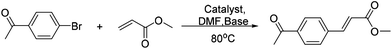
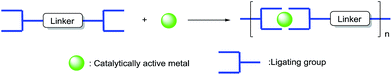A novel bisoxazoline/Pd composite microsphere: a highly active catalyst for Heck reactions†
Junke Wang a,
Yingxiao Zongab,
Guoren Yueb,
Yulai Hu*b and
Xicun Wang*b
a,
Yingxiao Zongab,
Guoren Yueb,
Yulai Hu*b and
Xicun Wang*b
aKey Laboratory of Hexi Corridor Resources Utilization of Gansu Universities, College of Chemistry and Chemical Engineering, Hexi University, Zhangye 734000, China
bGansu Key Laboratory of Polymer Materials, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, China. E-mail: wangjk@hxu.edu.cn; Fax: +86-936-8287080
First published on 2nd September 2015
Abstract
A novel bisoxazoline/Pd microsphere catalyst was successfully prepared. The structure of the solid catalyst was characterized by SEM, TGA and FT-IR. The catalyst exhibits excellent activity for the Heck reaction. Moreover, the catalyst shows outstanding stability and reusability, can be recovered simply and effectively and reused six times without any activity decrease.
The transition-metal-catalyzed C–C cross-coupling reaction is a key step in the synthesis of organic building blocks, natural products, pharmaceuticals, and agricultural derivatives.1 Certainly, Pd-catalyzed olefination of aryl halides (the Heck–Mizoroki reaction) is one of the most powerful tools for the construction of C–C bonds.2 In fact, the Pd-based catalytic systems are highly efficient, and they generally offer excellent product yields with good selectivity.3
In general, homogeneous palladium catalysts are mostly used in the Heck reaction owing to their high efficiency. However, the soluble palladium complexes suffer from drawbacks associated with the catalyst separation and recovery after the reaction. A promising route to solve the problems is the heterogenization of the homogeneous catalysts,4 and many support materials have been explored to immobilize palladium such as polymers, silica particles and metal oxides.5–8
In spite of their good catalytic activity in Heck coupling reactions, the scope and functional group tolerance of these catalytic systems are generally limited. More importantly, they show less effectiveness in the Heck olefination of less-active bromo- and chloroarenes. In addition, most of the catalytic systems suffered from the use of higher metal loadings (typically 5–10 mol%) and poor reusability. Therefore, the development of an efficient heterogeneous catalyst for Heck-type olefination is a challenging task.
Metal–organic polymerization through the formation of “self-assembled” systems is a promising strategy to realize heterogenization of the homogeneous catalysts or precursors.9 Compared with the other methods, the self-assembled strategy can produce heterogeneous catalysts without using any supports with high density of catalytically active units (Scheme 1).
Most recently, metal–organic polymer (MOP) were found to be an effective tool for the construction of C–C bonds.10 Bouchard et al.10a made the BDC-NH2 to be postmodified with salicylic aldehyde to obtain the binding catalytically active Pd(II) ions. The catalytic activity of the embedded Pd(II) ions was tested via heterogeneous Heck coupling. They found that a trade-off exists between the amount of metalation and pore blocking during catalytic testing. Bagherzadeh et al.10b prepared supported palladium nanoparticles on Mn-carboxylate coordination polymer (Pd/MnBDC) using solution impregnation method. This catalyst can provide high activity and selectivity to E-internal olefins for the Mizoroki–Heck cross-coupling reaction. However, bromo- and chloroarenes are less reactive. Moreover, these metal–organic polymers (MOPs) contain two kinds of metal and complicated ligands, which results in the trouble of preparation and the high price, so that they cannot be applied into the volume production. Hence, there is a continuous exploration for a better heterogeneous Pd-based metal–organic polymer (MOP) catalyst for the Heck coupling reaction.
According to Fujita et al.,11 the catalytically active center of the metal–organic polymers is the metal ion for the construction of metal–organic polymers, rather than the extra metal ion followed by the post-modification. Thus, the leaching of the catalytically active metal can be avoided, which may assure the reuses of the catalyst many times. We presumed that it could also be conceptually possible to generate self-assembled catalysts or catalyst precursors by using the self-assembled strategy. However, the catalysts could be recovered heterogeneously. In this study, we report the synthesis of a novel self-assembled bisoxazoline/Pd composite microsphere, and investigate the catalytical property for Mizoroki–Heck cross coupling reaction, reusability, and stability of bisoxazoline/Pd composite microsphere.
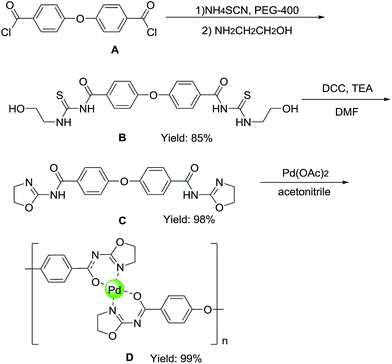
As illustrated in Scheme 2, the synthesis of the ligand (C) is a straightforward process starting from 4,4′-oxybisbenzoyl chloride and 2-aminoethanol, which are inexpensive.12 4,4′-Oxybisbenzoyl chloride (A) was treated with ammonium sulfocyanide in the presence of polyethylene glycol 400 (PEG-400) in CH2Cl2, followed by the addition of 2-aminoethanol, to obtain 4,4′-oxybis(N-((2-hydroxyethyl) carbamothioyl)benzamide) (B) in good yields (85%). This intermediate can be used to implement the intramolecular cyclization in the present of DCC to obtain the corresponding 4,4′-oxybis(N-(4,5-dihydrooxazol-2-yl)benzamide) (C) as the ligand in excellent yields (98%). By means of the self-assembled coordination, Pd(OAc)2 was added into the solution of the ligand in acetonitrile, and then the pale yellow powder was obtained as the catalyst (D). After washing with solvent thoroughly and drying, the solid remains pale yellow. However, the complex between ligand and palladium acetate was synthesized without using any deprotonating base.
The catalyst has a high melting point of above 200 °C, and it was observed to be insoluble in the polar solvents, which enable the catalyst to be a solid catalyst for the organic reactions.
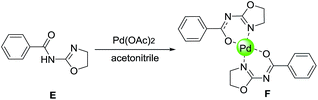
The structure of the synthesized palladium complex was characterized using different techniques such as 1H NMR, TGA, IR, and SEM. 1H NMR spectra difference between the novel oxazoline ligands and their metal complexes, is one the important methods for characterization of complexes in the literature.13,14 However, it is difficult to distinguish the 1H NMR spectra difference because of their relative insolubility in DMSO-D6. Therefore, we focused on the synthesis of N-(4,5-dihydrooxazol-2-yl)benzamide (E), and prepared its Pd complex (F) to confirm successful chelation of ligand to Pd (Scheme 3).
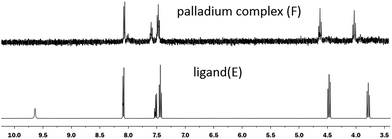
It is worth to note that, the 1H NMR spectra of the ligand (E) in DMSO-D6 and its palladium complex (F) in DMSO-D6 is similar, but the protons of oxazoline in the complex are shifted downfield relative to the protons of the ligand and the protons of N–H in benzamide disappeared (Fig. 1). These facts confirmed successful chelation to Pd. This result is accordant to our previous work.12
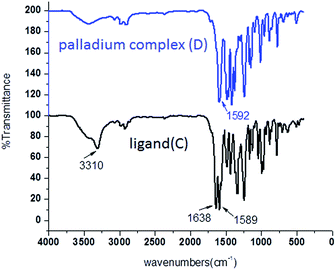
FT-IR was employed to give a detailed investigation of the ligand (C) and palladium complex (D). As can be seen from Fig. 2, the FT-IR spectra of the ligand shows that the strong peak at 1638 cm−1 could be due to the asymmetric and symmetric stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and that the broad peak at 3310 cm−1 could be due to N–H vibrations. However, there are not absorption peaks of the C
O, and that the broad peak at 3310 cm−1 could be due to N–H vibrations. However, there are not absorption peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H in palladium complex. Thus, the above results indicate that the ligand was successfully coordinated with palladium via coordination bond.
O and N–H in palladium complex. Thus, the above results indicate that the ligand was successfully coordinated with palladium via coordination bond.
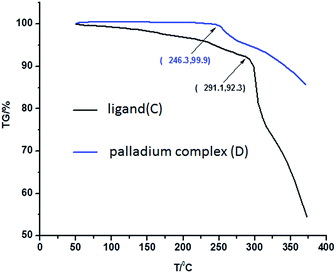
The stability of the ligand and palladium complex was compared by the thermogravimetric (TG) analysis (Fig. 3). The TG curve indicates that the decomposition temperature of the ligand was about 246.3 °C, which is lower than that of palladium complex (291.1 °C). This fact indicates the palladium complex is not a physical mixture of the ligand with palladium, but a metal complex.
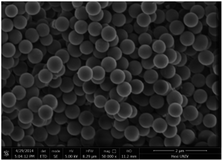
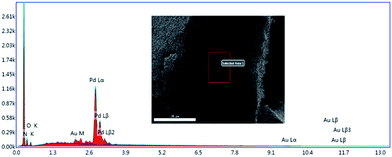
Fig. 4 depicts the scanning electron microscopy (SEM) images of the bisoxazoline/Pd composite microsphere catalyst, the catalyst presents with a monodispersed, uniform spherical morphology with the glabrate outer surface. The particle diameter is ranging from 0.53 to 0.55 μm. EDAX analysis offers both quantitative and qualitative information. The qualitative results are those “maps” or images which indicate the distribution of chemical elements in a sample (Fig. 5).
Following the successful preparation of bisoxazoline/Pd microsphere, it was then applied to the Heck reaction of various aryl halides and olefins. In a model reaction of p-bromoacetophenone and methyl acrylate in DMF solvent, different reaction parameters such as base, catalyst dosage and reaction time were optimized. As shown in Table 1, the target products were obtained when TEA and pyridine were used as base (Table 1, entries 5 and 6). However, when inorganic bases like K2CO3 or Na2CO3 were used (Table 1, entries 1 and 2), the isolated yields were excellent. Under the same conditions, when CH3COOK and CH3COONa was used as bases, the yield of the cross-coupled product was considerably reduced (Table 1, entries 3 and 4). Thus, K2CO3 has a pronounced effect on the product yield. In order to show the efficiency of the catalyst, a controlled experiment indicated that no cross-coupling product was observed in the absence of the catalyst (Table 1, entries 7).
| Entry | Catalyst (Pd mol%) | Base | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: bisoxazoline/Pd microsphere, 1 mmol of p-bromoacetophenone, 1 mmol of methyl acrylate, 2 mmol of base, 5 ml of solvent, 80 °C in air.b Isolated yield. | ||||
| 1 | 0.05 | Na2CO3 | 2 | 56 |
| 2 | 0.05 | K2CO3 | 2 | 72 |
| 3 | 0.05 | KOAc | 2 | 40 |
| 4 | 0.05 | NaOAc | 2 | 30 |
| 5 | 0.05 | TEA | 2 | 21 |
| 6 | 0.05 | Pyridine | 2 | 14 |
| 7 | 0 | K2CO3 | 2 | 0 |
| 8 | 0.075 | K2CO3 | 2 | 80 |
| 9 | 0.10 | K2CO3 | 2 | 90 |
| 10 | 0.15 | K2CO3 | 2 | 90 |
| 11 | 0.10 | K2CO3 | 3 | 94 |
| 12 | 0.10 | K2CO3 | 4 | 98 |
| 13 | 0.10 | K2CO3 | 5 | 98 |
Next, the effect of catalyst dosage was investigated (Table 1, entries 8–10): when the amount of catalyst was raised from 0.05 mol% to 0.10 mol%, the isolated yields increased from 72% to 90%; using more catalyst did not improve the yield further. Moreover, the reaction time were optimized (Table 1, entries 11 and 12). Isolated yields rose from 90% to 98% as reaction time rose from 2 h to 4 h. By prolonging reaction time to 5 h, the yield did not increase any more (Table 1, entries 13), which means that 4 h was sufficient for the present reaction.
With the optimum reaction conditions, a range of substituted aryl halides and olefins were investigated to check the feasibility of this protocol whose results are tabulated in Table 2. In general, the reaction afforded the desired products in good to excellent isolated yields (82–98%) for aryl bromide and olefins (Table 2, entries 1–14). Generally, the reaction of aryl bromide bearing electron-withdrawing group (–CN, –COOCH3, –COCH3) with olefins can afforded the coupling products in higher yields than aryl bromide bearing electron donating group (–OCH3).
| Entry | R | X | R′ | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: bisoxazoline/Pd microsphere containing 0.10 mol% Pd, 1.0 mmol of p-bromoacetophenone, 1.2 mmol of olefins, 2 mmol of base, 5 ml of solvent, 80 °C in air.b Isolated yield. | |||||
| 1 | –COCH3 | Br | C6H5 | 4 | 96 |
| 2 | –COCH3 | Br | –COOCH3 | 4 | 98 |
| 3 | –COCH3 | Br | –COOtBu | 4 | 98 |
| 4 | –CHO | Br | –COOCH3 | 4 | 95 |
| 5 | –CHO | Br | –COOtBu | 4 | 95 |
| 6 | –CHO | Br | –COOiBu | 4 | 97 |
| 7 | H | Br | –COOCH3 | 4 | 93 |
| 8 | H | Br | –COOtBu | 4 | 94 |
| 9 | –CN | Br | C6H5 | 4 | 99 |
| 10 | –CN | Br | –COOCH3 | 4 | 97 |
| 11 | –CN | Br | –COOtBu | 4 | 93 |
| 12 | –CN | Br | –COOiBu | 4 | 96 |
| 13 | H | Br | C6H5 | 4 | 92 |
| 14 | –OCH3 | Br | C6H5 | 4 | 82 |
| 15 | –OCH3 | I | C6H5 | 2 | 98 |
| 16 | H | I | C6H5 | 2 | 96 |
| 17 | –OCH3 | Cl | C6H5 | 4 | Trace |
| 18 | –CHO | Cl | –COOCH3 | 4 | Trace |
When this catalyst was applied to catalyze the Heck reaction of aryl iodides and aryl chloride with t-butyl acrylate at 80 °C (Table 2, entries 15–18). 4-Iodoanisole and lodobenzene reacted with styrene under the same conditions to give satisfying conversion in 98 and 96% within 2 h.
Generally, a hot filtration test can determine if the catalysis was derived from the bisoxazoline/Pd composite microsphere or from leaching palladium.15 Thus, we chose 4-bromoanisole with methyl acrylate as model reaction to investigate the fact. After the reaction was carried out for 2 h (the conversion of 4-bromoanisole reached 73%, and the yield of the crude cross coupling product is almost 70%), the catalyst was separated by a hot rapid filtration. No further reaction was observed by GC analysis, and no palladium could be detected in the hot liquid mixture by atomic absorption spectrometry (AAS) (the detection limit of the AAS experiment is 11 μg L−1). These results demonstrate that the palladium atom remains into the ligand during the reaction.
The reusability of the catalysts is an important parameter for practical application. Therefore, the reusability of bisoxazoline/Pd composite microsphere for the Heck reaction of p-bromoacetophenone and t-butyl acrylate was also investigated. In detail, after the reaction was completed, the catalyst particles were collected simply and effectively by centrifugation and then the recovered catalyst particles were reused in the next round of reaction by mixing them with new substrate, base, and solvent. It is noteworthy that the catalytic activity was maintained at least until the six uses, with almost the excellent yield of product in each run (Table 3). Furthermore, according to the IR shown in Fig. 6 the used bisoxazoline/Pd microsphere catalyst can retain its structure, indicating a good stability and durable structure of bisoxazoline/Pd microsphere in liquid reaction. Undoubtedly, this result is very promising and encouraging from a practical point of view.
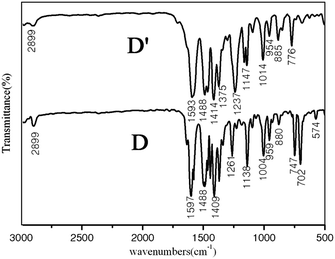 | ||
| Fig. 6 Comparison between the IR of bisoxazoline/Pd composite microsphere (D′) after recycled eight times and the IR of the fresh bisoxazoline/Pd composite microsphere (D). | ||
Conclusions
By the self-assembly of the bisoxazoline and Pd(AcO)2, a novel and non-phosphine solid palladium catalyst, bisoxazoline/Pd composite microsphere, was successfully prepared. The synthesized bisoxazoline/Pd composite microsphere shown high catalytic activity for Heck cross-coupling reactions of various aryl bromides and olefins even at catalyst loadings of 0.1 mol%. Moreover, the catalyst shows outstanding stability and reusability, can be recovered simply and effectively and reused six times without any activity decrease. Further investigation of the detailed mechanism and application of this chemistry are in progress in our lab.Experimental section
General information
Melting points were determined on a Perkin-Elmer differential scanning calorimeter and were uncorrected. The IR spectra were run on a Nicolet spectrometer (KBr). NMR spectra were recorded at 400 (1H) and 100 (13C) MHz, respectively, on a Varian Mercury plus-400 instrument using CDCl3 as solvent and TMS as the internal standard. Scanning electron microscopy (SEM) was performed on a FEI Quanta 450 FEG FESEM instrument. High resolution mass spectra (HRMS) were obtained on an Agilent LC-MSD-Trap-XCT spectrometer with micromass MS software using electrospray ionisation (ESI). All the solvents used were strictly dried according to standard operation and stored on 4 Å molecular sieves.All other chemicals (AR grade) were commercially available and used without further purification.
Synthesis of bisacylthiourea B
To a solution of 4,4′-oxybisbenzoyl chloride A (2 mmol) in CH2Cl2 (10 mL) was added ammonium thiocyanate (2.6 mmol) and PEG-400 (0.2 mmol). The mixture was then stirred at room temperature for 60 min and cooled to 0 °C, and the solution of 2-aminoethanol (1.8 mmol) in CH2Cl2 (2 mL) was added. The mixture was continuously stirred for 60 min. After the completion of the reaction, the solvent was removed by distillation, and water (10 mL) was added to obtain a white solid. The analytical sample was produced by flash chromatography (acetone and petroleum ether) to give a white solid B. Yield: 85%. Melting point: 209–211 °C. Spectral data: IR (KBr) (cm−1): ν 3337, 3225, 2944, 1670, 1531. 1H NMR (400 MHz, DMSO) δ 11.35 (s, 2H), 11.05 (s, 2H), 8.02 (d, J = 8.8 Hz, 4H), 7.17 (d, J = 8.8 Hz, 4H), 4.98 (s, 2H), 3.83–3.44 (m, 8H). 13C NMR (100 MHz, DMSO) δ 180.71, 167.65, 159.87, 131.68, 128.22, 118.95, 58.75, 47.97, 40.38, 40.17, 39.96, 39.75, 39.54. HR-MS: m/z calcd for C20H21N2O5S2 [M + H]+: 433.0892; found: 433.0889.Synthesis of bisoxazoline C
To a solution of compound B (1 mmol) in DMF (5 mL) was added dicyclohexylcarbodiimide (DCC) (1 mmol) and TEA (1 mmol). The mixture was stirred for 2 h at 80 °C, and cooled to room temperature. After the addition of water (5 mL), the white solid was obtained by the filtration. This solid was added into CH3CN (5 mL) to be dissolved, followed by the filtration and concentration to afford the target compound C. Yield: 98%. Melting point: 195–196 °C. Spectral data: IR (KBr) (cm−1): ν 3310, 2921, 1638, 1548. 1H NMR (400 MHz, DMSO) δ 9.61 (s, 2H), 8.28–7.99 (m, 4H), 7.20–6.98 (m, 4H), 4.47 (t, J = 8.6 Hz, 4H), 3.78 (t, J = 8.6 Hz, 4H). 13C NMR (100 MHz, DMSO) δ 180.71, 167.65, 159.87, 131.68, 128.22, 118.95, 58.75, 47.97, 40.59, 40.38, 40.17, 39.96, 39.75, 39.54, 39.33. HR-MS: m/z calcd for C20H19N4O5 [M + H]+: 395.1355; found: 395.1395.Synthesis of catalyst D
To the solution of Pd(AcO)2 (2 mmol) in CH3CN (5 mL) was added dropwise into the obtained compound C (1.36 g, 6 mmol) in CH3CN (2 mL), followed by the stirring for 10 h. On completion, the filtration was conducted to a yellow solid. Washing with commercial anhydrous CH3CN (3 × 5 mL) and drying at 50 °C overnight gave bisoxazoline/Pd microsphere as a pale yellow powder (compound D). IR (KBr) (cm−1): ν 3443, 2907, 1592. The Pd content of the bisoxazoline/Pd microsphere catalyst is 20.01 wt% (1.8 mmol g−1) measured by atomic absorption spectroscopy (AAS).General experimental procedures for Heck couplings
In a typical experiment, the bisoxazoline/Pd microsphere catalyst (0.10 mmol of Pd) was added to a mixture of aryl halide (1.0 mmol), olefins (1.2 mmol), and K2CO3 (2.0 mmol) in DMF (5.0 mL), and the reaction mixture was stirred at 80 °C. After the reaction was monitored to be complete by TLC analysis, the catalyst was removed by filtration, washed with ethanol (3 × 3 mL), and dried under vacuum for the next run. The organic fractions were then concentrated on a rotary evaporator to afford the desired compound in excellent yield. The crude products were purified by column chromatography on silica gel using hexane/ethyl acetate. All of the products are known compounds, and their 1H NMR data were identical to those reported in literature.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21462016, 21262010), Natural Science Foundation of Gansu Province and the Advanced Research Fund of Jinchuan Group Co., Ltd.Notes and references
- (a) F. Diederich and P. J. Stang, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, Weinheim, Germany, 1998 Search PubMed; (b) J. A. Baur and D. A. Sinclair, Nat. Rev. Drug Discovery, 2006, 5, 493 CrossRef CAS PubMed.
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (b) N. J. Whitcombe, K. K. Hii and S. E. Gibson, Tetrahedron, 2001, 57, 7449 CrossRef CAS; (c) H. U. Blaser, A. Indolese, F. Naud, U. Nettekoven and A. Schnyder, Adv. Synth. Catal., 2004, 346, 1812 CrossRef PubMed; (d) K. H. Shaughnessy, P. Kim and J. F. Hartwig, J. Am. Chem. Soc., 1999, 121, 2123 CrossRef CAS; (e) A. F. Littke and G. C. Fu, J. Am. Chem. Soc., 2001, 123, 6989 CrossRef CAS PubMed.
- (a) K. Okamoto, R. Akiyama, H. Yoshida, T. Yoshida and S. J. Kobayashi, J. Am. Chem. Soc., 2005, 127, 2125 CrossRef CAS PubMed; (b) A. Dahan and M. Portnoy, Org. Lett., 2003, 5, 1197 CrossRef CAS PubMed; (c) Y. C. Yang and T. Y. Luh, J. Org. Chem., 2003, 68, 9870 CrossRef CAS PubMed; (d) J. C. Garcia-Martinez, R. Lezutekong and R. M. Crooks, J. Am. Chem. Soc., 2005, 127, 5097 CrossRef CAS PubMed; (e) V. Calo, A. Nacci, A. Monopoli, A. Fornaro, L. Sabbatini, N. Cioffi and N. Ditaranto, Organometallics, 2004, 23, 5154 CrossRef CAS; (f) J. Huang, T. Jiang, H. Gao, B. Han, Z. Liu, W. Wu, Y. Chang and G. Zhao, Angew. Chem., Int. Ed., 2004, 43, 1397 CrossRef CAS PubMed; (g) A. Desforges, R. Backov, H. Deleuze and O. Mondain-Monval, Adv. Funct. Mater., 2005, 15, 1689 CrossRef CAS PubMed; (h) R. K. Arvela, N. E. Leadbeater, M. S. Sangi, V. A. Williams, P. Granados and R. D. Singer, J. Org. Chem., 2005, 70, 161 CrossRef CAS PubMed; (i) W. Kleist, Catal. Lett., 2008, 125, 197 CrossRef CAS.
- L. X. Yin and J. Liebscher, Chem. Rev., 2006, 107, 133 CrossRef PubMed.
- M. Králik and A. Biffis, J. Mol. Catal. A: Chem., 2001, 177, 113 CrossRef.
- R. Bernini, S. Cacchi, G. Fabrizi, G. Forte, F. Petrucci, A. Prastaro, S. Niembro, A. Shafir and A. Vallribera, Green Chem., 2010, 12, 150 RSC.
- S. Zhou, M. Johnson and J. G. Veinot, Chem. Commun., 2010, 46, 2411 RSC.
- V. Polshettiwar, C. Len and A. Fihri, Coord. Chem. Rev., 2009, 253, 2599 CrossRef CAS PubMed.
- (a) M. A. Yoichi, S. M. S. Yamada and U. Yasuhiro, J. Am. Chem. Soc., 2012, 134, 9285 CrossRef PubMed; (b) M. A. Yoichi, S. M. S. Yamada and U. Yasuhiro, J. Am. Chem. Soc., 2012, 134, 3190 CrossRef PubMed; (c) J. Gascon, A. Corma, F. Kapteijn and F. X. L. Xamena, ACS Catal., 2014, 4, 361 CrossRef CAS; (d) Y. Liang, Q. Jing, X. Li, L. Shi and K. Ding, J. Am. Chem. Soc., 2005, 127, 7694 CrossRef CAS PubMed.
- (a) J. W. Brown, N. N. Jarenwattananon, T. Otto, J. L. Wang, S. Glöggler and L. Bouchard, Chem. Commun., 2015, 65, 105 CAS; (b) M. Bagherzadeh, F. Ashouri, L. Hashemi and A. Morsali, Inorg. Chem. Commun., 2014, 44, 10 CrossRef CAS PubMed.
- O. Ohmori and M. Fujita, Chem. Commun., 2004, 1586 RSC.
- J. Wang, Y. Zong, S. Weib and Y. Pan, Appl. Organomet. Chem., 2014, 28, 351 CrossRef CAS PubMed.
- A. Sarkar, S. Bhattacharyya, S. K. Dey, S. Karmakar and A. Mukherjee, New J. Chem., 2014, 38, 817 RSC.
- Y. M. Lee, E. S. Kim, H. J. Kim, H. J. Choi, Y. I. Kim, S. K. Kang and S. N. Choi, Dalton Trans., 2009, 126 RSC.
- S. Mohanty, D. Suresh, M. S. Balakrishna and J. T. Mague, Tetrahedron, 2008, 64, 240 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16510e |
| This journal is © The Royal Society of Chemistry 2015 |