Thermo- and glucose-responsive micelles self-assembled from phenylborate ester-containing brush block copolymer for controlled release of insulin at physiological pH†
Weizhong Yuan*,
Lulin Li and
Hui Zou
School of Materials Science and Engineering, Key Laboratory of Advanced Civil Materials of Ministry of Education, Tongji University, 201804, People's Republic of China. E-mail: yuanwz@tongji.edu.cn
First published on 18th September 2015
Abstract
An amphiphilic brush block copolymer P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) was prepared by ATRP, RAFT and click chemistry. The copolymer micelles show temperature and glucose responses. The controlled release of insulin could be achieved by adjusting temperature and glucose concentration.
Considerable attention has been paid to glucose-responsive polymers due to their potential applications in drug releasing carriers for glucose-related human disorders such as diabetes.1 The conventional treatments including regular monitoring of blood glucose concentrations and multiple insulin injection were used to control the glucose concentration to the normal level. However, it is difficult to real-time adjust the insulin dosage with these methods. As a result, it is essential to prepare novel delivery system for intelligent release of insulin. Currently, glucose-responsive macromolecules have gradually attracted dramatic attention because they could achieve the self-regulated release of insulin. Among various glucose-responsive polymeric systems, phenylboronic acid (PBA)-based polymers have been extensively investigated owing to their superiority of further designs and advantages of better stability, compared to protein-based glucose oxidase and concanavalin A.2 However, the apparent pKa value of PBA is around 8.2–8.6, which is higher than human physiological pH 7.4. PBA-based materials could be obtained only slightly above the pKa of PBA about pH 9.3 Therefore, reducing the pKa of PBA-based polymer and improving the sensitivity to glucose for response to the fluctuation of blood glucose level are the ultimate goal of this area.4
Recently, the polymers containing pinacolboronate ester structure attracted much interest because the rigid cis-diol in glucose exhibit higher affinities with organoboronic acids than acyclic diols, which provides glucose molecules with strong competition to take away the PBA moiety from the pinacolboronate ester.5 Moreover, the lower pKa of boronate ester than that of boronic acid6 is helpful for construct smart glucose-responsive polymers at physiological pH. Thus, the advantages of higher affinities with organoboronic acids and lower pKa of boronate ester would cause the polymers containing boronate ester moieties as the ideal intelligent glucose-responsive system under physiological conditions.7
Increasing interest has been attracted to dual and multi-responsive polymeric systems as dual/multi-responsive properties will endow the polymers with more extensive functions than single response.8 Clinical practice shows that fever of diabetes patients will cause the increase of blood glucose concentration to a considerable extent and lead to exacerbations. Therefore, it is important to introduce thermo-responsive segments into glucose-responsive polymer and prepare temperature and glucose dual responsive copolymer. Therefore, the thermo-responsive segments would detect the fever and trigger the rapid release of insulin. The high blood glucose concentration and temperature will affect the structure and morphologies of the dual-responsive copolymer and the micelles self-assembled from the copolymer. Under this condition, glucose molecules entered into the core of micelles more easily and reacted with boronate ester to trigger the release of insulin. Among the temperature-responsive polymers, the random copolymers of 2-(2-methoxyethoxy)ethyl methacrylate (MEO2MA) and oligo(ethylene glycol)methacrylate (OEGMA) (P(MEO2MA-co-OEGMA)) have attracted increasing interest, because they exhibit tunable lower critical solution temperature (LCST) in water9 and present good biocompatibility, nontoxicity and nonimmunogenicity.10
In order to explore the effect of the copolymer morphology and the content of glucose-responsive segment in copolymer on the self-assembly behavior and glucose-responsive property, the brush block copolymer P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) was synthesized by the combination of atom transfer radical polymerization (ATRP), reversible addition-fragmentation chain transfer polymerization (RAFT) and click chemistry (Scheme S1, ESI†). PCMS was synthesized by RAFT polymerization with DDMAT as the RAFT agent. Then, the block copolymer of PCMS-b-P(MEO2MA-co-OEGMA) was prepared by RAFT reaction. After reaction with excess of NaN3, PAMS-b-P(MEO2MA-co-OEGMA) was obtained. Moreover, alkynyl PPBDEMA was prepared by ATRP of PBDEMA with propargyl 2-bromoisobutyrate as the initiator. Finally, the brush block copolymer of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) was synthesized by click chemistry of alkynyl PPBDEMA with PAMS-b-P(MEO2MA-co-OEGMA). Fig. S1–S3 (ESI†) show the 1H NMR spectra of PBDEMA monomer, propargyl 2-bromoisobutyrate and alkynyl PPBDEMA. Fig. S4 and S5 (ESI†) reveal the 1H NMR and ATR FT-IR spectra of PCMS and PAMS, respectively. The chemical shift of protons linked with Cl atom and N3 group changed in NMR spectra, companied with new absorption peak at 2091 cm−1 (character peak of N3) in ATR FT-IR spectrum, indicating the success transformation of Cl to N3. Fig. S6 and S7 (ESI†) show the 1H NMR spectra of PAMS-b-P(MEO2MA-co-OEGMA) and P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA). The click reaction efficiency can be calculated from the 1H NMR spectrum of the brush block copolymer. GPC traces (Fig. S8, ESI†) indicated that the molecular weight of PAMS, PAMS-b-P(MEO2MA-co-OEGMA) and P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) were 2260 g mol−1, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 and 55
500 g mol−1 and 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 respectively.
800 g mol−1 respectively.
The amphiphilic brush block copolymer P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) can self-assemble to micelles with P(MEO2MA-co-OEGMA) corona and P(St-g-PBDEMA) core at 25 °C in water. The CMC value was 0.017 mg mL−1 (Fig. S9, ESI†), indicating that the copolymer can self-assemble to micelles at comparative low concentrations. Fig. 1 shows the schematic illustration of the temperature- and glucose-responsive properties of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles. Due to the glucose-responsiveness of PPBDEMA segments, the core of micelles will swell after adding glucose molecules into micelle solution. Due to the temperature responsiveness of P(MEO2MA-co-OEGMA) segments, the shell of micelle will transform from stretch state to shrinkage state when the temperature is above the LCST of the copolymer.
 | ||
| Fig. 1 Schematic illustration of the temperature- and glucose-responsive properties of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles and the controlled release of insulin. | ||
The effect of pH and glucose on the morphologies of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles was investigated by dynamic light scattering (DLS) (Fig. 2). At pH 4.0, the micelle core formed from P(St-g-PBDEMA) was very hydrophobic and the micelles tend to aggregate into compounding micelles with comparatively large size. The hydrodynamic radius (Rh) of micelles was 306.4 nm (PDI = 0.359). After adding glucose (16 mg mL−1) to the micelle solution, the Rh of micelles was 308.4 nm (PDI = 0.173), indicating that glucose molecules can't react with pinacolboronate ester at acidic condition. The pinacolboronate ester was very stable at pH 4.0. When the pH value increased to 7.4, the Rh value of micelles decreased to 99.4 nm (PDI = 0.274), because the ionization of some pinacolboronate ester groups and the hydrophilicity of P(St-g-PBDEMA) increased, and the aggregation of micelles relieved to some degree. Therefore, the size of micelles decreased. After adding glucose to the solution, the Rh value increased to 287.8 nm (PDI = 0.093), which should be attributed to the structure transformation of micelles. In this case, the micelles do not adopt spherical core–shell structure after glucose reaction. When pH value was raised to 8.8, the ionization behavior of pinacolboronate ester groups became more obvious, and the Rh value of micelles decreased to 76.8 nm (PDI = 0.291). If adding glucose to the solution, it can be found that the Rh value increased to 166.0 nm (PDI = 0.186). The variability of Rh at pH 8.8 was less than that at pH 7.4, before and after adding glucose to the solutions. At pH 12.0, the Rh value decreased to 46.0 nm (PDI = 0.309). After adding glucose to the solution, the Rh value was 47.0 nm (PDI = 0.291), indicating that the addition of glucose can't change the morphology of micelles at pH 12.0. The hydroxide ions of NaOH caused the breakage of most PBA from the pinacolboronate ester and the PBA molecules were ionized by hydroxide ions. Therefore, the hydrophilicity of the copolymer was greatly improved, leading to the decrease of size of micelles. According to the results of Rh value of micelles at different pH values and before/after adding glucose, the very obvious glucose response occurred at pH 7.4, indicating that the P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles were suitable for use at physiological pH.
The morphologies of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles can be intuitively observed by transmission electron microscope (TEM). The micelles presented regularly spherical morphology at 25 °C and pH 7.4, as shown in Fig. 3(a). The average size of these micelles was about 160 nm. After adding glucose (16 mg mL−1), the morphology of micelles changed a lot (Fig. 3(b)). The micelles became “loose” and the morphology was transformed from spherical state to “flower” state. Besides, the average size of these micelles was expanded to about 360 nm. After adding glucose to the micelle solution (25 °C, pH 7.4), the glucose molecules tended to combine with PBA molecules broken from pinacolboronate ester groups of PPBDEMA. Therefore, the micelle core became hydrophilic and swelled, and the micelles tended to disassemble.
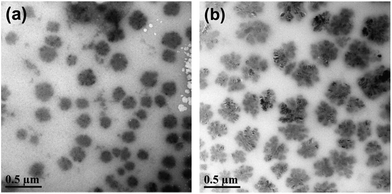 | ||
| Fig. 3 TEM images of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer micelles at 25 °C, pH 7.4: (a) without adding glucose, and (b) adding glucose: 16 mg mL−1 (concentration: 2 mg mL−1). | ||
The temperature-responsiveness of P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) brush block copolymer was characterized by 1H NMR spectra from 20 °C to 50 °C, as shown in Fig. 4(a). It can be seen that the intensities of proton peaks in P(MEO2MA-co-OEGMA) changed slightly from 20 °C to 35 °C. When the temperature increased to 40 °C and 50 °C, the intensities of the proton peaks were weakened, indicating that the transition from hydrophilicity to hydrophobicity of P(MEO2MA-co-OEGMA) segments when the temperature increased from 35 °C to 40 °C. The lower critical solution temperature (LCST) of the brush block copolymer micelle solution was ensured by transmittance measurement. Fig. 4(b) shows the transmittance curve of the brush block copolymer micelle solution. It can be seen that the transmittance curves show sharp transition during heating process. The LCST of the micelle solution was 38 °C. Fig. 4(b) also shows the plots of the Rh of micelles in water as a function of temperature. In the lower temperature ranges, the Rh values are relatively small and change slightly. In contrast, the values increase in the higher temperature ranges. At low temperatures, the P(MEO2MA-co-OEGMA) chains exist in random coil conformation owing to the hydrogen-bonding interaction between the chains and water molecules. When the temperature increases to a critical value, the chains shrink into a globular structure because the hydrogen bonds between the ether oxygen of P(MEO2MA-co-OEGMA) chains collapse and become hydrophobic. Therefore, the intermolecular hydrophobic attractions are thermodynamically favored and a micelle aggregate occurs, which results in the increase in Rh.
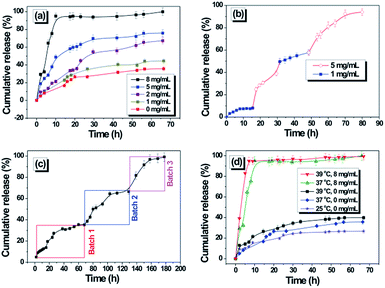
The copolymer micelles present the glucose-responsiveness at pH 7.4 and the LCST of 38 °C, and therefore could be used as a carrier to conduct the controlled release of insulin. Fig. 5(a) shows the release behavior of insulin at different glucose concentrations. With 0 mg mL−1 and 1 mg mL−1 of glucose, the insulin release was comparatively low. In the human body, the normal glucose concentration is about 0.8–1.4 mg mL−1. The investigation result confirmed that few insulin molecules would be released at normal glucose concentration. When the glucose increased to 2 mg mL−1, the release was raised obviously. For example, the cumulative release amount was 56.0% after 42 h. Further increasing the concentration to 5 mg mL−1, the insulin release also increased. After releasing for 42 h, the cumulative release amount reached 70.5%. Especially for the glucose concentration of 8 mg mL−1, the release rate and cumulative amount both significantly increased. For instance, the cumulative release amount of insulin reached 95.0% after 10 h. Obviously, the relatively high glucose concentration would cause the rapid and substantial release of insulin at 37 °C and pH 7.4.
To further explore accurate glucose-responsiveness of the copolymer micelles under normal human body glucose concentration and exceeding glucose responsiveness, the pulsed release of insulin was investigated at 1 mg mL−1 and 5 mg mL−1 (Fig. 5(b)). When insulin-loaded copolymer micelles were incubated in pH 7.4 and 1 mg mL−1 of glucose for first 15 h, the insulin release was very slow. When the micelles were incubated in pH 7.4 and 5 mg mL−1 of glucose, the rapid release of insulin was detected. Two apparent cycles of on–off insulin release were detected by changing the glucose concentration after specific time.
To better understand the insulin release behavior controlled by glucose concentration, the glucose concentration was maintained at 0.4 mg mL−1. As shown in Fig. 5(c), it can be found that only 37% of the encapsulated insulin was released during the first 70 h of incubation at pH 7.4 and 37 °C, and then followed by one release plateau. Upon the glucose concentration maintaining 0.4 mg mL−1, another insulin release behavior occurred during the second 60 h. Such insulin phase-releasing behavior was observed by three batch addition of glucose.
Fig. 5(d) shows the release profiles of insulin from micelles at different temperature with the glucose concentration of 8 mg mL−1 and 0 mg mL−1 at pH 7.4. At 25 °C, the highly hydrated P(MEO2MA-co-OEGMA) segments can stabilize the hydrophobic/hydrophilic core–shell structure of micelles, allowing very small amount of insulin diffused out of the micelles. When the temperature increased to 37 °C and 39 °C, the amount of insulin released from micelles was some higher than that at 25 °C, but many insulin molecules remain in the core of the micelles. This result revealed that only the change of temperature couldn't result in the rapid diffusion of insulin. But the P(MEO2MA-co-OEGMA) shell becomes hydrophobic at high temperature and the micellar core–shell structure was deformed, which would lead to the quick penetration of glucose molecules to the micelle core. Therefore, compared to the release plots of insulin from the micelles at 37 °C (below LCST) and pH 7.4, the release of insulin at 39 °C (above LCST) was more rapidly. For example, the cumulative release amount of insulin reached 64.6% for 6 h at 37 °C, but reached 88.7% for 6 h at 39 °C. The rapid trigger and release of insulin is very important for the disease control of diabetic patients.
In conclusion, the brush block copolymer P(St-g-PBDEMA)-b-P(MEO2MA-co-OEGMA) was synthesized by the combination of ATRP, RAFT and click chemistry. The micelles self-assembled form the copolymer presented temperature and glucose responsive properties. And the insulin could be controlled released from the micelles through altering the temperature and glucose concentration, indicating that the amphiphilic copolymer is expected to be used as the carrier of insulin for the treatment of diabetes.
Acknowledgements
The authors thank the financial supports of the National High Technology Research and Development Program (no. 2013AA032202) and the Project Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.Notes and references
- (a) Q. Wu, L. Wang, H. J. Yu, J. J. Wang and Z. F. Chen, Chem. Rev., 2011, 111, 7855 CrossRef CAS PubMed; (b) V. Lapeyre, I. Gosse, S. Chevreux and V. Ravaine, Biomacromolecules, 2006, 7, 3356 CrossRef CAS PubMed; (c) Z. Gu, T. T. Dang, M. L. Ma, B. C. Tang, H. Cheng, S. Jiang, Y. Z. Dong, Y. L. Zhang and D. G. Anderson, ACS Nano, 2013, 7, 6758 CrossRef CAS PubMed; (d) A. Sinha, A. Chakraborty and N. R. Jana, ACS Appl. Mater. Interfaces, 2014, 6, 22183 CrossRef CAS PubMed; (e) R. J. Ma and L. Q. Shi, Polym. Chem., 2014, 5, 1503 RSC.
- (a) I. Otsuka, T. Hongo, H. Nakade, A. Narumi, R. Sakai, T. Satoh, H. Kaga and T. Kakuchi, Macromolecules, 2007, 40, 8930 CrossRef CAS; (b) W. Qi, X. Yan, J. Fei, A. Wang, Y. Cui and J. Li, Biomaterials, 2009, 30, 2799 CrossRef CAS PubMed; (c) R. J. Ma, H. Yang, Z. Li, G. Liu, X. C. Sun, X. J. Liu, Y. L. An and L. Q. Shi, Biomacromolecules, 2012, 13, 3409 CrossRef CAS PubMed; (d) C. Y. Zhou, W. X. Gao, K. W. Yang, L. Xu, J. C. Ding, J. X. Chen, M. C. Liu, X. B. Huang, S. Wang and H. Y. Wu, Langmuir, 2013, 29, 13568 CrossRef CAS PubMed; (e) C. J. Zhang, M. D. Losego and P. V. Braun, Chem. Mater., 2013, 25, 3239 CrossRef CAS; (f) M. M. Zhou, J. D. Xie, S. T. Yan, X. M. Jiang, T. Ye and W. T. Wu, Macromolecules, 2014, 47, 6055 CrossRef CAS; (g) D. Roy and B. S. Sumerlin, ACS Macro Lett., 2012, 1, 529 CrossRef CAS.
- (a) A. Matsumoto, S. Ikeda, A. Harada and K. Kataoka, Biomacromolecules, 2003, 4, 1410 CrossRef CAS PubMed; (b) S. J. Hwang, C. Fernandez, J. P. Amoureux, J. W. Han, J. Cho, S. W. Martin and M. Pruski, J. Am. Chem. Soc., 1998, 120, 7337 CrossRef CAS.
- (a) E. Uchimura, H. Otsuka, T. Okano, Y. Sakurai and K. Kataoka, Biotechnol. Bioeng., 2001, 72, 307 CrossRef CAS; (b) D. Shiino, Y. Murata, A. Kubo, Y. J. Kim, K. Kataoka, Y. Koyama, A. Kikuchi, M. Yokoyama, Y. Skurai and T. Okano, J. Controlled Release, 1995, 37, 269 CrossRef CAS; (c) T. Hoare and R. Pelton, Biomacromolecules, 2008, 9, 733 CrossRef CAS PubMed; (d) H. R. Mulla, N. J. Agard and A. Basu, Bioorg. Med. Chem. Lett., 2004, 14, 25 CrossRef CAS PubMed; (e) T. Hoeg-Jensen, S. Ridderberg, S. Havelund, L. Schäffer, P. Balschmidt and I. Jonassen, J. Pept. Sci., 2005, 11, 339 CrossRef CAS PubMed.
- Y. Yao, X. M. Wang, T. W. Tan and J. Yang, Soft Matter, 2011, 7, 7948 RSC.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769 CrossRef CAS.
- Y. Yao, L. Y. Zhao, J. J. Yang and J. Yang, Biomacromolecules, 2012, 13, 1837 CrossRef CAS PubMed.
- (a) D. Wang, T. Liu, J. Yin and S. Y. Liu, Macromolecules, 2011, 44, 2282 CrossRef CAS; (b) P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25 RSC; (c) W. Z. Yuan, T. X. Shen, J. J. Wang and H. Zou, Polym. Chem., 2014, 5, 3968 RSC.
- (a) W. Z. Yuan and W. Guo, Polym. Chem., 2014, 5, 4259 RSC; (b) J. F. Lutz, Ö. Akdemir and A. Hoth, J. Am. Chem. Soc., 2006, 128, 13046 CrossRef CAS PubMed; (c) W. Z. Yuan, J. C. Zhang, H. Zou, T. X. Shen and J. Ren, Polymer, 2012, 53, 956 CrossRef CAS PubMed; (d) Z. K. Wang, D. Wang, H. Wang, J. J. Yan, Y. Z. You and Z. G. Wang, J. Mater. Chem., 2011, 21, 15950 RSC.
- J. F. Lutz, J. Andrieu, S. Üzgün, C. Rudolph and S. Agarwal, Macromolecules, 2007, 40, 8540 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and descriptions of the synthesis and characterization of copolymers. See DOI: 10.1039/c5ra16701a |
| This journal is © The Royal Society of Chemistry 2015 |