).
In order to investigate the repeatability of the sensors. The fabrication and measurement of sensors were repeated 5 times, the results showed the sensors had similar properties.
3. Results and discussion
3.1 Characterization of the products
The crystal phase of In2O3 microflowers, SnO2 nanoparticles and In2O3–SnO2 composites (S1) were investigated by XRD with a Rigaku D/max-2500 diffractometer using Cu-Kα1 radiation. From the Fig. 1, we can see that In2O3–SnO2 composites has all peaks of In2O3 and SnO2. They were in good agreement with the JCPDS file of In2O3 (JCPDS no. 71-2195) and SnO2 (JCPDS no. 77-451).It proved that In2O3 microflowers and SnO2 nanoparticles were mixed entirely by physical route and they had no chemical reacts. No diffraction peaks from any other impurities were observed, indicating the high purity of the products.3.2 Structural and morphological characteristics
The morphologies and structures of In2O3 microflowers, SnO2 nanoparticles and In2O3–SnO2 composites (S1) were identified by SEM using a JEOL JSM-7500F microscope with an accelerating voltage of 15 kV as shown in Fig. 2. Fig. 2(a) presents the SEM image of SnO2 particles, it can be seen that SnO2 particles are composed of relatively uniform SnO2 nanoparticles with a length of 20–30 nm. It can be seen that the surfaces of nanoparticles are coarse. As is shown in Fig. 2(b), the In2O3 microflowers have a good dispersion and uniform size of 1–1.5 μm, it can be seen from the image that the In2O3 microflowers are composed of aggregated nanosheets. Fig. 2(c) shows In2O3–SnO2 composites, SnO2 nanoparticles seems to be surrounded by In2O3 microflowers, SnO2 and In2O3 was marked with boxes. Composition of In2O3–SnO2 composites (S1) had been characterized using EDS as shown in Fig. 2(d). The EDS spectrum showed that components of the materials are In, Sn and O. The Si was attributed to the substrate used in the SEM measurement. | ||
| Fig. 2 SEM images of (a) SnO2 nanoparticles, (b) In2O3 microflowers and (c) In2O3–SnO2 composites (d) EDS spectra of In2O3–SnO2 composites. | ||
Fig. 3 shows nitrogen adsorption–desorption isotherm and pore size distribution (inset of Fig. 3) of the In2O3 microflowers, SnO2 nanoparticles and In2O3–SnO2 composites (S1). Pore size distribution curves were calculated from the desorption branch of a nitrogen isotherm by the BJH method using the Halsey equation. The BET surface area of the In2O3 microflowers, SnO2 nanoparticles and In2O3–SnO2 composites were calculated to be 19.5 m2 g−1, 41.0 m2 g−1 and 34.4 m2 g−1 respectively by the Brunauer–Emmett–Teller (BET) method with a Micromeritics Gemini VII apparatus (Surface Area and Porosity System).
 | ||
| Fig. 3 Typical N2 adsorption–desorption isotherms and pore size distribution of In2O3 microflowers, SnO2 nanoparticles and In2O3–SnO2 composites. | ||
3.3 Gas-sensing properties
As we know, the mass ratio of In2O3 microflowers and SnO2 nanoparticles and the working temperature both influenced greatly gas responses in this research, so the gas sensing properties of S0 to S3 to 100 ppm ethanol under different temperatures were investigated, the results were shown in Fig. 4.The VOCs were got from liquid evaporation, concentration calculation method is according to ideal gas equation of state (PV = nRT). The sensors based the In2O3–SnO2 composites (S1) showed the best response while the mass ratio of In2O3 and SnO2 was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The best response to 100 ppm ethanol was 53.2 at 250 °C. A comparison between the sensing performances of the sensor and literature reports26,27,30–32 were summarized in Table 1.
1. The best response to 100 ppm ethanol was 53.2 at 250 °C. A comparison between the sensing performances of the sensor and literature reports26,27,30–32 were summarized in Table 1.
| Material | Fabrication approach | Ethanol concentration (ppm) | Temperature (°C) | Sensor response | References |
|---|---|---|---|---|---|
| In2O3 microflowers | Hydrothermal route | 100 ppm | RT | ∼1.5 | 26 |
| SnO2 nanostructures | Hydrothermal route | 100 ppm | 275 °C | ∼9.6 | 27 |
| SnO2–In2O3 | Sol–gel and electrospinning | 100 ppm | RT | ∼3.0 | 30 |
| In2O3 nanospheres | Hydrothermal route | 100 ppm | 275 °C | ∼21.0 | 31 |
| In2O3–SnO2 nanocomposites | Mechanochemical reaction | 100 ppm | RT | ∼18.7 | 32 |
| In2O3–SnO2 composites | Hydrothermal route | 100 ppm | 250 °C | 53.2 | This work |
Fig. 5 shows the responses of the sensors based on S1 to different concentrations ethanol at 250 °C. Before 500 ppm, the responses increase with the ethanol concentration like a line, when the ethanol concentration is more than 500 ppm, the response is nearly saturated. Dynamic response towards 100–1000 ppm ethanol at 250 °C was shown in the inset of Fig. 5.
 | ||
| Fig. 5 Relationship between responses of sensors based on S1 and ethanol concentration at 250 °C, the inset displaying dynamic response. | ||
The response and recovery characteristics were investigated which were shown in the Fig. 6(a).The results indicate that the In2O3–SnO2 composites sensor based on S1 has fast response–recovery kinetics. The response and recovery times of the In2O3–SnO2 composites sensors are about 15 s and 60 s, respectively. Five reversible cycles of the response curve indicate a stable and repeatable characteristic, as shown in Fig. 6(b).
 | ||
| Fig. 6 (a) Dynamic response resistance of the sensor (S1) and (b) five periods of response curve of the sensor to 100 ppm ethanol at 250 °C (20% RH). | ||
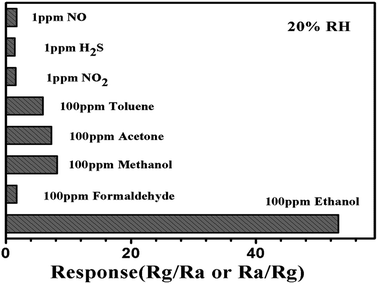
Selectivity is also an important performance of the sensor based on the as-prepared In2O3–SnO2 composites to various gases, such as NO, H2S, NO2, C6H5CH3, C3H6O, CH3OH, HCHO and CH3CH2OH.All of the gases were tested at an operating temperature of 250 °C as shown in Fig. 7. The response of the sensors based on In2O3–SnO2 composites (S1) to toluene, acetone and methanol is much lower than ethanol, and the response to other gases or vapours such as NO, H2S, NO2 and formaldehyde is negligible. The results indicate that the as-prepared In2O3–SnO2 composites display superior selectivity to ethanol against the other interference gases and was very suitable for sensing ethanol at 250 °C.
UV illumination was used to enhance the performance of the sensors (S1). Although the sensitivity and the response and recovery times were almost not affected by UV illumination, UV illumination greatly reduced the influence of humidity on the sensitivity of the sensors, the results were shown in Fig. 8. It can be seen that the sensitivity is almost stable below 80% RH under UV illumination. When the relative humidity is even up to 95% RH, the sensitivity still maintains 75%, which is higher than that without UV illumination. UV illumination can decompose the water vapour on the surface according to the mechanism of photochemical water splitting (water can be decomposed into hydrogen and oxygen under UV illumination33).
 | ||
| Fig. 8 Responses of the sensors (S1) in different relative humidity, the inset is the resistances in air vs. relative humidity. | ||
3.4 Gas mechanism
The formation of metal oxide composites is known to increase the response and selectivity to gases. For this work, both In2O3 and SnO2 increase the response and selectivity to gases. For this work, both In2O3 and SnO2 are n-type semiconductor oxides. The surface of the sensor material in air is covered with chemisorbed oxygen ions such as O−, O2− and O22−, the electrons on chemisorbed oxygen ions come from the material, which decrease the electron density in n-type material and in turn increase the resistance of the oxide. If reducing gas is introduced, it reacts with the surface adsorbed oxygen, the electron will be donated back into the semiconductor that causes a decrease in the resistance. Adsorbed ethanol undergoes two possible mechanisms of dehydrogenation to an aldehyde and dehydration to an alkene, shown in reactions (1) and (2).34| C2H5OH (g) →CH3CHO (g) + H2 (g) | (1) |
| C2H5OH (g) → C2H4 (g) + H2O (g) | (2) |
Following this process consecutive reactions happen which consume ionic oxygen species and release electrons, so the resistance of the oxide is reduced. The electrons only removed to a certain depth from the surface known as the depletion region. The depletion region width may change as the test gas or oxygen is adsorbed on the surface, which in turn caused a measurable change in the resistance. This process was shown in Fig. 9(a).
 | ||
| Fig. 9 (a) Schematic diagram of the proposed mechanism of In2O3–SnO2 composites and (b) Energy band structure of In2O3, SnO2 and In2O3–SnO2 composites. | ||
The enhancement in gas-sensing property on In2O3 and SnO2 composites may be mainly ascribed to heterostructure.35,36 The SnO2 has a lower Fermi level than that of In2O3, In2O3 would receive electrons from SnO2, leading to the formation of an accumulation layer at the In2O3–SnO2 interface, as shown in Fig. 9(b). The increase of electrons on the surface of In2O3 is in favour of adsorption of O2 and decrease of resistance.
Besides, In2O3 and SnO2 were both active site, but each promoted different breakdown profiles of the ethanol vapours being sensed. Because of their complementary catalytic activity (catalytic activity reaches an optimum level when the mass ratio of In2O3 and SnO2 is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the mixed oxides permitted a more complete breakdown of the ethanol vapours, leading to the observed enhancement in sensitivity. In addition, the porous structure is helpful for gas diffusion and its reaction on the surface of the sensing materials.
1), the mixed oxides permitted a more complete breakdown of the ethanol vapours, leading to the observed enhancement in sensitivity. In addition, the porous structure is helpful for gas diffusion and its reaction on the surface of the sensing materials.
4. Conclusions
In summary, In2O3–SnO2 composites had been successfully obtained through a mixing of In2O3 microflowers and SnO2 nanoparticles. The nanostructure is in favour of permeation of the tested gas. The In2O3–SnO2 sensors exhibit high response and good selectivity to ethanol at 250 °C. It can detect 100 ppm ethanol with a response value of about 53.2 and with little interference from other gases. The enhancement in gas-sensing property on In2O3 and SnO2 composites may be ascribed to heterostructure. Because of the depletion layer manipulation and complementary catalytic activity, the composite oxides permitted a more complete breakdown of the ethanol vapours, leading to the observed enhancement in sensitivity and selectivity. The sensitivity is stable while the relative humidity increasing under UV illumination due to photochemical water splitting.Acknowledgements
This work is supported by the National Nature Science Foundation of China (nos 61074172, 61134010, and 61327804) and Program for Chang Jiang Scholars and Innovative Research Team in University (no. IRT1017). National High-Tech Research and Development Program of China (863 Program, no. 2013AA030902).Notes and references
- K. Sutter, Chimia, 2002, 56, 59–62 CrossRef CAS.
- M. Seo, M. Yuasa and T. Kida, J. Ceram. Soc. Jpn., 2011, 119, 884–889 CrossRef CAS.
- K. Tetsuya, H. Hiroaki and M. Takuya, et al., J. Phys. Chem. C, 2010, 114, 15141–15148 Search PubMed.
- J. Wang and M. Musameh, Anal. Chem., 2003, 75, 2075–2079 CrossRef CAS PubMed.
- M. Penza, F. Antolini and M. Vittori-Antisari, Thin Solid Films, 2005, 472, 246–252 CrossRef CAS PubMed.
- P. Sun, X. He, W. Wang, J. Ma, Y. Sun and G. Lu, CrystEngComm, 2012, 14, 2229–2234 RSC.
- C. Wang, X. Cheng, X. Zhou, P. Sun, X. Hu, K. Shimanoe, G. Lu and N. Yamazoe, ACS Appl. Mater. Interfaces, 2014, 6, 12031–12037 CAS.
- P. Sun, X. Zhou, C. Wang, K. Shimanoe, G. Lu and N. Yamazoe, J. Mater. Chem. A, 2014, 2, 1302–1308 CAS.
- Y. Guan, D. Wang, X. Zhou, P. Sun, H. Wang, J. Ma and G. Lu, Sens. Actuators, B, 2014, 191, 45–52 CrossRef CAS PubMed.
- C. Wang, X. Li, B. Wang, J. Ma, Y. Cao, Y. Sun and G. Lu, RSC Adv., 2014, 4, 18365–18369 RSC.
- X. Zhou, C. Wang, W. Feng, P. Sun, X. Li and G. Lu, Mater. Lett., 2014, 120, 5–8 CrossRef CAS PubMed.
- S. I. Rembeza, N. N. Kosheleva and E. S. Rembeza, et al., Semiconductors, 2014, 48, 1118–1122 CrossRef CAS.
- J. Susanna, P. Katrin and G. Jordi, et al., Adv. Funct. Mater., 2007, 17, 3339–3347 CrossRef PubMed.
- P. M. Dominic, F. E. P. Keith and T. Paraskeva, et al., IEEE Sens. J., 2007, 7, 551–556 CrossRef.
- D. R. Miller, S. A. Akbar and P. A. Morris, Sens. Actuators, B, 2014, 204, 250–272 CrossRef CAS PubMed.
- S. Hemmatia, A. A. Firooz, A. A. Khodadadi and Y. Mortazavic, Sens. Actuators, B, 2011, 160, 1298–1303 CrossRef PubMed.
- C. Zhao, W. Hu, Z. Zhang, J. Zhou, X. Pan and E. Xie, Sens. Actuators, B, 2014, 195, 486–493 CrossRef CAS PubMed.
- A. Kusior, J. Klich-Kafel, A. Trenczek-Zajac, K. Swierczek, M. Radecka and K. Zakrzewska, J. Eur. Ceram. Soc., 2013, 33, 2285–2290 CrossRef CAS PubMed.
- G. N. Gerasimov, V. F. Gromov and L. I. Trakhtenberg, et al., Russ. J. Phys. Chem., 2014, 88, 503–508 CrossRef CAS.
- J. Zhao, M. Zheng, X. Lai, H. Lu, N. Li, Z. Ling and J. Cao, Mater. Lett., 2012, 75, 126–129 CrossRef CAS PubMed.
- X. Xu, D. Wang, J. Liu, P. Sun, Y. Guan, H. Zhang, Y. Sun, F. Liu, X. Liang, Y. Gao and G. Lu, Sens. Actuators, B, 2013, 185, 32–38 CrossRef CAS PubMed.
- P. Song, D. Han, H. Zhang, J. Li, Z. Yang and Q. Wang, Sens. Actuators, B, 2014, 196, 434–439 CrossRef CAS PubMed.
- X. Wang, S. Qiu, J. Liu, C. He, G. Lu and W. Liu, Eur. J. Inorg. Chem., 2014, 5, 863–869 CrossRef PubMed.
- J. T. McCue and J. Y. Ying, Chem. Mater., 2007, 19, 1009–1015 CrossRef CAS.
- H. Yang, X. Zhang and A. Tang, Nanotechnology, 2006, 17, 2860–2864 CrossRef CAS.
- X. Xu, D. Wang, W. Wang, P. Sun, J. Ma, X. Liang, Y. Sun, Y. Ma and G. Lu, Sens. Actuators, B, 2012, 171, 1066–1072 CrossRef PubMed.
- P. Sun, X. Zhou, C. Wang, B. Wang, X. Xu and G. Lu, Sens. Actuators, B, 2014, 190, 32–39 CrossRef CAS PubMed.
- G. Lu, J. Xu, J. Sun, Y. Yu, Y. Zhang and F. Liu, Sens. Actuators, B, 2012, 162, 82–88 CrossRef CAS PubMed.
- J. Sun, J. Xu, Y. Yu, P. Sun, F. Liu and G. Lu, Sens. Actuators, B, 2012, 169, 291–296 CrossRef CAS PubMed.
- Q. Qi, P. Wang, J. Zhao, L. Feng, L. Zhou, R. Xuan, Y. Liu and G. Li, Sens. Actuators, B, 2014, 194, 440–446 CrossRef CAS PubMed.
- P. Song, D. Han, H. Zhang, J. Li, Z. Yang and Q. Wang, Sens. Actuators, B, 2014, 196, 434–439 CrossRef CAS PubMed.
- H. Yang, X. Zhang and A. Tang, Nanotechnology, 2006, 17, 2860–2864 CrossRef CAS.
- X. Yang, Z. Li, G. Liu, J. Xing, C. Sun, H. Yang and C. Li, CrystEngComm, 2011, 13, 1378–1383 RSC.
- T. Jinkawa, G. Sakai, J. Tamaki, N. Miura and N. Yamazoe, J. Mol. Catal. A: Chem., 2000, 155, 193–200 CrossRef CAS.
- W. Li, S. Ma and Y. Li, Sens. Actuators, B, 2015, 211, 392–402 CrossRef CAS PubMed.
- S. Yan and Q. Wu, Sens. Actuators, B, 2014, 205, 329–337 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of preparations and characterizations; the SEM images using a JEOL JSM-7500F microscope with an accelerating voltage of 15 kV; the XRD measurements with a Rigaku D/max-2500 diffractometer using Cu-Kα1 radiation; the BET measurements with a Micromeritics Gemini VII apparatus (surface area and porosity system). See DOI: 10.1039/c5ra07213a |
| This journal is © The Royal Society of Chemistry 2015 |