Open Access Article
Open Access ArticleSwitchable silver mirrors with long memory effects†
Chihyun
Park
,
Seogjae
Seo
,
Haijin
Shin
,
Bhimrao D.
Sarwade
,
Jongbeom
Na
and
Eunkyoung
Kim
*
Department of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Korea. E-mail: eunkim@yosei.ac.kr; Web: http://web.yonsei.ac.kr/eunkim/
First published on 29th September 2014
Abstract
An electrochemically stable and bistable switchable mirror was achieved for the first time by introducing (1) a thiol-modified indium tin oxide (ITO) electrode for the stabilization of the metallic film and (2) ionic liquids as an anion-blocking layer, to achieve a long memory effect. The growth of the metallic film was denser and faster at the thiol-modified ITO electrode than at a bare ITO electrode. The electrochemical stability of the metallic film on the thiol-modified ITO was enhanced, maintaining the metallic state without rupture. In the voltage-off state, the metal film maintained bistability for a long period (>2 h) when ionic liquids were introduced as electrolytes for the switchable mirror. The electrical double layer in the highly viscous ionic liquid electrolyte seemed to effectively form a barrier to the bromide ions, to protect the metal thin film from them when in the voltage-off state.
1. Introduction
An ordinary silver mirror, coated on its back surface with silver, reflects light to produce high quality images by reflection, owing to the high reflectivity of silver. Recently, tunable mirrors, including liquid–liquid interfacial mirrors, have started to gather attention due to their high applicability for use as smart windows, light modulators, and chemical sensors.1–3 On the other hand, reversible electrochemical mirrors (REMs) are designed to modulate their reflectance from a highly reflective state, enough to mirror a subject, to a highly transparent state, according to external stimuli such as electricity, light, or heat.4–6 A number of REMs have been suggested, including metallic thin films,7,8 conducting polymers,9–11 metal hydrides,12–14 and colloidal electrochemical devices.15 None, however, has reached widespread practical application because of critical problems such as the poor stability of the mirror state and a lack of bistability in reflectance. Thus, there remain very important challenges for developing switchable silver mirrors with long memory effects, to afford bistable reversible electrochemical mirrors (BREMs).Previous studies have employed electrochemical deposition of a metal (e.g. Cu, Ag, Bi, etc.) onto a transparent conducting substrate to achieve a reflective state.16–19 The optical properties of these REMs are switched according to the redox states of the metals, as well as their morphologies. Thus, much effort has been directed towards improving the properties of REMs, especially for Ag film-based electrochemical devices,8,20 with the addition of Cu ions that stabilize deposited Ag nanoparticles.17 However, no studies have yet reported on the stabilization of the mirror state over a long period, either in the electricity-on or -off state.
In electrochemical metallic mirrors, the reversible reflectance change originates from the electrodeposition of the Ag film, to achieve a mirror state, and the dissolution of Ag as an ion into an electrolyte, to achieve a transparent state. While the transparent state is quite stable, the mirror state is unstable because the deposited Ag film is dissolved into the electrolyte solution as anions diffuse into the metallic film at the open-circuit state. To maintain the mirror state, it is necessary to apply a reduction voltage continuously to avoid the dissolution of Ag film into the electrolyte. However, Ag nanoparticles with aggregated structures and poor adhesion onto a substrate (e.g. ITO) generally yield cracks and wrinkles on the metal films under a prolonged supply of electrical charge. Therefore, it is important to stabilize the metallic film during the mirror formation.21
The ultimate goal of these studies is to obtain bistability in switchable mirrors—i.e., stability in both the reflective and transparent states—particularly when the electrical power is turned off. This bistable status is critical in order for new switchable mirrors to have technological promise for applications such as optic devices, memory, as well as energy saving smart windows. The poor bistability of REMs originates from the oxidation of metallic silver to soluble AgBrn(1−n) then to Ag(I), as described well in the literature.22,23 High concentrations of halides increase the rate of Ag dissolution.24 To protect the metal film from anions, we attempted to introduce an electrical double layer (EDL), which can block ion diffusion.25,26 Highly capacitive ionic liquids (ILs) have been successfully used to stabilize metal nanoparticles27,28 or electrochemical devices.29–31 Herein, we report an electrochemically stable and bistable reversible electrochemical mirror (BREM) for the first time, by using reversible silver deposition on a thiol-modified ITO electrode in ionic liquids as the electrolyte media.
2. Experimental
2.1 Materials
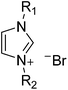
Dimethyl sulfoxide (DMSO), silver nitrate (AgNO3), copper chloride (CuCl2), (3-mercaptopropyl)trimethoxysilane (MPTMS), 1-methyl imidazole, 1-butyl imidazole, bromoethane, 1-bromohexane, tetrabutylammonium bromide (TBABr), and polyvinyl butyral (PVB, Butvar® B-98) were purchased from Aldrich. All chemicals were used as received. 1-Methyl-4-hexylimidazolium bromide (MHImBr), 1-butyl-4-ethylimidazolium bromide (BEImBr), and 1-butyl-4-hexylimidazolium bromide (BHImBr) were synthesized and purified according to previous reports.32–34 The structures of synthesized ILs are listed in Table 1.2.2 Preparation of surface-modified ITO electrodes (TI)
The surface-modified ITO electrode was prepared by a previously reported procedure.35 In brief, an ITO electrode was cleaned by scrubbing with a soft cloth and sonication in ethanol and acetone for at least 10 min each, and then dried under nitrogen. To get a sufficient amount of the surface treatment reagent on the ITO electrode, hydroxyl groups were formed by treating the bare ITO glass surface with oxygen plasma. The surface of the cleaned ITO glass was modified by oxygen plasma treatment for 5 min with a power of 6.8 W (CUTE-MP, Femto Science, USA). Then, the plasma-treated ITO electrode was placed in a vacuum chamber with a few drops of MPTMS, and then evacuated for 1 h.2.3 Preparation of electrochemical silver mirrors
The electrochemical mirrors consist of an electrolyte between the surface-modified ITO glass (TI) and the bare ITO (UI) electrode. The TBABr based electrolyte solution (TBAB) was prepared as follows: 0.5 mmol of AgNO3, 0.1 mmol of CuCl2 and 2.5 mmol of TBABr were dissolved in 11 g of DMSO with 1.2 g of PVB as the host polymer. The ionic liquid-based electrolyte solutions (MHIB, BEIB, and BHIB) were prepared by dissolving 0.5 mmol of AgNO3 and 0.1 mmol of CuCl2 in 10 wt% of DMSO and 45 mmol of MHImBr, BEImBr and BHImBr, respectively. The prepared electrolyte was carefully coated on to the bare ITO electrode with a polyimide spacer of 500 μm thickness and a window size of 2.0 × 2.0 cm2, and then assembled with a surface-modified ITO electrode.2.4 Measurement
Electrochemical measurements for the ionic liquids and prepared REMs were recorded using a universal potentiostat [model CHI 624B (CH Instruments, Inc.)]. UV-Vis. spectra were obtained using a PerkinElmer Lambda750 UV/Vis/NIR Spectrophotometer. The figures of merit for reflectance and thickness were calculated by dividing reflectance recorded at 650 nm (%) and thickness (nm) by consumed charge density (C cm−2). SEM images and EDS mapping were obtained using a JEOL-JSM-7001F, and TEM images were obtained using a JEOL-JEM-2100. TEM sample (Fig. S1c†) of the Ag/Cu alloy particle was obtained by collecting alloy particles from the sonication of the electrodeposited Ag and Cu electrode. The resistances and capacitances of the ILs were determined from an impedance analyzer (Ivium B08016, Ivium technology) as a function of frequency (from 10 to 105 Hz) using simulation software (Zview 2.8d, Scribner Associates Inc.). A 50 μm thick IL layer was sandwiched between two ITO electrodes and a laminated top silver contact. For obtaining confocal fluorescence images, the thiol-modified ITO glass was cut into a 5 cm × 1 cm piece. Then the two ITO glasses were separated by 500 μm by a polyimide tape spacer and adhered by a hot-melt adhesive. The electrolytes, TBAB and BEIB, containing a fluorophore (pyrene 1%) were injected between the ITO glasses and then covered with cover glasses. A confocal microscope (Axio Imager Z2, LSM 700, Carl Zeiss) was used for obtaining images of the side view. A laser (wavelength 405 nm) was used as the excitation source for pyrene.3. Results and discussion
3.1 Electrochemical stability of the REM device
A highly reflective mirror with a smooth surface was obtained from an electrolyte solution containing AgNO3 and CuCl2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
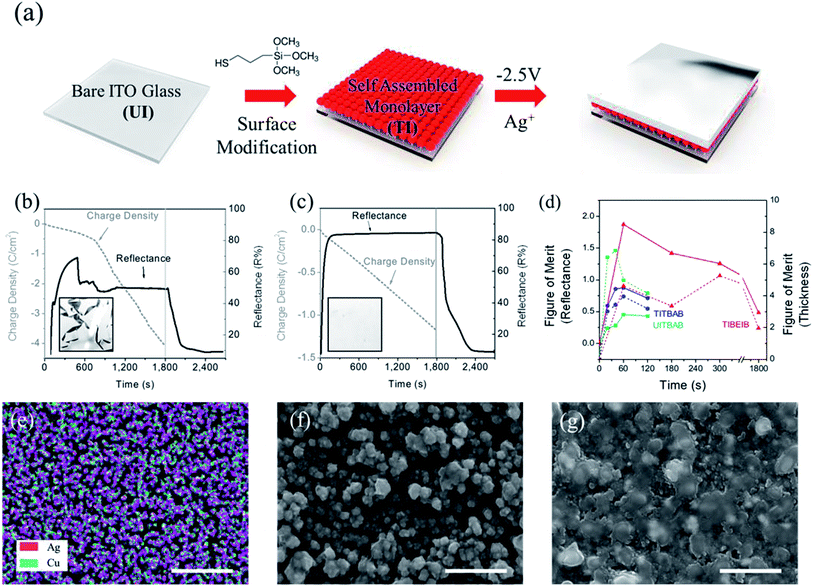
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio), which was optimized according to the previous study.20 The other components of the electrolyte were tetrabutylammonium bromide (TBABr) and polyvinyl butyral (PVB) in dimethyl sulfoxide (DMSO), as described in the experimental section. This electrolyte composition is abbreviated as TBAB hereafter. The distribution of metallic nanoparticles, formed upon application of −2.5 V on a 2-electrode REM device, was relatively homogeneous and denser in the presence of Cu2+ than without it. In the SEM images, along with EDS mapping, the homogeneous distribution of the Ag–Cu bimetallic films was clearly observed (Fig. 1e). This improved morphology of the metallic thin film is attributed to the fact that Cu induces aggregation of Ag particles. The Cu content on the electrodeposited Ag–Cu surface was determined as ∼24% of the Ag content from EDS mapping, which was a smaller amount than in the feed, possibly due to the charge difference between the two atoms (Table S1†).
1 molar ratio), which was optimized according to the previous study.20 The other components of the electrolyte were tetrabutylammonium bromide (TBABr) and polyvinyl butyral (PVB) in dimethyl sulfoxide (DMSO), as described in the experimental section. This electrolyte composition is abbreviated as TBAB hereafter. The distribution of metallic nanoparticles, formed upon application of −2.5 V on a 2-electrode REM device, was relatively homogeneous and denser in the presence of Cu2+ than without it. In the SEM images, along with EDS mapping, the homogeneous distribution of the Ag–Cu bimetallic films was clearly observed (Fig. 1e). This improved morphology of the metallic thin film is attributed to the fact that Cu induces aggregation of Ag particles. The Cu content on the electrodeposited Ag–Cu surface was determined as ∼24% of the Ag content from EDS mapping, which was a smaller amount than in the feed, possibly due to the charge difference between the two atoms (Table S1†).
A thiol-modified electrode (TI) was prepared by anchoring the plasma-treated ITO electrode with (3-mercaptopropyl)trimethoxysilane (MPTMS) (Fig. 1a). The metallic film was grown on TI upon application of a reduction potential (−2.5 V), using the solution of TBAB (Fig. S1d–f†). It was noteworthy that the deposited metallic film was denser on the surface-modified ITO (TI) than on the bare unmodified ITO (UI), which can be observed in Fig. S1d and e† and can be inferred from the figures of merit for the thicknesses of TITBAB and UITBAB (Fig. 1d). The average roughness of the Ag film on UI was 66 nm, which is twice that on TI (33 nm), as compared in Fig. S2.† This effect can be explained by the strong bonds between thiol groups and the metal ions. When TI is prepared with MPTMS, the trimethoxysilane groups are anchored onto the surface hydroxyl groups of the ITO electrode, while the terminal thiols are left for metal interaction. These surface thiols could enable the formation of strong interactions with the deposited Ag and Cu metals. Thus, the electrodeposited metal film on TI is more stable, denser and has lower roughness than that on UI.
The REM with TI as the working electrode, using the TBAB electrolyte (TITBAB), showed a characteristic cathodic current as the potential moved from zero to the negative direction (Fig. S3†). When the reduction potential reached −2.5 V, the formation of a reflective mirror was observed in the device, as metal ions were reduced. On the other hand, with the application of a potential in the positive direction, the anodic current appeared and peaked at +0.2 V. This anodic peak should correspond to the oxidation of the electrodeposited metal particles, because the transmittance of the cell was increased by the dissolution of metallic particles into electrolyte solution. The REM returned to its initial transparent state upon application of >1.0 V for less than 1 min, upon oxidation of Cu1+ to Cu2+, which mediates the oxidation of the Ag.36 The electro-reflectance change of TITBAB was similar to that of the REM using an unmodified ITO (UITBAB), which is the same mirror system reported in the literature,20 except for the electrochemical stability, described below.
Although it was possible to switch between the reflective and transparent states in UITBAB and TITBAB, the mirror state disappeared when the electricity was disconnected, possibly because the deposited Ag film dissolved into the solution immediately. To maintain the mirror state, a continuous reduction potential (−2.5 V) should be applied to the REM. As shown in Fig. 1b and c, the Ag–Cu metallic film formed on TI (TITBAB) showed a dramatic enhancement in its long-term electrochemical stability. The reflectance increased up to 82% within 3 min, and it was maintained constantly with the prolonged application of −2.5 V for 30 min. The metallic film was also stable, showing a highly reflective state without rupture, as shown in Fig. 1c. On the other hand, the metallic film grown on the bare electrode (UITBAB) showed instability after a few minutes at −2.5 V. The metallic films were ruptured, and the reflectance dropped significantly, as shown in Fig. 1b, and Movie S1.† Also, the charge density recorded with the application of −2.5 V increased abruptly when the metallic film was ruptured in the UITBAB, while the charge density in the TITBAB increased linearly.
Therefore, the ITO surface modification with thiol groups, which is well known to stabilize silver,21 significantly improved the electrochemical stability of the REM device, resulting in a stable mirror status. The reflectance of the TITBAB was higher than that of UITBAB, as shown in Fig. 1b and c, due to the formation of a highly dense metallic film in the TITBAB compared to the UITBAB, as described above. The result indicates that the surface modification afforded a viable method for a dramatic enhancement of long-term stability as well as high reflectance. Thus, the surface-treated electrodes were found to be essential for long-term electrochemical stability, and they were used in further experiments.
After metal deposition, the mirror state in the UITBAB disappeared immediately after the applied voltage was shut off. The mirror state in the TITBAB was maintained longer than in the UITBAB, possibly due to the denser film, but it lasted only 60 s after the electricity was disconnected. In switchable mirrors, the open-circuit memory property—i.e., the ability to maintain the mirror state without further energy consumption—is hard to achieve, although this is commonly observed for at least an hour in other electrochemical color switching systems (e.g., electrochromism).37–40
3.2 Bistable reversible electrochemical mirror
The low open-circuit memory in switchable mirrors is inevitable because the electrodeposited metal particles are designed to be dissolved in electrolytes containing excess halide ions, to achieve reversible switching. The mechanism for the Ag dissolution is based on the initial oxidation of metallic silver to form soluble AgBrn(1−n) then to Ag(I), as described well in the literature.22,23 There is a possibility that chemisorbed Br− ions may catalytically dissolve silver by oxygen dissolved in the electrolyte.41 However, the effect of this oxidative dissolution is negligible due to the low gas solubility in ionic liquids.42 The soluble anionic complexes (AgX2− and AgX32−, where X is Cl, Br, or I) have relative stabilities of I− > Br− > Cl− and their formation is accelerated under high concentrations of halides to increase the rate of Ag dissolution.24 Therefore, the mirror state rapidly disappears in the open circuit, accompanied by dissolution and diffusion of the electrodeposited mirror whenever Br anions are available.To protect the metal film in the open-circuit state, we introduced an electrical double layer (EDL), which can block anion diffusion into the metallic film. A cationic layer was reported to expel anions from the electrode.25 Therefore, we used an ionic liquid (IL) as an electrolyte for the switching mirrors, taking advantage of the large specific capacitances of ILs. In addition, the nonvolatile, nonflammable, and highly viscous natures of ILs confer advantages on REMs.30 The structures of the ILs and their electrochemical properties are summarized in Table 1.
| R1 | R2 | Name | Electrolyte resistance | Charge transport resistance | Capacitance | Constant phase element | χ 2 | |
|---|---|---|---|---|---|---|---|---|
| (Re) [Ω] | (Rct) [Ω] | (C) [μF] | (Q) [μF] | |||||
| a The equivalent circuit consisted of electrolyte resistance (Re), charge transport resistance (Rct), capacitance (C), and constant phase element (Q) for measuring the properties of the ionic liquids (the value in parentheses is for electrolytes containing AgNO3 and CuCl2 ions). | ||||||||
| 1 | Methyl- | n-Hexyl- | 1-Methyl-4-hexylimidazolium bromide (MHImBr) | 33.6 (24.1) | 14.9 (15.2) | 57.0 (69.1) | 13.0 (13.1) | 0.16 (0.10) |
| 2 | n-Butyl- | n-Ethyl- | 1-Butyl-4-ethylimidazolium bromide (BEImBr) | 29.2 (18.3) | 14.8 (13.6) | 78.0 (79.2) | 12.0 (11.8) | 0.11 (0.10) |
| 3 | n-Butyl- | n-Hexyl- | 1-Butyl-4-hexylimidazolium bromide (BEImBr) | 51.4 (26.4) | 17.1 (16.5) | 45.0 (62.7) | 12.0 (13.2) | 0.19 (0.11) |
| 4 | Tetrabutylammonium bromide (TBABr) 0.25 M solution in DMSO | 23.8 (17.3) | 7.15 (18.9) | 164.3 (81.7) | 11.8 (12.7) | 0.05 (0.10) | ||
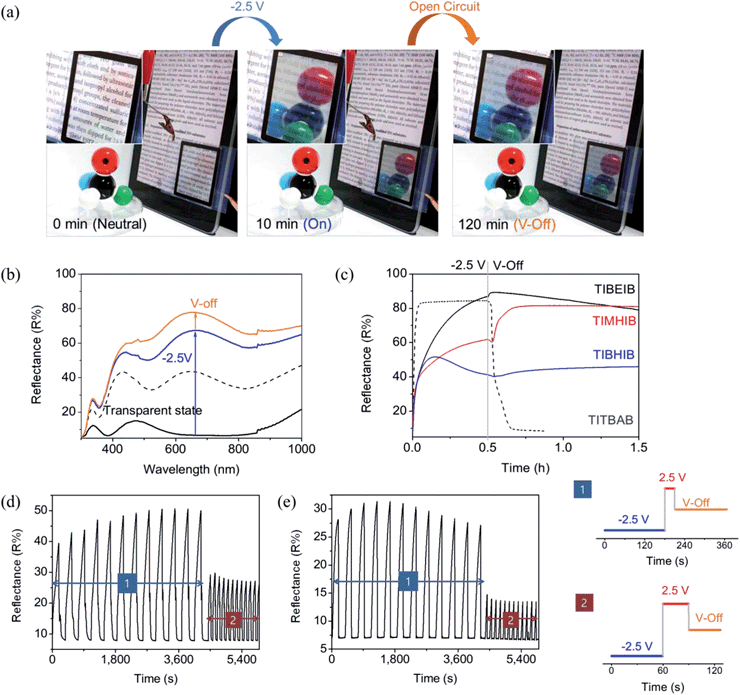
After metal deposition, the mirror state in the TIBEIB disappeared immediately upon application of a positive potential (>2.0 V), indicating an electrochemically switchable mirror similar to the TITBAB. The metallic mirror formation was accompanied by concomitant spectral growth from the UV to the IR region, as shown in Fig. 2b for the TIBEIB. Surprisingly, the reflectance of the TIBEIB was maintained after electricity was disconnected (V-off). The reflectance of the TIBEIB, following application of −2.5 V for 30 min, had a slight further increase to 89.3% immediately after switching to the V-off state (Fig. 2c, black solid line). The reflectance increase of the TIBEIB immediately in the V-off state may be attributed to the additional reduction of Ag–Cu due to the immediate polarization change. Importantly, even after 2 hours in the V-off state, the high reflectance was maintained (Movie S2†). Fig. 2c shows the memory effect (recorded at 650 nm) of the switching mirrors in different ILs having different alkyl chain lengths. In all of the ILs, the reflectance of the mirrors was maintained for longer than 30 min in the V-off state. The reflectance of the TIMHIB, which used MHImBr as an IL, increased to almost 80% immediately after the electricity was disconnected, and the memory effect was longer than 2 h (Fig. 2a) without energy consumption or any physical damage.
The mirror switching in an ionic liquid containing BEImBr, abbreviated as TIBEIB, was observed similarly, as expected from its similar CV to the TITBAB (Fig. S3†). The Cu content on the electrodeposited Ag–Cu surface from TIBEIB was determined as ∼24% of the Ag content, which was almost the same as that in UITBAB and TITBAB (Table S1†), as determined from EDS mapping (Fig. S1g†). Although the use of ILs enabled dramatically increased bistability without a loss in the reflectance of the device, the growth of the metallic film in ILs was slower than with TITBAB, possibly due to the highly viscous natures and high capacitances of ILs (Fig. 2c). But interestingly, the figure of merit for the thickness of TIBEIB was similar to that of TITBAB. Moreover, the figure of merit for reflectance was two times larger in the REM with ILs than with TBAB (Fig. 1d). The mechanism for Ag film deposition can be explained by the reaction of the Ag+ ions with Br− ions to form AgBrn(1−n) (n = 2–4), which is then reduced to give Ag metal on an electrode. It has been reported that immidazolium cations can stabilize AgBrn(1−n) and metallic granules.28,43,44 This stabilizing interaction could generate a dense film and thus result in a high figure of merit for reflectance in the mirrors using ILs.
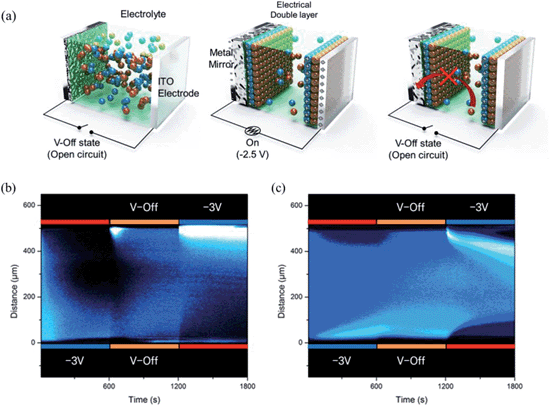
Fig. 3a shows a schematic diagram for the working mechanism of a bistable reversible electrochemical mirror (BREM), where Br− diffusion onto the metal film is forbidden due to the electrical double layer (EDL) developed during the switch-on process, allowing the bistability of the switching mirror. The growth of the metallic film, the switching response, and the reflectance increase at V-off were dependent on the alkyl chain lengths of the ionic liquids. Nonetheless, all of the mirrors in the EDL conditions showed long bistability. The EDL is immediately removed upon application of the reverse potential (oxidation) so that the reversible reaction toward the transparent state can be achieved repeatedly and reliably. Thus, the mirror state of the TIBEIB switched to a transparent state only when an oxidation potential was applied (Fig. S4 and S5†). The mirror with the IL was reversibly switched between the mirror and transparent states (Movie S3†) by an alternating potential cycle, as shown in Fig. 2d and e. Table S2† summarizes the electro-reflectance changes of the mirrors in this study.
In order to gain further insight on the bistability, we examined the ion transport in TITBAB and TIBEIB with a confocal microscope (Fig. 3b and c and S6†). Pyrene was used to elucidate the ion transport, taking advantage of its strong interactions with metal cations,45 which mean it would move in the same direction as metal ions did. As shown in Fig. S6a,† the fluorescence intensities of the TITBAB and TIBEIB were higher near the working electrode, forming a layer of pyrene crowding when the working electrode was applied with a reduction voltage (−3 V). Pyrenes (with metal ions) moved toward the working electrode over time and formed a ∼150 μm fluorescent layer after 10 min at −3 V, as clearly shown in Fig. 3b and c, and S6a and b.† Interestingly, the fluorescent layer was quite stable in the TIBEIB, while it diffused entirely into the electrolyte in the TITBAB at V-off within 10 min. These results verify that there is no ion transfer from the metallic film (coated at the working electrode upon reduction) into the electrolyte, and vice versa, in TIBEIB at V-off. On the other hand, the metallic films are dissolved as ions, which return into electrolyte in the TITBAB at V-off. The localized fluorescent layer in the TIBEIB was fully diffused only when an oxidation potential (+3 V) was applied as seen in Fig. 3c. The growth and diffusion of the fluorescent layer, and thus ion transfer, are more visible in Movies S4 and S5.†
4. Conclusions
An electrochemically stable and bistable electrochemical mirror was achieved for the first time by introducing (1) a thiol-modified ITO electrode for the stabilization of the Ag–Cu metallic film and (2) ionic liquids as an anion-blocking layer to achieve bistability in the switching mirror. Although the timescale on which this transition occurs in the BREM is rather slow (several min) at present, there appears to be considerable room for improvement through the choice of ionic liquids and organically-modified electrodes. In view of their long memory effects and electrochemical stabilities, the switchable mirrors could find numerous applications, such as smart windows for energy saving buildings and automobiles, wireless heaters, reflective displays, and switchable mirrors for optical systems.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2007-0056091).Notes and references
- V. A. Turek, M. P. Cecchini, J. Paget, A. R. Kucernak, A. A. Kornyshev and J. B. Edel, ACS Nano, 2012, 6, 7789–7799 CrossRef CAS PubMed.
- P.-P. Fang, S. Chen, H. Deng, M. D. Scanlon, F. Gumy, H. J. Lee, D. Momotenko, V. Amstutz, F. Cortés-Salazar, C. M. Pereira, Z. Yang and H. H. Girault, ACS Nano, 2013, 7, 9241–9248 CrossRef CAS PubMed.
- J. B. Edel, A. A. Kornyshev and M. Urbakh, ACS Nano, 2013, 7, 9526–9532 CrossRef CAS PubMed.
- R. A. M. Hikmet and H. Kemperman, Nature, 1998, 392, 476–479 CrossRef CAS PubMed.
- D. M. Tench and L. F. Warren Jr., US Pat., 6552843, 2003.
- A. Llordes, G. Garcia, J. Gazquez and D. J. Milliron, Nature, 2013, 500, 323–326 CrossRef CAS PubMed.
- S. Zaromb and J. Y. Chang, J. Electrochem. Soc., 1962, 109, 1034–1040 CrossRef CAS PubMed.
- S. Zaromb, J. Electrochem. Soc., 1962, 109, 903–912 CrossRef CAS PubMed.
- H. Shin, Y. Kim, T. Bhuvana, J. Lee, X. Yang, C. Park and E. Kim, ACS Appl. Mater. Interfaces, 2012, 4, 185–191 CAS.
- T. Bhuvana, B. Kim, X. Yang, H. Shin and E. Kim, Nanoscale, 2012, 4, 3679–3686 RSC.
- T. Bhuvana, B. Kim, X. Yang, H. Shin and E. Kim, Angew. Chem., Int. Ed., 2013, 52, 1180–1184 CrossRef CAS PubMed.
- J. N. Huiberts, R. Griessen, J. H. Rector, R. J. Wijngaarden, J. P. Dekker, D. G. de Groot and N. J. Koeman, Nature, 1996, 380, 231–234 CrossRef CAS.
- A. T. M. van Gogh, E. S. Kooij and R. Griessen, Phys. Rev. Lett., 1999, 83, 4614–4617 CrossRef CAS.
- J. L. Slack, J. C. W. Locke, S.-W. Song, J. Ona and T. J. Richardson, Sol. Energy Mater. Sol. Cells, 2006, 90, 485–490 CrossRef CAS PubMed.
- J. N. Richardson, Z. Aguilar, N. Kaval, S. E. Andria, T. Shtoyko, C. J. Seliskar and W. R. Heineman, Electrochim. Acta, 2003, 48, 4291–4299 CrossRef CAS PubMed.
- C. M. Lampert, Mater. Today, 2004, 7, 28–35 CrossRef CAS.
- C. O. Avellaneda, M. A. Napolitano, E. K. Kaibara and L. O. S. Bulhões, Electrochim. Acta, 2005, 50, 1317–1321 CrossRef CAS PubMed.
- G. A. Niklasson and C. G. Granqvist, J. Mater. Chem., 2007, 17, 127–156 RSC.
- M. Nakashima, T. Ebine, M. Shishikura, K. Hoshino, K. Kawai and K. Hatsusaka, ACS Appl. Mater. Interfaces, 2010, 2, 1471–1482 CAS.
- S. Araki, K. Nakamura, K. Kobayashi, A. Tsuboi and N. Kobayashi, Adv. Mater., 2012, 24, OP122–OP126 CAS.
- M. J. Esplandiu and H. Hagenström, Solid State Ionics, 2002, 150, 39–52 CrossRef CAS.
- A. Gupta, M. Maynes and S. Silver, Appl. Environ. Microbiol., 1998, 64, 5042–5045 CAS.
- C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry and G. E. Brown, Environ. Sci. Technol., 2013, 47, 5738–5745 CrossRef CAS PubMed.
- X. Li, J. J. Lenhart and H. W. Walker, Langmuir, 2010, 26, 16690–16698 CrossRef CAS PubMed.
- M. V. Fedorov, N. Georgi and A. A. Kornyshev, Electrochem. Commun., 2010, 12, 296–299 CrossRef CAS PubMed.
- M. V. Fedorov and A. A. Kornyshev, Chem. Rev., 2014, 114, 2978–3036 CrossRef CAS PubMed.
- W. Dobbs, J.-M. Suisse, L. Douce and R. Welter, Angew. Chem., 2006, 118, 4285–4288 CrossRef.
- R. Fukui, Y. Katayama and T. Miura, J. Electrochem. Soc., 2011, 158, D567–D572 CrossRef CAS PubMed.
- W. Lu, A. G. Fadeev, B. Qi, E. Smela, B. R. Mattes, J. Ding, G. M. Spinks, J. Mazurkiewicz, D. Zhou, G. G. Wallace, D. R. MacFarlane, S. A. Forsyth and M. Forsyth, Science, 2002, 297, 983–987 CrossRef CAS PubMed.
- J. H. Cho, J. Lee, Y. He, B. S. Kim, T. P. Lodge and C. D. Frisbie, Adv. Mater., 2008, 20, 686–690 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Q. Q. Baltazar, J. Chandawalla, K. Sawyer and J. L. Anderson, Colloids Surf., A, 2007, 302, 150–156 CrossRef CAS PubMed.
- Y. Sun and L. Shi, Fuel, 2012, 99, 83–87 CrossRef CAS PubMed.
- A. S. Borges, J. D. L. Dutra, R. O. Freire, R. T. Moura, J. G. Da Silva, O. L. Malta, M. H. Araujo and H. F. Brito, Inorg. Chem., 2012, 51, 12867–12878 CrossRef CAS PubMed.
- A. Doron, E. Katz and I. Willner, Langmuir, 1995, 11, 1313–1317 CrossRef CAS.
- A. Tsuboi, K. Nakamura and N. Kobayashi, Adv. Mater., 2013, 25, 3197–3201 CrossRef CAS PubMed.
- R. D. Rauh, Electrochim. Acta, 1999, 44, 3165–3176 CrossRef CAS.
- J. Kim, J. You, B. Kim, T. Park and E. Kim, Adv. Mater., 2011, 23, 4168–4173 CrossRef CAS PubMed.
- E. Kim, J. Korean Soc. Imaging Sci. Technol., 2013, 19, 39–49 CrossRef.
- S. Seo, H. Shin, C. Park, H. Lim and E. Kim, Macromol. Res., 2013, 21, 284–289 CrossRef CAS PubMed.
- A. Henglein, J. Phys. Chem., 1993, 97, 5457–5471 CrossRef CAS.
- Z. Lei, C. Dai and B. Chen, Chem. Rev., 2014, 114, 1289–1326 CrossRef CAS PubMed.
- D. G. Foster, Y. Shapir and J. Jorne, J. Electrochem. Soc., 2005, 152, C462–C465 CrossRef CAS PubMed.
- N. Serizawa, Y. Katayama and T. Miura, J. Electrochem. Soc., 2009, 156, D503–D507 CrossRef CAS PubMed.
- J. H. Lee, E. R. Carraway, M. A. Schlautman, S. Yim and B. E. Herbert, J. Photochem. Photobiol., A, 2004, 167, 141–148 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4sc01912a |
| This journal is © The Royal Society of Chemistry 2015 |