Oxidative and membrane stress-mediated antibacterial activity of WS2 and rGO-WS2 nanosheets†
Govinda R. Navalea,
Chandra Sekhar Routb,
Kushal N. Gohilc,
Mahesh S. Dharne*c,
Dattatray J. Late*d and
Sandip S. Shinde*a
aOrganic Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune 411008, Maharashtra, India. E-mail: ss.shinde@ncl.res.in
bSchool of Basic Sciences, Indian Institute of Technology Bhubaneswar, Bhubaneswar 751013, Odisha, India. E-mail: csrout@iitbbs.ac.in
cNCIM Resource Centre, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune 411008, Maharashtra, India. E-mail: ms.dharne@ncl.res.in
dPhysical & Materials Chemistry Division, CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune 411008, Maharashtra, India. E-mail: dj.late@ncl.res.in
First published on 27th August 2015
Abstract
Graphene-based materials have strong cytotoxic attributes against bacteria due to their unique physicochemical properties. We examined the antibacterial activities of nanosheets of the graphene analogue tungsten disulphide (WS2) and a composite of reduced graphene oxide-tungsten disulphide (rGO-WS2), comparing them with reduced graphene oxide (rGO) by a time and concentration dependent viability assay and growth curve studies against four bacterial strains: Gram negative Escherichia coli (E. coli) and Salmonella typhimurium (S. typhimurium), and Gram positive Bacillus subtilis (B. subtilis) and Staphylococcus epidermidis (S. epidermidis). The nanosheets of the rGO-WS2 composite caused a more significant retardation in bacterial growth and inhibitory effect on the tested bacterial strains than WS2, followed by rGO. The tested E. coli and B. subtilis strains were more susceptible than the other strains. A mechanistic study revealed that rGO and WS2 did not produce the superoxide anion (O2˙−) or reactive oxygen species (ROS), but the nanocomposite of rGO-WS2 did produce both. However, all these materials did oxidize glutathione, which serves as a redox state mediator in bacteria. We conclude that the antimicrobial mechanism is due to the combined effect of initial cell deposition on the rGO-WS2 materials, the membrane stress due to direct contact with the nanosheets, and the produced superoxide anion-independent oxidation mechanisms. The beneficial aspects of the physicochemical properties of rGO-WS2, such as its size and conductivity, can be precisely customized to reduce its health and environmental risk factors.
1. Introduction
Graphene, the 2D nanosheet of carbon atoms, has attracted wide interest due to its unusual electrical, magnetic and optical properties exhibited by a single layer or a few layers of nanosheets.1–6 This has attracted attention from physicists, chemists, material scientists and biologists for a variety of fundamental phenomena and new exciting applications. The zero bandgap of graphene hinders its wider application in various fields. The atomically thin two-dimensional metal dichalcogenide (TMD) materials such as molybdenum disulfide (MoS2), tungsten disulfide (WS2), molybdenum diselenide (MoSe2), tungsten diselenide (WSe2), etc. and their composites with graphene have been utilized as graphene analogue materials due to their semiconducting nature, tunable bandgap and optical properties.7–17 Bulk WS2 is a layered material with an indirect bandgap of 1.3 eV, whereas single layered WS2 has a direct bandgap of 2 eV. Reduced graphene oxide (rGO)18,19 and MoS2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 have been utilized in biological applications for antibacterial studies due to their intrinsic chemical, physical and optical properties,5,15 and have also been utilized widely in the biomedical field for cell imaging and for DNA detection.21–23 The electronic, magnetic, sensing, hydrogen evolution, and photonic properties of rGO, a few layers of WS2 and its composite materials have been widely studied.5,17,24–26 Recently, 2D WS2 nanosheets have been used in medical diagnostics such as cancer therapy and drug delivery.27–29 Compared to the intensive study of the biological effects of graphene and 2D MoS2 sheets, there is an absence of studies on the possible toxicity effects of WS2 and its composite with rGO materials, to realize the potential applications of pristine and composite nanosheets.
20 have been utilized in biological applications for antibacterial studies due to their intrinsic chemical, physical and optical properties,5,15 and have also been utilized widely in the biomedical field for cell imaging and for DNA detection.21–23 The electronic, magnetic, sensing, hydrogen evolution, and photonic properties of rGO, a few layers of WS2 and its composite materials have been widely studied.5,17,24–26 Recently, 2D WS2 nanosheets have been used in medical diagnostics such as cancer therapy and drug delivery.27–29 Compared to the intensive study of the biological effects of graphene and 2D MoS2 sheets, there is an absence of studies on the possible toxicity effects of WS2 and its composite with rGO materials, to realize the potential applications of pristine and composite nanosheets.
In view of the lack of studies on the antibacterial activity of WS2 and a nanocomposite of rGO-WS2 nanosheets, we considered it important to carry out detailed investigations on the antibacterial activities of nanosheets of emerging 2D pristine WS2 and a rGO-WS2 composite. The composite nanosheets intensively inhibited the growth activities of Gram negative Escherichia coli DH5α, Salmonella typhimurium and Gram positive Bacillus subtilis, Staphylococcus epidermidis bacterial cells.
In this article we are reporting for the first time the antibacterial activities of 2D nanosheets of WS2 and its composite with rGO against S. typhimurium and S. epidermidis respectively, as both are well known pathogens to cause nosocomial infections.30,31 The possibility of the production of superoxide anion (O2˙−) induced reactive oxygen species (ROS) was evaluated by the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5 carboxanilide (XTT) method. In vitro γ-L-glutamyl-L-cysteinyl-glycine (glutathione, GSH) oxidation was used to examine the superoxide anion independent oxidative stress. On the basis of these results, material characteristics related to their antibacterial activities were identified.
2. Experimental
2.1. Preparation of rGO, WS2 and rGO-WS2 nanosheets
A graphene oxide (GO) synthesis was performed using a modified Hummers’ method.17 In the general synthesis process, concentrated H2SO4 (50 mL) was added to graphite powder (SP-1, Bay carbon), K2S2O8 (1 g), and P2O5 (1 g) in a round-bottomed flask and heated at 80 °C. The obtained mixture was stirred using a magnetic stirrer and maintained at a temperature of 0 °C in an ice bath. Potassium permanganate (KMnO4, 6 g) was slowly added to the solution and stirred for 2 h. The reaction was terminated by the addition of an excess amount of distilled water and 5 mL H2O2 solution. The mixture was filtered and washed with excess HCl. The resulting graphite oxide was suspended in distilled water again, followed by dialysis (Dialysis membrane: Spectrum Laboratories, MWCO-12-14000) to remove excess HCl. The graphite oxide was exfoliated to give ∼5 mg mL−1 GO solution by ultrasonication. After exfoliation, the solution was centrifuged at 3500 rpm for 10 min to remove the non-exfoliated graphite oxide and the top supernatant GO solution was used for the hydrothermal reaction. The presence of oxygen functional groups makes the few-layered GO sheets highly hydrophilic and a stable dispersion was obtained.The few layered WS2 nanosheets were synthesized by a one-step hydrothermal reaction method as reported earlier.9,17 In a typical experiment, 3 mM WCl6 (Sigma-Aldrich, 99.98%) and 15 mM thioacetamide (C2H5NS, Sigma-Aldrich, ≥99%) were dissolved in 40 mL DI water and stirred for 1 h at room temperature using a magnetic stirrer. The solution was then transferred to a 50 mL stainless steel autoclave, followed by heating at 265 °C for 24 hours. After cooling the autoclave naturally, the as-synthesized product was filtered, washed with DI water and then dried under vacuum at 60 °C for 6 hours.
The rGO-WS2 composite nanosheets were prepared by similar hydrothermal reaction conditions to those for the WS2 sheets. 8 mL of 5 mg mL−1 GO solution were added to the mixture of WCl6 and thioacetamide and then the total volume of the solution was maintained at 40 mL. Then a similar procedure to the synthesis of the WS2 nanosheets was followed. During the hydrothermal synthesis process, the smaller size WS2 nanosheets were found to be epitaxially formed on the GO and subsequently the GO was transformed into rGO.
2.2. Microbial strains, culture conditions and cell preparation
Four representative bacterial strains were selected for this study: E. coli DH5α (dlacZ Delta M15 Delta (lacZYA-argF) U169 recA1 endA1 hsdR17(rK−mK+) supE44 thi-1 gyrA96 relA1 genotype; procured from Life technologies, USA), S. typhimurium NCIM 2501, B. subtilis NCIM 2063 and S. epidermidis NCIM 2493 were procured from the National Collection of Industrial Microorganisms (NCIM) Pune, India. All strains were grown in a Luria Bertani (LB) broth (Hi-Media, Mumbai) medium at 37 °C, and harvested in the mid exponential growth phase as and when required. The cell culture was centrifuged at 6000 rpm for 10 min to collect cells, and the cells were washed three times with isotonic saline solution to remove residual macromolecules and other growth medium constituents. The bacterial cell suspension was diluted in isotonic saline solution to obtain cell samples containing 107–108 colony forming units (CFU) mL−1 for antibacterial evaluations.2.3. Bacterial cell growth
The bacterial growth kinetics were assayed as reported by Wang et al.,32 with minor modifications. 200 μL of the diluted cell suspensions of all four bacteria (107 to 108 CFU mL−1) were mixed with 20 μL of five different concentrations of rGO, WS2 and the rGO-WS2 composite nanosheets (10, 50, 100 and 250 μg mL−1) and incubated under shaking conditions at 37 °C for 2 h at 150 rpm. A control sample contained 200 μL of the cell suspensions and 20 μL of saline water. The mixture was then transferred to 5 mL tubes, each containing 2 mL LB medium, and the tubes were inoculated on a rotary shaker at 150 rpm and 37 °C. The value of optical density (OD) at a wavelength of 620 nm was measured after every 2 h on a Multiscan EX UV-VIS spectrometer (Thermo scientific, USA). Bacterial growth curves were generated by plotting OD620 nm values versus time. All tests were prepared in triplicate.2.4. Cell viability assessment
All three nanosheets were dispersed in nuclease free (NF) water with different concentrations of nanosheets (10, 50, 100, 250 μg mL−1) for use. Dilutions ranging between 107–108 CFU mL−1 of B. subtilis, E. coli DH5α, S. typhimurium and S. epidermidis cells were incubated and dispersed in the desired concentration of nanosheets in NF water at 37 °C under 150 rpm shaking speed for a length of time up to 6 h. All the bacterial samples in NF water without nanosheets were used as a control. The loss of viability of all the strains was evaluated by the time and concentration dependent viability (colony counting) method. Briefly, a series of 10−4, 10−5 and 10−6 cell dilutions (100 μL each) were spread onto LB plates after every 2 h, and left to grow overnight (12–16 h) at 37 °C. The colonies were counted and compared with those on control plates to calculate the total viable count (TVC) and percentage of non-viable cells. All tests were prepared in duplicate, and repeated on at least two separate occasions.2.5. Cell morphology observation with TEM
The morphological changes of S. epidermidis bacteria (used as a model for TEM analysis) were further investigated using TEM after treatment with the rGO, WS2 and rGO-WS2 nanosheets. The bacterial suspensions were treated with all three nanosheets for 2 h at 37 °C. After centrifugation at 3000 rpm, the bacterial cells were fixed with 2.5% glutaraldehyde for 30 min and washed with phosphate buffer saline (pH 7.4). Subsequently, the samples were dehydrated in an ascending ethanol series (30, 50, 70, 80, 90 and 100%) for 15 min, and dried in a vacuum oven. Finally, diluted samples containing the bacterial cells were placed on the TEM grids and observed under a TEM FEI TECNAI TF-30 (FEG) instrument.2.6. Detection of reactive oxygen species (O2 ˙−)
To find out the reactive oxygen species’ antibacterial paths, the possibility of superoxide radical anion (O2˙−) production was evaluated by measuring the absorption of XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-arboxanilide, Sigma Aldrich). XTT can be reduced by the superoxide radical anion (O2˙−) to form water-soluble XTT-formazan that has its maximum absorption at 470 nm.20,33 XTT (0.4 mM) with menadione (0.25 mM) was used as a positive control. The detailed protocol is described in the ESI.†2.7. Thiol oxidation and quantification
Following the method used in a previous study,19,20 the concentration of thiols in GSH was quantified by an Ellman’s assay.36 rGO, WS2 and the rGO-WS2 composite (225 μL at 100 μg mL−1) in 50 mM bicarbonate buffer (pH 8.6) were added into 225 μL of GSH (0.8 mM in the bicarbonate buffer) to initiate oxidation. All samples were prepared in triplicate. The GSH or three nanosheet mixtures were transferred into a 24-well plate. The 24-well plate was covered with alumina foil to prevent illumination, and then placed in a shaker with a speed of 150 rpm at room temperature for an incubation time of 2 h. After incubation, 785 μL of 0.05 M Tris–HCl and 15 μL of DNTB (Ellman’s reagent, 5, 50-dithio-bis-(2-nitrobenzoic acid), Sigma-Aldrich) were added into the mixtures to yield a yellow product. All three were removed from the mixtures by filtration through a 0.22 μm syringe filter with a membrane (Hi-media, India). A 250 μL aliquot of filtered solutions from each sample was then placed in a 96-well plate. Their absorbance at 412 nm was measured on a Multiscan EX UV-VIS spectrometer (Thermo scientific, USA). A GSH solution without graphene-based materials was used as a negative control. GSH (0.4 mM) oxidized by H2O2 (1 mM) was used as a positive control. The loss of GSH was calculated by the following formula: loss of GSH % = (absorbance of negative control − absorbance of sample)/absorbance of negative control × 100. After 2 h incubation at room temperature, 98% of GSH in the positive control sample was lost, which is consistent with previous studies.19,343. Results and discussion
3.1. Antibacterial activity of rGO, WS2 and rGO-WS2 nanosheets
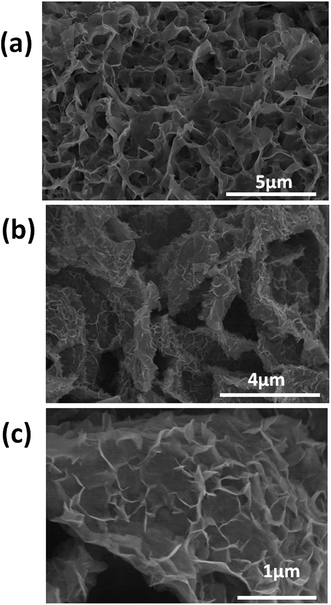
Fig. 1 shows a field emission scanning electron microscope (FE-SEM) image of the rGO nanosheets (Fig. 1a), few-layered WS2 sheets (Fig. 1b) and nanocomposite rGO-WS2 (Fig. 1c) nanosheets. The FE-SEM images show that the WS2 sheets are ∼1–5 nm thick and their length is in the range of ∼1–3 μm. The FE-SEM images of the rGO-WS2 composite show a large number of 3D architectures compared to the WS2 and rGO sheets. [For more detailed structural and morphological characterizations such as transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDAX), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, etc., please see the ESI†].The antibacterial effect of the rGO, WS2 and rGO-WS2 nanosheets against four bacterial strains including two Gram negative (E. coli DH5α (strain devoid of restriction-modification system, non pathogenic), S. typhimurium (pathogenic and biofilm forming)) and two Gram positive (B. subtilis, S. epidermidis (pathogenic and biofilm forming)) strains were used. Initially, the growth kinetics study experiment was carried out using various concentrations of the three nanosheets (0 (control), 10, 50, 100 and 250 μg mL−1). The death phases of all the bacteria (including pathogenic) at 250 μg mL−1 concentration were shown after 18 h of incubation with the WS2 and rGO-WS2 composite materials and little extension with rGO. These results show that the WS2 and rGO-WS2 nanosheets have better inhibitory effects on the growth kinetics towards all the tested bacterial strains than the rGO nanosheets (see ESI, Fig. S1†).
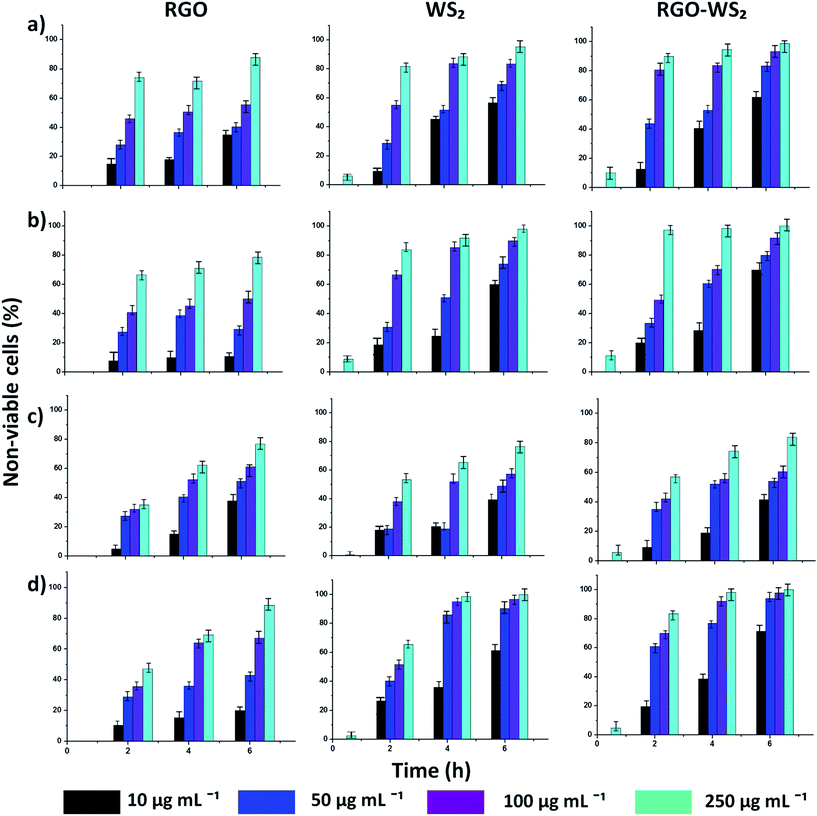
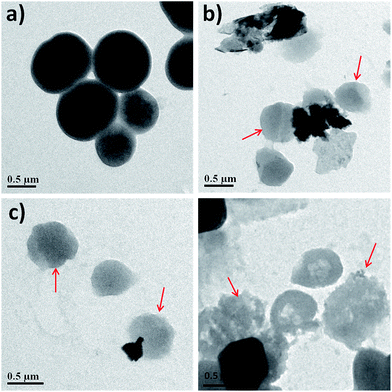
Fig. 2 shows the time and concentration dependent cell viability of all four bacterial strains with the three nanosheets using the same concentrations as mentioned above for 6 h. The loss of viability (death rate) of the bacterial cells was determined by the colony counting method, after each 2 h interval (see materials and method). Fig. 2 is summarized in tabular form in Table 1, and shows the comparison of viability loss percentage at the highest concentration (250 μg mL−1) for all the nanosheets against four bacteria. It was concluded that the bacterial cell loss viability steadily increases with the concentration of rGO, WS2 and rGO-WS2, as well as the incubation time. Among all the bacterial strains, S. epidermidis was more pathogenic which was used as a model organism for TEM analysis. The morphological changes of S. epidermidis after treatment with the three nanosheets were observed by TEM analysis (Fig. 3) and optical microscopic images of the disorted morphology of other strains of bacteria were shown in the ESI (see ESI Fig. S2†). However, the tested nanosheets exhibited antibacterial activity in a concentration and time dependent manner, and the composite rGO-WS2 nanosheets had a potentially better antibacterial activity than the rGO and WS2 nanosheets.
| Bacteria | Nanosheets | |||||
|---|---|---|---|---|---|---|
| Loss of viabilitya (%) | ||||||
| rGO | WS2 | rGO-WS2 | ||||
| 2 h | 6 h | 2 h | 6 h | 2 h | 6 h | |
| a Data extracted from Fig. 1. | ||||||
| E. coli | 64.23 | 87.7 | 81.88 | 96.67 | 90.22 | 98.67 |
| S. typhimurium | 35.12 | 62.5 | 53.43 | 76.54 | 57.05 | 83.89 |
| B. subtilis | 66.67 | 78.58 | 84.02 | 97.11 | 97.34 | 99.98 |
| S. epidermidis | 47.13 | 88.58 | 65.51 | 99.97 | 83.44 | 99.97 |
3.2. Antibacterial mechanism of rGO, WS2 and rGO-WS2
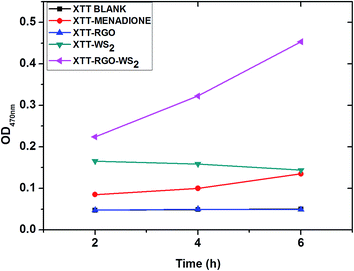
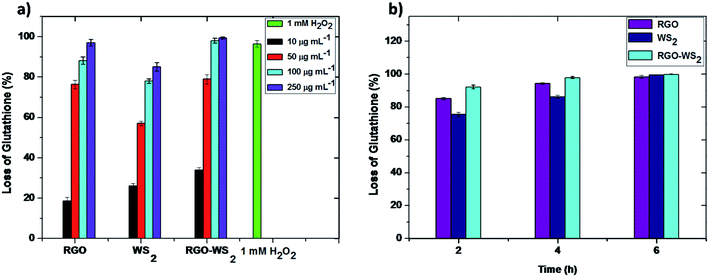
The morphology and oxidative stress play important roles in the antibacterial activity of graphene35 and other nanomaterials,36 due to the similarity in the structural and physicochemical properties of nanomaterials. Therefore, it is necessary to thoroughly evaluate the possibility of cellular oxidative stress produced by rGO, WS2 and the composite of the rGO-WS2 nanosheets. It is evidenced that oxidative stress mediated by graphene based materials may arise from two paths; one is reactive oxygen species (ROS) mediated oxidative stress, in which the oxidative stress mechanism is induced by ROS generated graphene nanomaterials.27 The second path is ROS-independent oxidative stress, in which nanomaterials may disrupt a specific microbial process by disturbing or oxidizing a vital cellular structure or component without ROS.36 To evaluate the oxidative stress paths for rGO, WS2 and rGO-WS2, we initially measured the possibility of superoxide anion (O2˙−) production using the XTT menadione mediated assay (see materials and methods) during an incubation period of 6 h; the results are shown in Fig. 4. The composite of the rGO-WS2 nanosheets shows high ROS production compared to rGO and WS2, which have no significant absorption during the 6 h period. XTT with menadione was used as a positive control to validate our XTT tests.37 Compared to the absorbance of XTT along with these three nanosheets, the rGO-WS2 composite enhanced the absorbance at 470 nm throughout the entire 6 h incubation period. These results suggested that the composite materials could produce ROS. In contrast, no significant absorbance was detected for the rGO and WS2 nanomaterials. These results were consistent with a previous study of rGO, which showed a low superoxide anion production due to single oxygen and hydroxyl radicals derived from superoxide anions.19,38 For the reduction of XTT, rGO and WS2 took longer than 24 h (see ESI Fig. S3†). We detected ROS produced by the rGO-WS2 composite nanosheets after 2 h of incubation and an orange colour formed after 4 h of dark incubation (see image S4 in ESI†). The rGO-WS2 composite nanosheets reduced XTT faster than menadione (a positive control). These results indicated that the mechanism of the nanomaterials could not only alter the morphological properties but also the mechanistic property of antibacterial activity.Further, we used in vitro GSH oxidation to examine the possibility of ROS-independent oxidative stress mediated by the rGO, WS2 and rGO-WS2 nanocomposites by Ellman’s assay20,38 and the results are summarized in Fig. 5a and b; and in Table 2. In general, graphene based nanomaterials like rGO, GO, Gt, and MoS2 are known for GSH oxidation.19,20 GSH is an antioxidant to bacteria and fungi of plants; to examine the possibility of ROS-independent oxidative stress is important because this path could play a significant role in the antimicrobial activity, as well as in the toxicity of nanoparticles.39
Mechanistically, GSH is a tri-peptide with HS-groups that can be oxidized to form disulphide (–S–S–), converting GSH to glutathione disulphide. In bacteria, GSH concentration is in the range of 0.1 to 10.0 mM, preventing damages to cellular components.40 GSH can prevent damage to cellular components caused by oxidative stress and it is an oxidative stress indicator in cells.41,42
To evaluate the oxidation of GSH, it was incubated with rGO, WS2 and composite of rGO-WS2, where GSH (0.8 mM) in a bicarbonate buffer (50.0 mM at pH 8.6) was used as a negative control and H2O2 (1.0 mM) separately without the three different nanosheets was used as a positive control in the GSH oxidation experiment. The negative control suggests that our incubation conditions could not cause GSH oxidation. The oxidation capacity of the three nanosheets toward GSH was examined by taking the absorbance at 412 nm.19,20 As shown in Fig. 5a, GSH oxidation by rGO, WS2 and rGO-WS2 was compared over several concentrations (10, 50, 100 and 250 μg mL−1) for 2 h; as the concentration increases, the glutathione oxidation also increases. The percentages of GSH oxidized after exposure to concentrations of 0, 50, 100 and 250 μg mL−1 rGO were 18.6 ± 2.5%, 76.4 ± 1.5%, 88.2 ± 0.5% and 97.2 ± 3.4% respectively, and when exposed to WS2, they were 26.4 ± 1.4%, 57.7 ± 2.6%, 78.2 ± 3.5% and 85.5 ± 1.5% respectively, and finally, when exposed to the composite of rGO-WS2 nanosheets they were 34.3 ± 2.3%, 79.2 ± 2.1%, 98.2 ± 1.5% and 99.3 ± 0.5% respectively.
Among the three types of graphene-based materials, the rGO-WS2 composite has the highest oxidation capacity towards GSH, followed by rGO and WS2. When 0.8 mM GSH was incubated with 100 μg mL−1 of all these three nanosheets separately, the oxidation of GSH gradually increased with extending reaction time. Fig. 4b shows the fraction of GSH oxidized by the three nanosheets after 6 h of incubation.
The different oxidation capacities toward GSH between rGO, WS2 and the rGO-WS2 composite can also be attributed to their different electronic properties. rGO, WS2 and rGO-WS2 represent electrical conductors, whereas graphene materials are a zero-gap semiconductor with excellent electrical conductivity.5,17 The conductivity of rGO is much higher than that of WS2. Materials with higher conductivity, such as rGO and graphite, do not display higher oxidation capacities to GSH compared to materials with lower conductivity, such as WS2. Our observation suggests that rGO and rGO-WS2 might share a similar mechanism to metallic SWCNTs.34 They could act as a conductive bridge over the insulating lipid bi-layer to release cellular energy.42 The oxidation capacities of the rGO, WS2 and rGO-WS2 nanosheets towards GSH have shown time and concentration dependence. Overall, these results indicate that all three nanosheets are capable of inducing superoxide anion independent oxidative stress and can oxidize cellular components such as proteins, and DNA and RNA materials.
4. Conclusions
The antibacterial activities of WS2 and rGO-WS2 composite nanosheets were evaluated by a colony counting method and growth curve studies against four bacterial strains: Gram positive B. subtilis and S. epidermidis, and Gram negative E. coli DH5α and S. typhimurium. The composite of the rGO-WS2 nanosheets caused significant bacterial growth retardation and an inhibitory effect on the tested bacterial strains compared to WS2. We demonstrated that the detailed mechanism of the production of oxygen stress induced reactive oxygen species (ROS) was only effective with rGO-WS2, but the GSH membrane mechanism stress was shown with all three nanosheets due to the direct contact with nanosheets, and superoxide anion-independent oxidation. The results suggested that the antimicrobial actions are contributed to by both membrane and oxidation stress. We believe that the antimicrobial activity of the rGO-WS2 composite nanosheets will be interesting in medical and pharmaceutical industrial applications.Acknowledgements
The work was supported by the Department of Science and Technology/Science and Engineering Research Board (Government of India) for Fellowship and grants to CSR, DJL, SSS and MSD, (Grant no. SR/S2/RJN-21/2012; SR/S2/RJN-130/2012 and SR/S2/RJN-111/2012, SB/YS/LS-347/2013, SB/FT/CS-116/2013), CSIR-NCL-MLP project grant 028626 BRNS 34/14/20/2015.Notes and references
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- (a) F. Schwierz, Nat. Nanotechnol., 2010, 5, 487 CrossRef CAS PubMed; (b) D. J. Late, Adv. Device Mater., 2015, 1, 52 CrossRef PubMed.
- A. Ghosh, D. J. Late, L. S. Panchakarla, A. Govindaraj and C. N. R. Rao, J. Exp. Nanosci., 2013, 4, 313 CrossRef PubMed.
- D. J. Late, U. Maitra, L. S. Panchakarla, U. V. Waghmare and C. N. R. Rao, J. Phys.: Condens. Matter, 2011, 23, 055303 CrossRef PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed.
- D. J. Late, B. Liu, H. S. S. R. Matte, V. P. Dravid and C. N. R. Rao, ACS Nano, 2012, 6, 5635 CrossRef CAS PubMed.
- M. Thripuranthaka, R. V. Kashid, C. S. Rout and D. J. Late, Appl. Phys. Lett., 2014, 104, 081911 CrossRef PubMed.
- H. S. S. R. Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059 CrossRef CAS PubMed.
- D. J. Late, B. Liu, H. S. S. R. Matte, C. N. R. Rao and V. P. Dravid, Adv. Funct. Mater., 2012, 22, 1894 CrossRef CAS PubMed.
- (a) D. J. Late, P. A. Shaikh, R. Khare, R. V. Kashid, M. Chaudhary, M. A. More and S. B. Ogale, ACS Appl. Mater. Interfaces, 2014, 6, 15881 CrossRef CAS PubMed; (b) D. Chakravarty and D. J. Late, Eur. J. Inorg. Chem., 2015, 1973 CrossRef CAS PubMed; (c) D. Chakravarty, D. J. Late, RSC Adv., 2015, 5, 21700 Search PubMed.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, ACS Nano, 2014, 8, 1102 CrossRef CAS PubMed.
- (a) D. J. Late, Y. Huang, B. Liu, J. Luo, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ACS Nano, 2013, 7, 4879 CrossRef CAS PubMed; (b) P. K. Kannan, D. J. Late, H. Morgan and C. S. Rout, Nanoscale, 2015, 7, 13293 RSC; (c) D. J. Late and C. S. Rout, J. Nanomed. Res., 2015, 2, 00015 Search PubMed.
- M. Thripuranthaka and D. J. Late, ACS Appl. Mater. Interfaces, 2013, 6, 1158 Search PubMed.
- D. J. Late, S. N. Shirodkar, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ChemPhysChem, 2014, 15, 1592 CrossRef CAS.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Sci. Rep., 2013, 3, 3282 Search PubMed.
- S. Liu, M. Hu, T. H. Zeng, R. Wu, R. Jiang, J. Wei, L. Wang, J. Kong and Y. Chen, Langmuir, 2012, 28, 12364 CrossRef CAS PubMed.
- S. Liu, T. H. Zeng, M. Hofmann, E. Burcombe, J. Wei, R. Jiang, J. Kong and Y. Chen, ACS Nano, 2011, 5, 6971 CrossRef CAS.
- X. Yang, J. Li, T. Liang, C. Ma, Y. Zhang, H. Chen, N. Hanagata, H. Su and M. Xu, Nanoscale, 2014, 6, 10126 RSC.
- L. Lin, Y. Xu, S. Zhang, I. M. Ross, A. C. M. Ong and D. A. Allwood, ACS Nano, 2013, 7, 8214 CrossRef CAS PubMed.
- S. Wang, Y. Zhang, Y. Ning and G. J. Zhang, Analyst, 2015, 140, 434 RSC.
- B. Cai, S. Wang, L. Huang, Y. Ning, Z. Zhang and G. J. Zhang, ACS Nano, 2014, 8, 2632 CrossRef CAS PubMed.
- Y. Ma, Y. Dai, M. Guo, C. Niu, J. Lu and B. Huang, Chem. Phys., 2011, 13, 15546 CAS.
- M. A. Lukowski, A. S. Daniel, C. R. English, F. Meng, A. Forticaux, R. J. Hamersa and S. Jin, Energy Environ. Sci., 2014, 7, 2608 CAS.
- M. Li, Q. Liu, Z. Jia, X. Xu, Y. Cheng, Y. Zheng, T. Xi and S. Wei, Carbon, 2014, 67, 185 CrossRef CAS PubMed.
- Y. B. Zhang, S. F. Ali, E. Dervishi, Y. Xu, Z. R. Li, D. Casciano and A. S. Biris, ACS Nano, 2010, 4, 3181 CrossRef CAS PubMed.
- Y. Yong, L. Zhou, Z. Gu, L. Yan, G. Tian, X. Zheng, X. Liu, X. Zhang, J. Shi, W. Cong, W. Yin and Y. Zhao, Nanoscale, 2014, 6, 10394 RSC.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886 CrossRef CAS PubMed.
- M. otto, Nat. Rev. Microbiol., 2009, 7, 555 CrossRef CAS PubMed.
- J. Borecka, M. Hocmannova and W. J. van Leeuwen, Zentralbl. Bakteriol. Orig. A, 1976, 236, 262 CAS.
- X. P. Wang, X. Q. Liu and H. Y. Han, Colloids Surf., B, 2013, 103, 136 CrossRef CAS PubMed.
- H. Ukeda, S. Maeda, T. Ishii and M. Sawamura, Anal. Biochem., 1997, 251, 206–209 CrossRef CAS PubMed.
- C. D. Vecitis, K. R. Zodrow, S. Kang and M. Elimelech, ACS Nano, 2010, 4, 5471 CrossRef CAS PubMed.
- (a) D. Y. Lyon and P. P. J. Alvarez, Environ. Sci. Technol., 2008, 42, 8127 CrossRef CAS; (b) D. Y. Lyon, L. Brunet, G. W. Hinkal, M. R. Wiesner and P. J. J. Alvarez, Nano Lett., 2008, 8, 1539 CrossRef CAS PubMed.
- G. R. Navale, M. Thripuranthaka, D. J. Late and S. S. Shinde, JSM Nanotechnol. Nanomed., 2015, 3, 1033 Search PubMed.
- J. Meletiadis, J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij and E. Network, J. Clin. Microbiol., 2001, 3402 CrossRef CAS.
- G. L. Ellman, Tissue sulfhydryl groups, Arch. Biochem. Biophys., 1959, 82, 70 CrossRef CAS.
- A. Pompella, A. Visvikis, A. Paolicchi, V. de Tata and A. F. Casini, Biochem. Pharmacol., 2003, 66, 1499 CrossRef CAS.
- R. C. Fahey, W. C. Brown, W. B. Adams and M. B. Worsham, J. Bacteriol., 1978, 133, 1126 CAS.
- A. Joshi, S. Punyani, S. S. Bale, H. C. Yang, T. Borca-Tasciuc and R. S. Kane, Nat. Nanotechnol., 2008, 3, 41 CrossRef CAS PubMed.
- O. Carmel-Harel and G. Storz, Annu. Rev. Microbiol., 2000, 54, 439–461 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Growth kinetics of four bacteria with different concentrations of rGO, WS2 and rGO-WS2; optical microscopic images of the bacterial cells exposed to rGO, WS2 and rGO-WS2 nanosheets at 100 μg mL−1 concentrations for 2 h; superoxide radical anion production by XTT experiments for 24 h by rGO, WS2 and rGO-WS2 nanosheets; ROS production by the rGO-WS2 composite detected by monitoring color change, and the detailed morphological and structural characterization data such as TEM, HRTEM, X-RD, Raman spectroscopy, XPS, SEM and EDAX etc. See DOI: 10.1039/c5ra15652a |
| This journal is © The Royal Society of Chemistry 2015 |