Observation of high-temperature magnetic transition and existence of ferromagnetic short-range correlations above transition in double perovskite La2FeMnO6
Jasnamol Pezhumkattil Palakkalab,
P. Neenu Lekshmib,
Senoy Thomasb,
K. G. Sureshc and
Manoj Raama Varma*ab
aAcademy of Scientific and Innovative Research (AcSIR), CSIR – National Institute for Interdisciplinary Science and Technology (CSIR-NIIST) Campus, Thiruvananthapuram-695 019, India. E-mail: manoj@niist.res.in
bMaterials Science and Technology Division, CSIR – National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram-695 019, India
cDepartment of Physics, Indian Institute of Technology Bombay, Mumbai 400 076, India
First published on 1st December 2015
Abstract
We present the observation of a high-temperature magnetic transition along with ferromagnetic short-range correlations (FSCs) in La2FeMnO6 perovskite system. XPS analysis confirmed the presence of Fe3+ and Mn3+ cations. Magnetization vs. temperature curves show two distinct transitions at TC1 ∼ 60 K and TC ∼ 425 K. Coercivity values of ∼1140 Oe and ∼35 Oe are observed at 2 K and 300 K respectively. Thermomagnetic analysis reveals the presence of FSCs in La2FeMnO6 up to T* = 570 K, well above the transition point, similar to a Griffiths-like phase (GP). The presence of ferromagnetic clusters in the paramagnetic region might be due to the intrinsic inhomogeneities associated with the structure, the quenched disorder related to the B-site cations and the antisite boundaries. The coefficient of lower temperature electronic specific heat is as high as 59.5 mJ mol−1 K−2. The electron spin resonance spectra show ferromagnetic resonance signals that point to the possibility of the presence of FSCs at room temperature. The material seems to be quite a promising candidate for some room temperature applications due to the possibility of the coexistence of functionalities like ferromagnetism, ferrimagnetism, GP, magnetotransport coupling, etc. in a single material.
Introduction
The perovskite oxides ABO3 (A = alkaline or rare earth elements (R), B = transition elements) possess a vast variety of physical properties like ferromagnetism (FM), ferrimagnetism (FiM), half metallicity, magnetoresistance, magnetodielectric (MD) coupling, etc.1–10 The manganite perovskites (AMnO3) have received considerable attention due to their magnetic, transport and magnetotransport properties linked with the change in the spin state.11,12 Introducing a second element to the B-site will provide further characteristics to the system [Double Perovskite (DP)] in accordance with the difference in cationic ordering, oxidation states, antiphase boundaries, multiple exchange interactions, lattice distortion, etc.1–4 Enormous studies are being carried out in nowadays on rare earth (RE) manganites with DP structure that exhibit multifunctional properties by virtue of their wide B/Mn cationic range.7–10 Among these compounds, La2NiMnO6 is quite attractive because it is a FM semiconductor with good magnetodielectricity and magnetoresistance at temperatures close to room temperature due to its frustrated spin system originating from Ni and Mn cations.13,14 Among the RE manganites, La2FeMnO6, the Fe counterpart of La2NiMnO6, remains unexplored fully, to a large extent.The perovskites LaFeO3 and LaMnO3 are antiferromagnetic (AFM) insulators in bulk form with Néel temperature (TN) 740 K and 140 K respectively, in which the superexchange interactions Fe3+–O–Fe3+ and Mn3+–O–Mn3+ lead to AFM coupling.15–17 There are reports pertaining to the preparation of La2FeMnO6 having perovskite structure.18,19 Many researchers continued the work on La2FeMnO6 both experimentally and theoretically, but a clear magnetic transition was not identified experimentally on bulk La2FeMnO6. The present work presents the observation of a high-temperature magnetic transition and the evidence for the existence of ferromagnetic short-range correlations (FSCs) far above room temperature in La2FeMnO6.
Experimental
Polycrystalline La2FeMnO6 is synthesized by employing Pechini method.19 High purity chemicals La(NO3)3·6H2O, Fe(NO3)3·9H2O and Mn(NO3)2 (Sigma-Aldrich, purity ∼99.99%) were weighed according to stoichiometry. Citric acid was added to this mixture such that the citric acid and cation ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The nitrates and citric acid were mixed in 1 L beaker in de-ionized water medium at 70 °C with continuous stirring. The temperature were slowly raised and finally the combustion happened at 200 °C. The powder was kept at same condition for one day. The precursor powder was ground well and loaded into a muffle furnace at 600 °C for 2 hours and 900 °C for 2 hours. The powders were ground well and pelletized using a hydraulic press. For pelletization, polyvinyl alcohol was used as a binder. The pellets were finally sintered at 900 °C for 6 hours. The sintered pellets were ground well, and the final material was confirmed as phase pure La2FeMnO6 by using the X-ray diffraction (XRD) data obtained by Philips PANalytical X'Pert Pro Powder X-Ray Diffractometer with a Ni filtered Cu Kα radiation (λ = 1.5406 Å). The Rietveld refinement of the XRD pattern was done using the software GSAS – EXPGUI.20 The crystallographic structure is framed using CrystalMaker®.21 X-ray photoelectron spectroscopy (XPS) measurements were carried out using an Omicron Nanotechnology Multiprobe Instrument. The XPS spectra corresponding to Fe 2p and Mn 2p of La2FeMnO6 are recorded by using a high resolution hemisphere analyzer EA 125 HR equipped with a detection system consisting of seven channeltrons. A monochromatic Al Kα source of energy, hν = 1486.6 eV was used to probe the La2FeMnO6 pellets, attached by a double-sided tape to the molybdenum sample holder. The pressure in the XPS chamber during the measurements was 5 × 10−10 mbar. A wide scan was collected to ensure that no foreign materials were present on the sample surface. Narrow scans of Fe 2p and Mn 2p regions were collected at analyzer pass energy of 20 eV. The peaks are fitted using the XPS Peak Fit software. The magnetization measurements were carried out on La2FeMnO6 powders by using a Vibrating Sample Magnetometer (VSM) attached to a Physical Property Measurement System (Dynacool, Quantum Design). The specific heat (C) is measured for the temperature range of 10–150 K by using the heat capacity option attached to the PPMS (Dynacool, Quantum Design). The electron spin resonance (ESR) spectra obtained using JES-FA200 ESR Spectrometer (ESR-JEOL, Japan) at temperatures 100 K, 170 K, and 300 K.
3. The nitrates and citric acid were mixed in 1 L beaker in de-ionized water medium at 70 °C with continuous stirring. The temperature were slowly raised and finally the combustion happened at 200 °C. The powder was kept at same condition for one day. The precursor powder was ground well and loaded into a muffle furnace at 600 °C for 2 hours and 900 °C for 2 hours. The powders were ground well and pelletized using a hydraulic press. For pelletization, polyvinyl alcohol was used as a binder. The pellets were finally sintered at 900 °C for 6 hours. The sintered pellets were ground well, and the final material was confirmed as phase pure La2FeMnO6 by using the X-ray diffraction (XRD) data obtained by Philips PANalytical X'Pert Pro Powder X-Ray Diffractometer with a Ni filtered Cu Kα radiation (λ = 1.5406 Å). The Rietveld refinement of the XRD pattern was done using the software GSAS – EXPGUI.20 The crystallographic structure is framed using CrystalMaker®.21 X-ray photoelectron spectroscopy (XPS) measurements were carried out using an Omicron Nanotechnology Multiprobe Instrument. The XPS spectra corresponding to Fe 2p and Mn 2p of La2FeMnO6 are recorded by using a high resolution hemisphere analyzer EA 125 HR equipped with a detection system consisting of seven channeltrons. A monochromatic Al Kα source of energy, hν = 1486.6 eV was used to probe the La2FeMnO6 pellets, attached by a double-sided tape to the molybdenum sample holder. The pressure in the XPS chamber during the measurements was 5 × 10−10 mbar. A wide scan was collected to ensure that no foreign materials were present on the sample surface. Narrow scans of Fe 2p and Mn 2p regions were collected at analyzer pass energy of 20 eV. The peaks are fitted using the XPS Peak Fit software. The magnetization measurements were carried out on La2FeMnO6 powders by using a Vibrating Sample Magnetometer (VSM) attached to a Physical Property Measurement System (Dynacool, Quantum Design). The specific heat (C) is measured for the temperature range of 10–150 K by using the heat capacity option attached to the PPMS (Dynacool, Quantum Design). The electron spin resonance (ESR) spectra obtained using JES-FA200 ESR Spectrometer (ESR-JEOL, Japan) at temperatures 100 K, 170 K, and 300 K.
Results and discussion
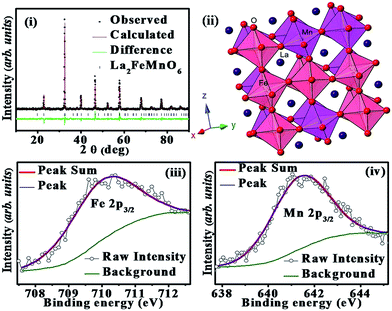
The orthorhombic, Pbnm crystal structure is confirmed for La2FeMnO6 by the Rietveld refinement using GSAS-EXPGUI20 (WRP = 4.64%). The observed, calculated and difference data are shown in Fig. 1(i) and the obtained lattice parameters are, a = 5.54 Å, b = 5.51 Å, c = 7.81 Å, and α, β, γ = 90° with 〈Fe–O–Mn〉 bond angle of 161.75°. The crystallographic structure framed using CrystalMaker® is shown in Fig. 1(ii).The XPS spectra corresponding to Fe 2p and Mn 2p of La2FeMnO6 are analyzed. The binding energies were corrected by C 1s as reference energy (C 1s = 284.8 eV). The peaks are fitted using the XPS Peak Fit software and the fitted curves are shown in Fig. 1(iii) and (iv). The fitted curves give single peak for Fe 2p3/2 and Mn 2p3/2 with peak positions at 710.14 eV and 641.36 eV respectively which confirms the oxidation states of cations as Fe3+ and Mn3+.22,23.
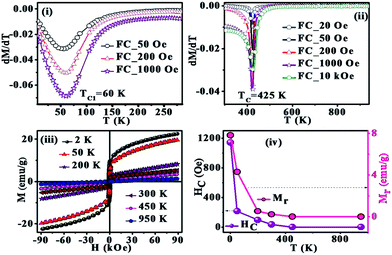
Based on the reported density-functional calculations, La2FeMnO6 could be either a FM semiconductor or a FM half-metal or a FM metal or a FiM semiconductor under different biaxial strain conditions.24 Also, de Lima et al. reported the magnetic properties of La2FeMnO6 bulk with TC = 65 K and coercivity (HC) of 1160 Oe at 2 K and 170 Oe at 300 K.25 Further, a hysteresis loop with small HC and remanence (Mr) at 300 K was also reported for La2FeMnO6.26 In the present study, zero field cooled (ZFC) and field cooled (FC) magnetizations were measured in the temperature range 5–385 K using the cryostat (inset of Fig. 2) and in the range 300–950 K using the oven (Fig. 2) in applied fields of 50, 200 and 1000 Oe. The temperature dependent magnetization (M–T) show irreversibility in magnetization as can be evidenced in the ZFC and FC bifurcation at lower temperatures and lower applied fields, which points towards the presence of spin frustration/spin glass state at lower temperatures in accordance with the previous reports on La2FeMnO6.27–29 The derivative dM/dT vs. T plot (Fig. 3(i)) indicates a broad transition at TC1 = 60 K, which corroborates the spin glass-like transition reported earlier.25,27–29
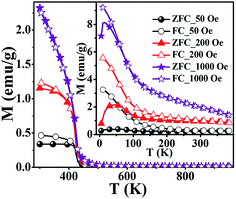 | ||
| Fig. 2 ZFC and FC curves at 50, 200, and 1000 Oe for 5–380 K. Inset: ZFC and FC curves at 50, 200, and 1000 Oe for 300–950 K. | ||
Bhame et al. observed positive values of Curie–Weiss constant (θ) as an indication of the presence of ferromagnetic exchange interactions and a HC of 1170 Oe at 12 K for La2FeMnO6.30 The M–T in the range 5–300 K is similar to that observed in previous cases, and a closer look at the plot shows that the moment does not go to zero even at room temperature.30,31 Further, the room temperature hysteresis loop is also reported for La2FeMnO6.25,26 Ueda et al. observed no transition in the M–T in the range of 50–400 K, and predicted that there might be an AFM transition above 400 K.33 They have also fabricated thin films of La2FeMnO6, by artificially creating superlattices and by natural growth and showed a FM to PM transition (TC) at ∼230 K and ∼380 K respectively.32,33.
This instigated us to carry out higher temperature (300–950 K) M–T measurements (Fig. 2), which showed a transition at TC ∼ 425 K as shown in dM/dT vs. T plot (Fig. 3(ii)). This evidence of the onset of a magnetic transition above room temperature is in line with the theoretical predictions and experimental observations in La2FeMnO6 thin films.34,35 The disordered cation arrangements generate antisite boundaries leading to competing exchange interactions viz. Fe3+–O–Mn3+ FiM interactions, Fe3+–O–Fe3+, and Mn3+–O–Mn3+ AFM interactions and Mn3+–O–Mn3+ dynamic FM interactions.17,26. Further, there is a key role played by the 〈B–O–B′〉 bond angle in determining the exchange interaction strength with the maximum in exchange interaction for 180°. The super exchange interaction is rather favored in this case since the 〈Fe–O–Mn〉 bond angle is as high as 161.75°.
Field dependent isothermal magnetization (M–H) was measured in the field range of −90 kOe to +90 kOe at different temperatures ranging from 2 to 950 K, and representative results are summarized in Fig. 3(iii). The M–H is non-saturating even at the highest field due to the inhomogeneities associated with its structure. Variations of HC and Mr with temperature are shown in Fig. 3(iv). It shows a hysteresis with HC = 1140 Oe and Mr = 7.8 emu g−1 at 2 K and HC = 35 Oe and Mr = 0.2 emu g−1 at 300 K that are in agreement with the earlier report25,30 and the loop disappears at higher temperatures. The room temperature loop is almost similar to that reported earlier.25,26 The coercivity value of 1140 Oe drops to 215 Oe as the sample is warmed to 50 K from 2 K and then a slow rate of decrease in HC with temperature is observed, which is an indication of the decrease of the strength of FM clusters and increase of the strength of PM matrix. The straight line plot at 950 K, with no HC, and Mr, shows the complete PM state. The M–H curves show a non-linear behavior for low fields (<2 kOe) even above TC, which is an indication of the presence of weak magnetization in the PM region.
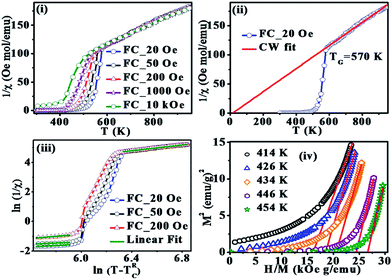
The inverse susceptibility derived from FC magnetization for 20, 50, 200, 1000 Oe and 10 kOe is shown in Fig. 4(i) and the fit to Curie–Weiss (CW) law 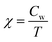 for 20 Oe is shown in Fig. 4(ii). A CW fit is obtained only above T* = 570 K and the presence of FSC is clearly evidenced bye the plot as a sharp downturn below T*, which is gradually reduced as the field is increased. This feature is usually considered as a manifestation of typical Griffiths-like phases (GP).36–51 GP was originally proposed as randomly distributed lsing FM regions with nearest neighbor exchange interactions J and 0 with probabilities p and (1 − p) respectively.52 The CW fit in PM region yields an effective magnetic moment,
for 20 Oe is shown in Fig. 4(ii). A CW fit is obtained only above T* = 570 K and the presence of FSC is clearly evidenced bye the plot as a sharp downturn below T*, which is gradually reduced as the field is increased. This feature is usually considered as a manifestation of typical Griffiths-like phases (GP).36–51 GP was originally proposed as randomly distributed lsing FM regions with nearest neighbor exchange interactions J and 0 with probabilities p and (1 − p) respectively.52 The CW fit in PM region yields an effective magnetic moment, 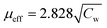 (Cw is the Curie constant) as 6.5 μB per F.U. The XPS spectra confirmed Fe3+ and Mn3+ spin states in La2FeMnO6. The theoretically expected μeff for La2FeMnO6 with Fe3+ and Mn3+ are given in Table 1. The obtained μeff proclaims that the Fe3+ and Mn3+ are not in a low spin state (LS) simultaneously, and if spin-orbit coupling is present, Fe3+ should not be in LS. The FM state may arise from the high spin state (HS) Fe3+ (t32g, e2g) and LS Mn3+ (t42g, e0g), whereas the FiM state arises from LS Fe3+ (t52g, e0g) and HS Mn3+ (t32g, e1g), under certain strain conditions.24 A recent study on La2FeMnO6 thin film confirmed AFM coupling of HS Fe3+ and HS Mn3+, leading to FiM and observed an inverse relationship in between saturation magnetization and Fe/Mn-order.34
(Cw is the Curie constant) as 6.5 μB per F.U. The XPS spectra confirmed Fe3+ and Mn3+ spin states in La2FeMnO6. The theoretically expected μeff for La2FeMnO6 with Fe3+ and Mn3+ are given in Table 1. The obtained μeff proclaims that the Fe3+ and Mn3+ are not in a low spin state (LS) simultaneously, and if spin-orbit coupling is present, Fe3+ should not be in LS. The FM state may arise from the high spin state (HS) Fe3+ (t32g, e2g) and LS Mn3+ (t42g, e0g), whereas the FiM state arises from LS Fe3+ (t52g, e0g) and HS Mn3+ (t32g, e1g), under certain strain conditions.24 A recent study on La2FeMnO6 thin film confirmed AFM coupling of HS Fe3+ and HS Mn3+, leading to FiM and observed an inverse relationship in between saturation magnetization and Fe/Mn-order.34
| Spin-orbit coupling | Mn3+ (HS) | Mn3+ (LS) |
|---|---|---|
| Fe3+ (HS) | 5.916 | 5.916 |
| Fe3+ (LS) | 1.732 | 1.732 |
| Spin only interaction | Mn3+ (HS) | Mn3+ (LS) |
|---|---|---|
| Fe3+ (HS) | 7.681 | 6.16 |
| Fe3+ (LS) | 5.196 | 3.317 |
The disordered perovskite causes a random dilution of FM with different exchange interactions viz. super exchange and double exchange interactions as described before and causes the evolution of FSCs above TC. The presence of inhomogeneous magnetic states, magnetic clusters and random competing exchange interactions in La2FeMnO6 are well reported previously.27–30 Moreover, the quenched disorder, triggered by the random chemical replacement of ions of different sizes can also induce intrinsic inhomogeneities in manganites.53–55 The disorder is quenched within the distorted structure of La2FeMnO6 due to the strong coupling of Jahn–Teller distortion of the Fe3+/Mn3+ ions and orthorhombic crystal lattice so that the FM bonds can be assumed as fixed within the lattice that form clusters and FSCs.54
Usually, the susceptibility of a Griffiths-like phase in low fields follows the power law,36−48 χ−1 ∝ (T − TRC)1−λ, where λ is the magnetic susceptibility exponent (0 ≤λ ≤1) and TRC is the random critical temperature. TRC is taken as the temperature for which the equation yields a λ (λPM) value close to zero above T*. The θ obtained from CW fit is 18 K for 20 Oe (Fig. 4(ii)), and assuming TRC at 18 K well establishes λPM ∼ 0.02. The linear part of the plot ln(χ−1) vs. ln(T − TRC) (Fig. 4(iii)) is fitted with the power law and estimated the susceptibility exponent λ as 0.54 at 20 Oe and 0.29 at 200 Oe. The value of λ lies in between 0 and 1 and is decreasing with increase in the field, a signature of Griffiths-like phases.36–48 The Arrot plots (M2 vs. H/M) at five different temperatures, below and above TC are shown in Fig. 4(iv). The linear extrapolation to the high field region of the Arrot plot gives a negative M2 axis intercept confirming the absence of spontaneous magnetization (Fig. 4(iv)) with the existence of finite-sized FM interrelated spins without any static long-range magnetization.40,41
The presence of inhomogeneous magnetic phases with co-existence of FM and AFM states in La2FeMnO6 was reported where, the FM phase originating from static Jahn–Teller distortions removed Mn3+–O–Mn3+ dynamic interactions and the AFM phase arising from Fe3+–O–Fe3+ interactions.27 De et al. reported a glassy complex system at low temperatures with two dynamical freezing points below room temperature (at 20 K and 255 K), in La2FeMnO6 due to phase separation that was observed in many mixed valence manganites and also due to the existence of several competing magnetic interactions induced by antiphase boundaries 28–29 Zhou et al. studied LaMn1−xFexO3 (0 < x < 1.0) system and concluded that Mn favors double exchange interaction whereas Fe favors AFM super exchange.35 Bhame et al. reported the material as a spin glass, with Fe3+ and Mn3+ ions having Mn-rich and/or Fe-rich clusters of different sizes, causing predominant FM or AFM interactions respectively.30 These clusters are distributed within the lattice according to the synthesis conditions; wherein the magnetism is predicted by a two-phase model.
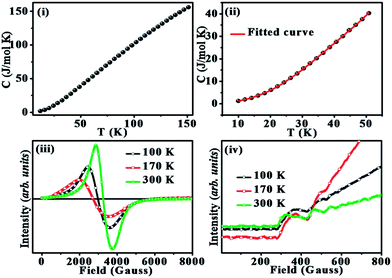
In order to get a clear idea about the inhomogeneities, the specific heat (C) was measured for the temperature range of 10–150 K as shown in Fig. 5(i). The low-temperature region from 10–50 K is fitted using the polynomial:40 C = γT + β3T3 + β5T5 + β7T7 as shown in Fig. 5(ii). The γ gives the coefficient of electronic specific heat due to free charge carriers. The higher order T terms are the lattice contribution arising from phonons. The obtained values are γ = 59.5 mJ mol−1 K−2 for the linear term, β3 = 0.694 mJ mol−1 K−4, β5 = −2.54 × 10−4 mJ mol−1 K−6 and β7 = 3.69 × 10−8 mJ mol−1 K−8. The high value of the electronic linear contribution to the low-temperature specific heat could be an indication of the coexistence of FM metallic and charge ordered state.44 A higher value of γ is obtained for materials having FSC with GP.40,43,44
The electron spin resonance spectra obtained at 100 K, 170 K, and 300 K are shown in Fig. 5(iii). ESR is highly sensitive to trivial magnetic correlations and is widely used for analyzing magnetic properties of manganites.55–57 The ferromagnetic resonance signals, seen in the enlarged low field region of ESR spectra as shown in Fig. 5(iv) clearly show the presence of FSCs. The coexistence of PM resonance and FM resonance (FMR) signal is an indication of co-existence of PM and FM clusters.57
Conclusions
In the present study, the disordered, orthorhombic La2FeMnO6 is found to possess double transition at TC1 = 60 K and, TC = 425 K. The inverse susceptibility show a sharp downturn with temperature, below T* = 570 K, as an indication of the occurrence of FSCs/GP. The oxidation states of cations are +3 (for Fe and Mn), and the effective PM moment is obtained as 6.5 μB per F.U. Fe3+–O–Mn3+, Fe3+–O–Fe3+ and Mn3+–O–Mn3+ super exchange interactions, and Mn3+–O–Mn3+ dynamic FM interactions could be present in the system. These competing interactions are responsible for the occurrence of glassy like transition at 60 K and Griffiths-like phase at higher temperatures. The total thermo-magnetic picture of La2FeMnO6 is as follows. At TRC = 18 K, random dilution of FM phases starts due to the transition of some of the magnetic phases. The FM clusters within the matrix interacts with each other and these clusters will undergo spin flipping with time and enters into a Griffiths-like phase above TRC. At TC1 = 60 K, most of the FM phase will undergo magnetic transition into PM state and at TC1 = 425 K, another magnetic transition occurs. Even though the TC has reached, some of the FM clusters remain in the PM matrix and eventually transform completely into a PM state at T* = 570 K, similar to a Griffiths-like phase. The Griffiths analysis yields a λ value 0.54 at 20 Oe and decreases to 0.29 at 200 Oe. Below TC, the FM clusters present themselves as a matrix in which PM clusters are embedded. Above TC, it seems the FM clusters are distributed within the PM matrix and eventually completely transforms to PM state. The intrinsic inhomogeneities associated with the structure, quenched disorder related to the B-site cations and the antisite boundaries cause the presence of FSCs. The coefficient of low temperature electronic specific heat is as high as γ = 59.5 mJ mol−1 K−2, and such a high value can be argued to be responsible for FSCs. The electron spin resonance spectra show ferromagnetic resonance signals pointing the possibility of the presence of FSCs at room temperature. Normally, magneto-transport properties co-exist with GP, which can be explored in our future research, and the material is a promising candidate for room temperature applications. The coexistence of functionalities like FM, FiM, GP, magnetotransport coupling, etc. in a single material is of great interest for both the scientific aspects and for practical applications.Acknowledgements
Jasnamol P. P. is thankful to the University Grants Commission (UGC), India and P. Neenu Lekshmi is thankful to the Council of Scientific and Industrial Research (CSIR), India for providing Senior Research Fellowships. S. Thomas acknowledge the financial support received from DST, India, via INSPIRE faculty award. M. R. Varma is pleased to acknowledge CSIR, India granted project SURE (No. CSC0132) and Kerala State Council for Science, Technology & Environment (KSCSTE) for financial support. The authors are thankful to Mr Ajeesh P Paulose for the technical support.References
- K.-I. Kobayashi, T. Kimura, H. Sawada, K. Terakura and Y. Tokura, Nature, 1998, 395, 677 CrossRef CAS.
- P. Baettig, C. Ederer and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 214105 CrossRef.
- M. T. Anderson, K. B. Greenwood, G. A. Taylor and K. R. Poeppelmeiert, Prog. Solid State Chem., 1993, 22, 197 CrossRef CAS.
- Ll. Balcells, J. Navarro, M. Bibes, A. Roig, B. Martínez and J. Fontcuberta, Appl. Phys. Lett., 2001, 78, 781 CrossRef.
- R. von Helmolt, J. Wecker, B. Holzapfel, L. Schultz and K. Samwer, Phys. Rev. Lett., 1993, 71, 2331 CrossRef CAS.
- M.-R. Li, M. Retuerto, Z. Deng, P. W. Stephens, M. Croft, Q. Huang, H. Wu, X. Deng, G. Kotliar, J. Sanchez-Benitez, J. Hadermann, D. Walker and M. Greenblatt, Angew. Chem., 2015, 127, 1 CrossRef.
- P. Neenu Lekshmi, M. Vasundhara, M. R. Varma, K. G. Suresh and M. Valant, Phys. B, 2014, 448, 285 CrossRef CAS.
- Y. Mao, J. Parsons and J. S. Mc Cloy, Nanoscale, 2013, 5, 4720 RSC.
- R. Yadav, H. S. Nair, A. Kumar, S. Adiga, H. L. Bhat, S. M. Yusuf and S. Elizabeth, J. Appl. Phys., 2015, 117, 093903 CrossRef.
- P. Barrozo and J. Albino Aguiar, J. Appl. Phys., 2013, 113, 17E309 CrossRef.
- J. M. D. Coey, M. Viret and L. Ranno, Phys. Rev. Lett., 1995, 75, 3910 CrossRef CAS.
- J. M. D. Coey and M. Viret, Adv. Phys., 1999, 48, 167 CrossRef CAS.
- D. Choudhury, P. Mandal, R. Mathieu, A. Hazarika, S. Rajan, A. Sundaresan, U. V. Waghmare, R. Knut, O. Karis, P. Nordblad and D. D. Sarma, Phys. Rev. Lett., 2012, 108, 127201 CrossRef CAS PubMed.
- Y. Guo, L. Shi, S. Zhou, J. Zhao and W. Liu, Appl. Phys. Lett., 2013, 102, 222401 CrossRef.
- J. W. Seo, E. E. Fullerton, F. Nolting, A. Scholl, J. Fompeyrine and J.-P. Locquet, J. Phys.: Condens. Matter, 2008, 20, 264014 CrossRef CAS PubMed.
- P. Mondal, D. Bhattacharya, P. Choudhury and P. Mandal, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 172403 CrossRef.
- J. Kanamori, J. Phys. Chem. Solids, 1959, 10, 87 CrossRef CAS.
- P. Ganguly, Bull. Mater. Sci., 1981, 3, 255 CrossRef CAS.
- Y. Wu, Z. Yu and S. Liu, J. Solid State Chem., 1994, 112, 157 CrossRef CAS.
- B. H. Toby, EXPGUI, a graphical user interface for GSAS, J. Appl. Crystallogr., 2001, 34, 210 CrossRef CAS.
- CrystalMaker Software Ltd, Oxford, England (www.crystalmaker.com).
- J. Blomquist, U. Helgeson, L. C. Moberg, B. Folkesson and R. Larsson, Inorg. Chim. Acta, 1983, 69, 17 CrossRef CAS.
- J. C. Carver, G. K. Schweitzer and T. A. Carlson, J. Chem. Phys., 1972, 57, 973 CrossRef CAS.
- Y. Qian, H. Wu, E. Kan, J. Lu, R. Lu, Y. Liu, W. Tan, C. Xiao and K. Deng, J. Appl. Phys., 2013, 114, 063713 CrossRef.
- O. F. de Lima, J. A. H. Coaquira, R. L. de Almeida, L. B. de Carvalho and S. K. Malik, J. Appl. Phys., 2009, 105, 013907 CrossRef.
- Z.-X. Wei, Y. Wang, J.-P. Liua, C.-M. Xiao and W.-W. Zeng, Mater. Chem. Phys., 2012, 136, 755 CrossRef CAS.
- D. V. Karpinsky, I. O. Troyanchuk and V. V. Sikolenko, J. Phys.: Condens. Matter, 2007, 19, 036220 CrossRef.
- K. de, M. Thakur, A. Manna and S. Giri, J. Appl. Phys., 2006, 99, 013908 CrossRef.
- K. De, R. Ray, R. N. Panda, S. Giri, H. Nakamura and T. Kohara, J. Magn. Magn. Mater., 2005, 288, 339 CrossRef CAS.
- S. D. Bhame, V. L. Joseph Joly and P. A. Joy, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 054426 CrossRef.
- P. Barrozo, N. O. Moreno and J. A. Aguiar, Adv. Mater. Res., 2014, 975, 122 CrossRef CAS.
- K. Ueda, H. Tabata and T. Kawai, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, R12 561 CrossRef CAS.
- K. Ueda, Y. Muraoka, H. Tabata and T. Kawai, Appl. Phys. Lett., 2001, 78, 512 CrossRef CAS.
- K. Yoshimatsu, K. Nogami, K. Watarai, K. Horiba, H. Kumigashira, O. Sakata, T. Oshima and A. Ohtomo, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 054421 CrossRef.
- X.-D. Zhou, L. R. Pederson, Q. Cai, J. Yang, B. J. Scarfino, M. Kim, W. B. Yelon, W. J. James, H. U. Anderson and C. Wang, J. Appl. Phys., 2006, 99, 08 M918 Search PubMed.
- S. K. Giri, S. M. Yusuf, M. D. Mukadam and T. K. Nath, J. Alloys Compd., 2014, 591, 181 CrossRef CAS.
- A. K. Pramanik and A. Banerjee, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 024431 CrossRef.
- W. Jiang, X. Z. Zhou and G. Williams, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 064424 CrossRef.
- P. Tong, B. Kim, D. Kwon, T. Qian, S. Lee, S.-W. Cheong and B. G. Kim, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 184432 CrossRef.
- H. S. Nair, D. Swain, N. S. Hariharan, S. Adiga, C. Narayana and S. Elzabeth, J. Appl. Phys., 2011, 110, 123919 CrossRef.
- A. Shahee, K. Singh, R. J. Choudhary and N. P. Lalla, Phys. Status Solidi B, 2015, 1, 1832 CrossRef.
- S. Zhou, Y. Guo, J. Zhao, L. He and L. Shi, J. Phys. Chem. C, 2011, 115, 1535 CAS.
- A. Slebarski, J. Goraus and M. Fijałkowski, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 075154 CrossRef.
- C. L. Lu, K. F. Wang, S. Dong, J. G. Wan, J. M. Liu and Z. F. Ren, J. Appl. Phys., 2008, 103, 07F714 Search PubMed.
- S. Guo, D. P. Young, R. T. Macaluso, D. A. Browne, N. L. Henderson, J. Y. Chan, L. L. Henry and J. F. DiTusa, Phys. Rev. Lett., 2008, 100, 017209 CrossRef CAS PubMed.
- W. Jiang, X. Z. Zhou and G. Williams, Europhys. Lett., 2008, 84, 47009 CrossRef.
- W. Jiang, X. Z. Zhou and G. Williams, Phys. Rev. Lett., 2007, 99, 177203 CrossRef PubMed.
- C. Magen, P. A. Algarabel, L. Morellon, J. P. Araujo, C. Ritter, M. R. Ibarra, A. M. Pereira and J. B. Sousa, Phys. Rev. Lett., 2006, 96, 167201 CrossRef CAS PubMed.
- A. K. Pathak, D. Paudyal, W. T. Jayasekara, S. Calder, A. Kreyssig, A. I. Goldman, K. A. Gschneidner Jr and V. K. Pecharsky, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 224411 CrossRef.
- O. Chauvet, G. Goglio, P. Molinie, B. Corraze and L. Brohan, Phys. Rev. Lett., 1998, 81, 1102 CrossRef CAS.
- R. B. Griffiths, Phys. Rev. Lett., 1969, 23, 17 CrossRef.
- A. Moreo, M. Mayr, A. Feiguin, S. Yunoki and E. Dagotto, Phys. Rev. Lett., 2000, 84, 5568 CrossRef CAS PubMed.
- Y. Ren-Fu, Y. Sun, W. He, Q.-A. Li and C. Zhao-Hua, Appl. Phys. Lett., 2007, 90, 032502 CrossRef.
- V. Markovich, R. Puzniak, I. Fita, A. Wisniewski, D. Mogilyansky, B. Dolgin, G. Gorodetsky and G. Jung, J. Appl. Phys., 2013, 113, 233911 CrossRef.
- X. J. Liu, Z. Q. Li, A. Yu, M. L. Liu, W. R. Li, B. L. Li, P. Wu, H. L. Bai and E. Y. Jiang, J. Magn. Magn. Mater., 2007, 313, 354 CrossRef CAS.
- S. Zhou, L. Shi, H. Yang and J. Zhao, Appl. Phys. Lett., 2007, 91, 172505 CrossRef.
- J. Deisenhofer, D. Braak, H.-A. Krug von Nidda, J. Hemberger, R. M. Eremina, V. A. Ivanshin, A. M. Balbashov, G. Jug, A. Loidl, T. Kimura and Y. Tokura, Phys. Rev. Lett., 2005, 95, 257202 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |