Panchromatic quasi-monolayer of Ag nanoparticles for high-efficiency dye-sensitized solar cells†
Hyun-Young Kim and
Jung Sang Suh*
Nano-materials Laboratory, Department of Chemistry, Seoul National University, Kwanakro 1, Kwanakgu, Seoul 151-742, Republic of Korea. E-mail: jssuh@snu.ac.kr; Fax: +82-2-875-6636; Tel: +82-2-880-7763
First published on 25th June 2015
Abstract
We developed a panchromatic quasi-monolayer of Ag nanoparticles (NPs) whose localized surface plasmon resonances all take place in the visible range and applied this technique to fabricate dye-sensitized solar cells (DSSCs). Three kinds of Ag NPs, whose λmax were at 540, 620 and 470 nm, were fabricated. We immobilized them on a photoactive film of TiO2 NPs coated with poly(4-vinylpyridine) (P4VP), then coated P4VP again, and then deposited a scattering layer. Most Ag NPs were immobilized individually without aggregation, and formed a quasi-monolayer. By constructing a panchromatic quasi-monolayer between the photoactive and scattering layers, the efficiency was enhanced from 8.9 ± 0.3% to 11.0 ± 0.4%, mainly by enhancing the photocurrent density. The photocurrent density might be enhanced by enhancement of light absorption and electron transfer yield. Absorption of dye molecules might be enhanced on or near the surface of Ag NPs by the localized surface plasmons. Absorption of dye molecules, particularly molecules not adsorbed on or near the surface, could be enhanced by the scattered light, since the quasi-monolayer of Ag NPs scattered light strongly. Owing to the enhanced dye absorption, we could reduce greatly the thickness of the photoactive layer. The thickness was about 4.5 μm, corresponding to about one-half the optimum length for DSSCs which did not include metal NPs. The electron transfer yield to the electrode might be enhanced by reducing the electron transfer length. Our general method could be used in the fabrication of other types of solar cells.
Introduction
Localized surface plasmon resonances (LSPRs) are non-propagating excitations of the conduction electrons of metal nanoparticles (NPs) coupled to the electromagnetic field.1,2 When the frequency of light photons matches the natural frequency of the collective oscillation of conduction electrons in metal NPs, LSPR is excited. LSPRs create sharp spectral scattering and absorption peaks as well as strong electromagnetic near-field enhancements. The principle of LSPR has been applied to increase the optical absorption and/or photocurrent in a wide range of solar cell configurations.3–25 However, there has been relatively less effort to apply the enhanced light scattering properties of metal NPs to improve the energy conversion efficiency of solar cells.For all the reports of plasmonic DSSCs, the energy conversion efficiency increased up to a certain weight percent of Ag or Au NPs to TiO2 NPs but then decreased when the weight percent was further increased.17–26 Recently, Kim et al. have proved that this is due to aggregation of metal NPs.25,26 In the fabrication of photoactive films, metal NPs and TiO2 NPs were mixed together and then coated on an ITO or FTO glass. Therefore, in principle, aggregations between metal NPs could not be prevented, particularly at a relatively high weight percent of metal NPs. When metal NPs are aggregated, the localized plasmon resonance frequency is red shifted.27,28 N719 dye, which is the most used dye in DSSCs, has two strong absorption bands centered at 393 and 533 nm. The solar intensity is much stronger at 533 nm than 393 nm, and the band centered at 533 nm could absorb more solar light than that centered at 393 nm. Spherical silver NPs have a LSPR band centered at near 400 nm, while gold NPs have one at near 520 nm with an increasingly strong background from 500 nm to UV.24,25,28 Therefore, to optimize the spectral overlap between the band at 533 nm of N719 dye and the LSPR band of metal NPs, Ag NPs should be aggregated to dimers or trimers, while Au NPs should remain individual without aggregation. When Au NPs are aggregated or the cluster size of Ag NPs becomes larger than dimer or trimer, the localized plasmon resonance is shifted to red. In this case, the spectral overlap between the absorption of N719 dye molecules and the LSPR of metal NPs may be reduced, and consequently the plasmon-enhanced absorption of the dye molecules will be reduced.
Ag nanoplates have two localized plasmon resonances, due to the in-plane and out-of-plane resonances, in the visible region. With increasing the size of Ag nanoplates, the wavelength of the in-plane plasmon near 530 nm is red shifted, while that of the out-of-plane plasmon near 400 nm is almost not shifted.26 By controlling their size and geometry, the LSPR of Ag nanoplates could be tuned. Colloid solutions like Ag or Au are very stable because their colloid particles have the same surface charge and repel each other. When Ag or Au NPs are immobilized on a plate coated with poly(4-vinyl pyridine) (P4VP), they are immobilized individually without aggregation due to this repulsion.28
Herein, we have fabricated three kinds of Ag NPs whose extinction maximum wavelengths are 540, 620 and 470 nm. By immobilizing them on the film of TiO2 NPs coated with P4VP without aggregation, we fabricated a panchromatic quasi-monolayer of Ag NPs whose LSPR took place in the entire visible region. DSSCs including a panchromatic quasi-monolayer of Ag NPs between the photoactive layer of TiO2 NPs and the scattering layer were very effective, and the power conversion efficiency was enhanced from 8.9 ± 0.3% to 11.0 ± 0.4%. The efficiency of 11.4% achieved here is the highest published so far for plasmonic DSSCs and about the highest value for DSSCs based on N719 dye and TiO2 NPs.
Experimental
Synthesis of colloidal Ag NPs
Ag NPs were synthesized by a rapid thermal process.29 25 mL of 0.1 mM silver nitrate, 1.5 mL of 30 mM trisodium citrate, 1.5 mL of 0.7 mM poly(vinylpyrolidone) (weight average molecular weight; ∼29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and 60 μL of 30 wt% hydrogen peroxide were mixed together. To this solution, 60 μL of X (60, 80, 100) mM sodium borohydride was rapidly injected and then stirred vigorously for 45 min. The colour of the colloid solutions became pale yellow after injection, and then slowly changed to orange or purple or blue, indicating the growth of Ag NPs. The size of Ag NPs was controlled by varying the concentration of the sodium borohydride solution injected.
000 g mol−1) and 60 μL of 30 wt% hydrogen peroxide were mixed together. To this solution, 60 μL of X (60, 80, 100) mM sodium borohydride was rapidly injected and then stirred vigorously for 45 min. The colour of the colloid solutions became pale yellow after injection, and then slowly changed to orange or purple or blue, indicating the growth of Ag NPs. The size of Ag NPs was controlled by varying the concentration of the sodium borohydride solution injected.
Fabrication of DSSCs
A TiO2 blocking layer was formed on the surface of fluorine-doped thin oxide (FTO) glass by spin-coating with 5 wt% of titanium di-isopropoxide bis(acetylacetonate) in butanol and annealed at 450 °C for 1 h. Conventional photoanodes were prepared by depositing TiO2 NPs paste (T/SP, solaronix) on the surface of the FTO glass using a doctor blade technique, and then heated gradually at 450 °C for 1 h. The scheme for constructing a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer is shown in Fig. 5. The surface of the conventional photoanodes was coated with poly(4-vinylpyridine) (P4VP) by dipping in a P4VP solution (0.3 g of P4VP was dissolved in ethanol 100 mL) for 1 h 30 min, then the surface was washed sufficiently with ethanol, and then dried in air. The photoanode coated with P4VP was put in a Petri dish containing 15 mL of Ag NPs solution for 12 h to immobilize Ag NPs on it, and then the surface was washed with H2O and ethanol. After drying, the surface was coated with P4VP again, and then a paste for the scattering layer (DSL 18NR-AO, Dyesol) was coated by doctor blade and then sintered at 450 °C for 30 min. The resulting films were treated in titanium isopropoxide (TIP) solutions (0.1 M in isopropyl alcohol) for 30 min at 90 °C, followed by annealing at 350 °C for 10 min. For the films including Ag NPs, additional hydrogen was flowed at 450 °C for 10 min. The photoanodes were immersed into an N719 (solaronix) dye solution (50 mM in EtOH) under heating to 50 °C for 12 h. Pt-layered counter-electrodes were prepared by drop-coating H2PtCl6 solution (0.5 M in EtOH) onto FTO glass and then sintered at 450 °C for 30 min. The working electrode was assembled with a Pt-coated counter electrode by using a 60 μm thick layer of Surlyn®. The composition of the electrolyte was as follows: 0.7 M of 1-butyl-3-methyl imidazolium iodide (BMII), 0.03 M of I2, 0.1 M of guanidium thiocyanate (GSCN), and 0.5 M of 4 tert-butyl pyridine (TBP) in a mixture of acetonitrile and valeronitrile (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v).
15 v/v).
Characterization of Ag NPs and DSSCs
The structure and size of Ag NPs were confirmed by an energy-filtering transmittance electron microscope (EF-TEM, Carl Zeiss, LIBRA 120) and a field emission scanning electron microscope (FE-SEM) (JSM-6330F, JEOL Inc.). Current density–voltage (J–V) characteristics of the DSSCs were measured using an electrometer (KEITHLEY 2400) under AM 1.5 illumination (100 mW cm−2) provided by a solar simulator (1 kW xenon with AM 1.5 filter, PEC-L01, Peccel Technologies). The incident photon-to-current conversion efficiency (IPCE) was measured using a McScience model K3100 with reference to the calibrated diode. A 300 W xenon lamp was used as a light source for the generation of a monochromatic beam. The bias light was supplied by a 150 W halogen lamp. Electrochemical impedance spectroscopy (EIS) was carried out with a potentiostat (Solartron 1287) equipped with a frequency response analyzer (Solartron 1260), with the frequency ranging from 10−1 to 105 Hz. The applied bias voltage and ac amplitude were set at the open circuit voltage (Voc) of the DSSCs and 10 mV, respectively. The impedance measurements were carried out at open-circuit potential under AM 1.5 one-sun light illumination.Results and discussion
Fig. 1 shows scanning electron microscopy (SEM) images of three kinds of Ag NPs immobilized on the surface of cover glasses coated with P4VP and a schematic of Ag NPs immobilized on P4VP. Most Ag NPs are isolated. The Ag NPs shown in Fig. 1a are more like spheres, while they are more like nanoplates in Fig. 1b and c. TEM images of the three kinds of Ag NPs are shown in Fig. S1,† and one is shown in Fig. 1b as an insert. Their size is about 15 ± 4, 24 ± 3, and 30 ± 4 nm, respectively. Ag NPs are immobilized on P4VP by an attractive interaction between the lone pair electrons on the nitrogen atom of the pyridine ring and Ag NPs (see Fig. 1d).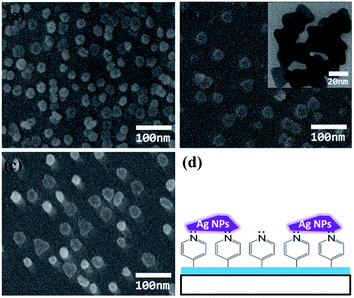 | ||
| Fig. 1 SEM images of three kinds of Ag NPs immobilized on the surface of cover glasses coated with P4VP and a schematic of silver NPs immobilized on P4VP. Insert is a TEM image. | ||
Fig. 2 shows the UV-visible extinction spectra of the three kinds of immobilized Ag NPs, whose SEM images are shown in Fig. 1a–c, and those of their solutions. The extinction maxima of the three kinds of immobilized Ag NPs are at 470, 540 and 620 nm, respectively. With increasing the size, the maximum wavelength is shifted to red and the extinction band becomes broad. The spectrum of each kind of immobilized Ag NPs is very similar to that measured in their solution. There is no significant shift in their extinction maximum wavelengths. The Ag nanoplates fabricated by seed-mediated processes have two localized plasmon resonance peaks, due to the in-plane and out-of-plane resonances, near 520 and 400 nm, respectively.30 The in-plane peak of Fig. 2a and b is much broader than that reported, while the out-of-plane peak is relatively much weaker and indistinct. For a comparison, the absorption spectrum of N719 dye is shown in Fig. 2c. There are two visible bands at 398 and 538 nm. The absorption of N719 dye is relatively weak near 450 nm and at longer wavelengths than 600 nm.
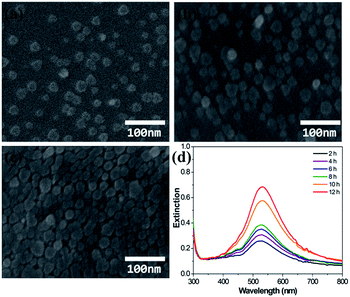
Fig. 3 shows the SEM images of Ag NPs immobilized for 4, 8, and 12 h and UV-visible extinction spectra measured by varying the immobilization time. Ag NPs are completely isolated when the immobilization time was relatively short. With increasing immobilization time the surface coverage is increased, and some seem to be aggregated. Although the extinction intensity is increased greatly with increasing immobilization time, the extinction maximum wavelength is only slightly red shifted. This may mean that aggregation of Ag NPs is quite limited in the process of immobilization. Aggregation could be prevented by the repulsion force between Ag NPs which have the same surface charge.
Fig. 4 shows the SEM images and extinction spectra of three kinds of Ag NPs immobilized on a cover glass coated with P4VP in three steps. It should be mentioned that Ag NPs were not distinguished from TiO2 NPs when they were immobilized on the film of TiO2 NPs (see Fig. S2†). When Ag NPs having λmax of 540 nm in the solution phase were immobilized for 4 h, the extinction maximum wavelength was near 540 nm, which was almost the same as that of the Ag NPs in the solution phase (see the black colored spectrum in Fig. 4d). The surface coverage of Ag NPs was greater than 50%. When two kinds of Ag NPs having λmax of 540 and 620 nm were immobilized, in sequence, for 4 h each, the λmax was near 575 nm, and a shoulder on the low energy side was significantly increased (see the blue spectrum). When three kinds of Ag NPs having λmax of 540, 620, and 470 nm were immobilized in sequence for 4 h each, the λmax was near 550 nm, and the shoulders on the high and low energy sides were significantly increased compared to the black spectrum (see the red spectrum). The extinction intensity was increased with increasing the total immobilization time.
A conventional photoanode based on TiO2 NPs is fabricated by formation of a blocking layer on FTO glass, then depositing a relatively thick layer of TiO2 NPs, and then depositing a scattering layer. We have included a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer. The schematics of the fabrication processes are shown in Fig. 5. P4VP was coated on the surface of the film of TiO2 NPs deposited on the FTO glass, which had formed a blocking layer on its surface, and then Ag NPs were immobilized on the surface of P4VP by placing the FTO glass coated with P4VP in Ag colloid solutions. After they were immobilized, P4VP was coated again to prevent movement of the Ag NPs, then the scattering layer was deposited by doctor blade printing, and then sintered. For a panchromatic quasi-monolayer of Ag NPs whose extinction takes place in the whole visible region, three kinds of Ag NPs, whose maximum absorptions were at 540, 620 and 470 nm, were immobilized in sequence. In the immobilization of Ag NPs, the surface coverage was increased with increasing immobilization time but not linearly. The increasing rate of the coverage was decreased with increasing immobilization time. Due to this non-linearity, it was very hard to optimize a quasi-monolayer consisting of three kinds of Ag NPs. To simplify, we immobilized each kind of Ag NPs for an equal time. In this case, the efficiency was critically affected by the immobilization order of the three kinds of Ag NPs. The efficiency was highest when Ag NPs of 540 nm in λmax were immobilized first, and then 620 nm, and finally 470 nm (see Table S7†). This could be related to the fact that among the films prepared by varying the immobilization order of three kinds of Ag NPs, the film prepared by immobilizing Ag NPs having λmax of 540 nm first, then 620 nm, and then 470 nm showed the broadest extinction spectrum, and had the highest overlap with the spectrum of N719 dye (see Fig. S3†). The efficiency of the DSSCs including a quasi-monolayer consisting of three kinds of Ag NPs was highest when the immobilization time of each type of Ag NPs was 4 h (12 h in total, see Table S6†). Also, the efficiency was slightly affected by the coating times of P4VP before and after immobilization of Ag NPs (see Tables S1–S5†). It was highest when the coating time of P4VP before and after immobilization of Ag NPs was 1.5 and 1 h, respectively.
To prevent corrosion of Ag NPs, TIP treatment was used. TIP treatment is known to be an efficient method to prevent silver corrosion and electron leakage from Ag NPs. Jeong et al. have studied the effect of treating Ag NPs with TIP in detail and proved that TIP treatment can sufficiently prevent silver corrosion and electron leakage from metal NPs.12 The thickness of the photoactive TiO2 film itself was about 4.5 μm in the DSSCs including a quasi-monolayer of Ag NPs, while 9 μm in the conventional DSSCs. Including the scattering layer, the thickness of the photoactive TiO2 film was about 13 μm for the DSSCs including a quasi-monolayer of Ag NPs and 17 μm for the conventional DSSCs.
It should be mentioned that the morphology of Ag nanoplates could be modified by sintering at 450 °C. When Ag nanoplates are sintered at a relatively high temperature, they will be changed ultimately to spherical silver to minimize their surface area. It is known that the LSPR band of spherical Ag NPs is centred near 400 nm.28 Therefore, a blue shift of the LSPR band of our Ag nanoplates could take place when the morphology was modified by sintering. However, the Ag nanoplates included in our DSSCs existed between the photoactive and scattering layers. This means that the Ag nanoplates were surrounded by a large amount of TiO2 NPs. Also, the surface of the Ag nanoplates was coated with TiO2 to prevent corrosion. Therefore, the morphology change of Ag nanoplates by sintering could be reduced greatly under our conditions. We tried to observe the blue shift of Ag nanoplates surrounded by TiO2 NPs by sintering. However, we could not observe the LSPR band of Ag nanoplates themselves due to the strong background of TiO2.
In Fig. 6a, the photocurrent density–voltage (J–V) curves measured in air mass 1.5 sunlight from the DSSCs based on films of TiO2 NPs, with and without a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer, are compared. The photovoltaic parameters are summarized in Table 1. The total immobilization time of a single kind or three kinds of Ag NPs was kept the same at 12 h. For a panchromatic quasi-monolayer, Ag NPs with λmax at 540 nm were immobilized first, then those at 620 nm, and then those at 470 nm. For the DSSC not including a monolayer of Ag NPs, the short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (ff), and overall conversion efficiency (η) were 16.23 ± 0.42 mA cm−2, 0.80 ± 0.02 V, 0.69 ± 0.01, and 8.9 ± 0.3%, respectively. Among the DSSCs including a single-type of Ag NPs as a quasi-monolayer, the one with Ag NPs whose λmax was 540 nm showed the highest efficiency. The photovoltaic parameters were 18.11 ± 0.55 mA cm−2, 0.79 ± 0.01 V, 0.70 ± 0.02, and 10.5 ± 0.3%, respectively. For the DSSC including a quasi-monolayer consisting of three kinds of Ag NPs, the photovoltaic parameters were 19.27 ± 0.53 mA cm−2, 0.79 ± 0.03 V, 0.71 ± 0.03, and 11.0 ± 0.4%, respectively. It is concluded that the DSSCs including a quasi-monolayer of three kinds of Ag NPs are more effective for light harvesting than the DSSCs including that of a single kind of Ag NPs or conventional DSSCs. The open-circuit voltages and fill factor are not changed significantly. The efficiency enhancement by inclusion of a quasi-monolayer of Ag NPs is mainly caused by the increase in photocurrent density. The efficiency of 11.4% achieved here is the highest published so far for plasmonic DSSCs.24,31
| Jsc (mA cm−2) | Voc (V) | ff (%) | η (%) | |
|---|---|---|---|---|
| 470 nm | 16.23 ± 0.42 | 0.80 ± 0.02 | 0.69 ± 0.01 | 9.3 ± 0.2 |
| 540 nm | 18.11 ± 0.55 | 0.79 ± 0.01 | 0.70 ± 0.02 | 10.5 ± 0.3 |
| 620 nm | 16.98 ± 0.48 | 0.78 ± 0.01 | 0.71 ± 0.02 | 9.7 ± 0.2 |
| Panchromatic | 19.27 ± 0.53 | 0.79 ± 0.03 | 0.71 ± 0.03 | 11.0 ± 0.4 |
| Reference | 16.20 ± 0.41 | 0.78 ± 0.02 | 0.70 ± 0.03 | 8.9 ± 0.3 |
The incident photon-to-current conversion efficiency (IPCE) spectra measured from the five DSSCs are shown in Fig. 6b. By construction of a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer, the IPCE over the wavelength range 400 to 700 nm is enhanced, exhibiting a maximum near 550 nm which is closely associated with N719 dye absorption. The DSSC including a quasi-monolayer of three kinds of Ag NPs shows highest intensity over the whole range of wavelengths (400–700 nm). The relative intensity of the IPCE spectra of the DSSCs including a quasi-monolayer of Ag NPs is closely related to the degree of the spectral overlap between the extinction bands of the Ag NPs included in fabrication of the DSSCs and the absorption bands of N719 dye. This is clearly seen in the IPCE improvement factor spectra shown in Fig. 6c. The factor has been calculated by the following equation: ΔIPCE (λ)/IPCE (λ)% = [(IPCEDSSC including a Ag NPs’ layer (λ) − IPCEDSSC not including a Ag NPs’ layer (λ))/IPCEDSSC not including a Ag NPs’ layer (λ)] × 100. In the IPCE improvement factor spectra, the intensity of the DSSC including a quasi-monolayer of Ag NPs whose λmax is 620 nm is stronger in the low energy region but weaker in the high energy region than that of the DSSC including a quasi-monolayer of Ag NPs whose λmax is 470 nm.
Fig. 7 shows the characteristic electrochemical impedance spectra (EIS) for the DSSCs based on TiO2 film and TiO2/Ag nanoplate film. They were recorded in the frequency range of 0.1 Hz to 100 KHz. Each spectrum contains three well-defined semicircles. The hemisphere in the high-frequency region is assigned to the parallel combination of the resistance and capacitance at the Pt-FTO/electrolyte and to the interface between the FTO and TiO2 layers, while those in the intermediate and low-frequency regions offer information on the resistance and capacitance at the TiO2/electrolyte interface and the Nernst diffusion of the electrolyte, respectively.32 On the Nyquist plot the two spectra are almost the same, while on the Bode plot the two spectra show a little difference in the low and high frequency regions. In both spectra, the position of the middle frequency (fmid) peak was the same, at 21.23 Hz. The value of the middle frequency (fmid) is related to the inverse of the electron lifetime (τ) as follows: τeff = 1/(2πfmid).33,34 The calculated electron lifetime was 7.49 ms for the DSSCs based on the photoactive film of TiO2 NPs with and without a panchromatic quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer. Also, the electron lifetime is related to the effective carrier diffusion length (Ln) as follows: Ln2 = Deff × τ, where Deff is the effective electron diffusion coefficient and τ is the electron lifetime. Based on the Bisquert model,35 Deff is described as Deff = (Rk/Rw)L2keff.33,34 Where Rw, Rk, L, and keff represent the resistance of electron transport in the photoanode, the resistance of charge transfer related to recombination, the thickness of the photoanode, and the constant of effective rate for recombination, respectively. The values of these parameters can be obtained from the semicircle of middle frequency in the Nyquist and Bode plots. Rw is estimated from the diameter of the middle semicircle in the Nyquist plot shown in Fig. 7a, and Rk is determined from the diameter of the middle semicircle in the Nyquist plot obtained under dark conditions (Fig. S4†). keff is obtained from the maximum peak frequency obtained from the Bode plot in Fig. 7b.34 The calculated values are summarized in Table S8.† The calculated value of Ln for the DSSCs based on the photoactive film of TiO2 NPs with and without a panchromatic quasi-monolayer of Ag NPs was 7.10 and 7.98 μm, respectively. The results indicate that there is no significant difference both in the electron lifetime and effective carrier diffusion length. This could be due to the fact that the panchromatic layer of Ag NPs was constructed on the surface of the photoactive layer, and it was not on the path of electron transfer. Therefore, basically the electron lifetime and effective carrier diffusion length could not be affected by the panchromatic layer.
By construction of a panchromatic quasi-monolayer of Ag NPs between the photoactive and scattering layers and reducing the thickness of the photoactive layer, the power conversion efficiency has been enhanced up to 11.4%, mainly by enhancing the photocurrent density. The efficiency of 11.4% achieved here is the highest published so far for plasmonic DSSCs.24 The photocurrent density might be enhanced by enhancement of light absorption and electron transfer yield to the electrode. An enhanced light absorption of dye molecules could take place on or near the surface of metal NPs by LSPRs. The LSPRs decay either radiatively or into (quasi) particles such as electron–hole (e–h) pairs.36 The former decay path gives rise to dramatic electromagnetic field enhancements, for instance, as in surface-enhanced Raman spectroscopy. By the latter decay path, the light absorption of dye molecules adsorbed on or near Ag NPs is enhanced.37 Absorption of dye molecules, particularly molecules not adsorbed on or near the surface of Ag NPs, could be enhanced by the scattered light, since the quasi-monolayer of Ag NPs scattered light strongly. Besides these two enhanced absorptions caused by Ag NPs, the normal absorption of the incident light by the dye molecules adsorbed on the surface of TiO2 NPs takes place. This normal absorption may increase with increasing the number of dye molecules up to a certain value, since the number of photons of solar light is limited. Therefore, this absorption will increase with increasing the thickness of the photoactive layer consisting of TiO2 NPs where dye molecules are adsorbed. The plasmon enhanced absorption takes place only to the molecules adsorbed on or near the surface of Ag NPs, and it is not affected directly by the thickness of the photoactive layer. However, it is affected by the light intensity reaching the quasi-monolayer. Therefore, the plasmon enhanced absorption may be critically affected by the location of the quasi-monolayer of Ag NPs, since the intensity of light reaching the quasi-monolayer is decreased with an increasing thickness of photoactive layer. With an increasing thickness of photoactive layer, absorption of the scattered light could be affected by two opposite factors. With an increasing thickness of the photoactive layer, the intensity of light reaching the quasi-monolayer is decreased, and that of the scattered light is also decreased. However, the number of dye molecules, which could absorb the scattered light, is increased with increased thickness of the photoactive layer. Consequently, the dye absorption could be affected by the thickness of the photoactive layer existing in front of the panchromatic quasi-monolayer. Actually, the efficiency of the DSSCs with a panchromatic quasi-monolayer between the photoactive and scattering layers was affected by the thickness of the photoactive layer. When the thickness of the photoactive layer was 3.0, 4.5, and 9.0 μm, the efficiency was 9.99, 11.4, and 10.7%, respectively. A more precise optimization of the thickness should be studied in detail. At least, a high efficiency was achieved by construction of a panchromatic quasi-monolayer of Ag NPs on the surface of a photoactive layer whose thickness was 4.5 μm. This thickness is much thinner than the optimum length (about 9 μm) for the DSSCs based on only TiO2 NPs.38 There is no doubt that the electron transfer yield to the electrode increases with decreasing the transfer length. Therefore, the high efficiency might be contributed partially by the reduced thickness of the photoactive film.
It is not a difficult process to construct a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer. The optical properties of the quasi-monolayer of Ag NPs are not critically affected by the fabrication technique, since most Ag NPs are immobilized without aggregation due to repulsion between Ag NPs. Also, the panchromatic property is made quite uniformly on the whole area of the quasi-monolayer, since Ag NPs are immobilized randomly. This general method to construct a quasi-monolayer of Ag NPs could be used in the fabrication of other types of solar cells.
Conclusions
We have developed a quasi-monolayer of Ag NPs, without causing aggregation, whose extinction takes place in all of the visible range and applied this technique to construct dye-sensitized solar cells (DSSCs). Three kinds of Ag NPs, whose λmax were at 540, 620 and 470 nm, were immobilized, in sequence, on a photoactive film of TiO2 NPs coated with P4VP, and then coated with P4VP again, then a scattering layer was deposited. The efficiency of the DSSCs including a quasi-monolayer of Ag NPs between the layer of TiO2 NPs and the scattering layer was enhanced up to 11.4%. The efficiency of 11.4% achieved here is the highest published so far for plasmonic DSSCs. A high efficiency is contributed by the increased light absorption and reduced electron transfer length. Our general and simple method could be used in the fabrication of other types of solar cells.Acknowledgements
This work was supported by Basic Study program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012R1A1A2003515), the New & Renewable Energy of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant (305-20110024) funded by the Ministry of Knowledge Economy, Republic of Korea, and the BK21 program.Notes and references
- M. E. Stewart, C. R. Anderton, L. B. Thompson, J. Maria, S. K. Gray, J. A. Rogers and R. G. Nuzzo, Chem. Rev., 2008, 108, 494–521 CrossRef CAS PubMed.
- N. Zhou, V. López-Puente, Q. Wang, L. Polavarapu, I. Pastoriza-Santos and Q.-H. Xu, RSC Adv., 2015, 5, 29076 RSC.
- M. Ihara, K. Tanaka, K. Sakaki, I. Honma and K. Yamada, J. Phys. Chem. B, 1997, 101, 5153–5157 CrossRef CAS.
- S. D. Standridge, G. C. Schatz and J. T. Hupp, Langmuir, 2009, 25, 2596–2600 CrossRef CAS.
- A. Baba, K. Wakatsuki, K. Shinbo, K. Kato and F. Kaneko, J. Mater. Chem., 2011, 21, 16436–16441 RSC.
- Y. Wang, J. Zhai and Y. Song, Phys. Chem. Chem. Phys., 2015, 17, 5051 RSC.
- Y. Wang, J. Zhai and Y. Song, RSC Adv., 2015, 5, 210 RSC.
- J.-L. Wu, F.-C. Chen, Y.-S. Hsiao, F.-C. Chien, P. Chen, C.-H. Kuo, M. H. Huang and C.-S. Hsu, ACS Nano, 2011, 5, 959–967 CrossRef CAS PubMed.
- W. Jiang, H. Liu, L. Yin and Y. Yin, J. Mater. Chem. A, 2013, 1, 6433–6440 CAS.
- S. D. Standridge, G. C. Schatz and J. T. Hupp, J. Am. Chem. Soc., 2009, 131, 8407–8409 CrossRef CAS PubMed.
- M. D. Brown, T. Suteewong, R. S. S. Kumar, V. D’Innocenzo, A. Petrozza, M. M. Lee, U. Wiesner and H. J. Snaith, Nano Lett., 2011, 11, 438–445 CrossRef CAS PubMed.
- N. C. Jeong, C. Prasittichai and J. T. Hupp, Langmuir, 2011, 27, 14609–14614 CrossRef CAS PubMed.
- Y.-C. Yen, P.-H. Chen, J.-Z. Chen, J.-A. Chen and K.-J. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 1892–1898 CAS.
- T. Kawawaki, Y. Takahashi and T. Tatsuma, J. Phys. Chem. C, 2013, 117, 5901–5907 CAS.
- S. W. Sheehan, H. Noh, G. W. Brudvig, H. Cao and C. A. Schmuttenmaer, J. Phys. Chem. C, 2013, 117, 927–934 CAS.
- J. T. Park, W. S. Chi, H. Jeon and J. H. Kim, Nanoscale, 2014, 6, 2718 RSC.
- J. Yun, S. H. Hwang and J. Jang, ACS Appl. Mater. Interfaces, 2015, 7, 2055–2063 CAS.
- Y. H. Jang, Y. J. Jang, S. T. Kochuveedu, M. Byun, Z. Lin and D. H. Kim, Nanoscale, 2014, 6, 1823 RSC.
- M. K. Gangishetty, R. W. J. Scott and T. L. Kelly, Langmuir, 2014, 30, 14352–14359 CrossRef CAS PubMed.
- H. Jung, B. Koo, J.-Y. Kim, T. Kim, H. J. Son, B. S. Kim, J. Y. Kim, D.-K. Lee, H. Kim, J. Cho and M. J. Ko, ACS Appl. Mater. Interfaces, 2014, 6, 19191–19200 CAS.
- H. F. Zarick, O. Hurd, J. A. Webb, C. Hungerford, W. R. Erwin and R. Bardhan, ACS Photonics, 2014, 1, 806–811 CrossRef CAS.
- M. K. Gangishetty, K. E. Lee, R. W. J. Scott and T. L. Kelly, ACS Appl. Mater. Interfaces, 2013, 5, 11044–11051 CAS.
- H. Choi, W. T. Chen and P. V. Kamat, ACS Nano, 2012, 6, 4418 CrossRef CAS PubMed.
- X. Dang, J. Qi, M. T. Klug, P.-Y. Chen, D. S. Yun, N. X. Fang, P. T. Hammond and A. M. Belcher, Nano Lett., 2013, 13, 637–642 CrossRef CAS PubMed.
- H.-Y. Kim, D. H. Song, H. Yoon and J. S. Suh, RSC Adv., 2015, 5, 27464 RSC.
- H.-Y. Kim, W.-Y. Rho, H. Y. Lee, Y. S. Park and J. S. Suh, Sol. Energy, 2014, 109, 61 CrossRef CAS PubMed.
- M. Rycenga, M. R. Langille, M. L. Personick, T. Ozel and C. A. Mirkin, Nano Lett., 2012, 12, 6218–6222 CrossRef CAS PubMed.
- S. Lee, G. H. Gu and J. S. Suh, Chem. Phys. Lett., 2011, 511, 121 CrossRef CAS PubMed.
- G. M. Metraux and C. A. Mirkin, Adv. Mater., 2005, 17, 412–415 CrossRef CAS PubMed.
- S. T. Gentry and M. W. J. Bezpalko, J. Phys. Chem. C, 2010, 114, 6989–6993 CAS.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- Y. J. Kim, M. H. Lee, H. J. Kim, G. Lim, Y. S. Choi, N.-G. Park, K. Kim and W. I. Lee, Adv. Mater., 2009, 21, 3668–3673 CrossRef CAS PubMed.
- J. Song, Z. Yin, Z. Yang, P. Amaladass, S. Wu, J. Ye, Y. Zhao, W.-Q. Deng, H. Zhang and X.-W. Liu, Chem.–Eur. J., 2011, 17, 10832–10837 CrossRef CAS PubMed.
- Y. Wang, J. Zhai and Y. Song, RSC Adv., 2015, 5, 210–214 RSC.
- J. Bisquert, J. Phys. Chem. B, 2002, 106, 325–333 CrossRef CAS.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer Series in Materials Science, Springer, NY, USA, 1995 Search PubMed.
- C. Langhammer, B. Kasemo and I. Zorić, J. Chem. Phys., 2007, 126, 194702 CrossRef PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information regarding the dependence of the photovoltaic parameters of the DSSCs including a quasi-monolayer of Ag NPs on the coating time of P4VP, and on the immobilization time of Ag NPs. See DOI: 10.1039/c5ra10858f |
| This journal is © The Royal Society of Chemistry 2015 |