Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stoichiometric to catalytic reactivity of the aryl cycloaurated species with arylboronic acids: insight into the mechanism of gold-catalyzed oxidative C(sp2)–H arylation†
Qian
Wu
,
Chenglong
Du
,
Yumin
Huang
,
Xingyan
Liu
,
Zhen
Long
,
Feijie
Song
and
Jingsong
You
*
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, West China Medical School, Sichuan University, 29 Wangjiang Road, Chengdu 610064, PR China. E-mail: jsyou@scu.edu.cn; jingbolan@scu.edu.cn; Fax: +86-28-85412203
First published on 9th October 2014
Abstract
Based on the well-defined five-membered aryl gold(III) complexes, [Au(tpy)X2] (3a and 3b) and [AuBr(Ph)(tpy)] (7), as well as the aryl gold(III) complex [AuCl2(Ph)(tpy)] (8) (tpy = 2-(o-tolyl)pyridine) as reliable models, we present a detailed study of the mechanism for gold(III)-catalyzed oxidative cross-coupling reactions between cycloaurable arenes and arylboronic acids. Here we report the direct evidence for a mechanistic proposal including arene C–H activation, transmetallation and biaryl reductive elimination. The chelation-assisted C–H activation strategy has been used for the development of the gold(III)-catalyzed C–H bond arylation of arenes with aryl reagents to forge extended π-conjugated systems.
Introduction
Gold-catalyzed organic transformations have been placed under the spotlight during the past decade. Gold salts and complexes are most commonly used as a redox-neutral, carbophilic π acid for the activation of carbon–carbon multiple bonds such as alkynes, alkenes, and allenes towards nucleophilic attack.1 In contrast, the gold-catalyzed oxidative coupling reactions proposed to involve Au(I)/Au(III) cycles are still underrepresented.2 In particular, gold-catalyzed C–H bond arylation of simple arenes to forge biaryl scaffolds is still in its infancy. In addition to a few examples of catalytic homo-coupling of simple arenes,3 stoichiometric homo- and hetero-coupling processes have nearly exclusively dominated the previous literature.4–6 It has proven to be a challenge to evolve a stoichiometric hetero-coupling between an unactivated arene and an aromatic nucleophile to a catalytic version. The only known catalytic scenario is the oxidative cross-coupling reaction between arylsilanes and simple arenes recently demonstrated by Lloyd-Jones, Russell and Ball.7,8 The development of such transformations may encounter two major obstacles: (1) the relatively high redox potential of the Au(I)/Au(III) couple (E0 = +1.41 V) traditionally precludes the catalytic cycles;9 and (2) there are considerable uncertainties in the reaction mechanism, due in part to the difficulties of accessing appropriate aryl Au(III) models and the use of a strong external oxidant that complicates the catalytic system.In 2008, the seminal work of Gouverneur and co-workers demonstrated the potential of electrophilic fluorinating reagents in oxidizing Au(I) to Au(III) species.10 Subsequently, substantial progress has been made in the gold-catalyzed oxidative heteroarylation (e.g. oxyarylation and aminoarylation) of alkenes with various arylating reagents, using electrophilic fluorinating reagents as oxidants,11 in which alkylgold(III) fluorides have been proposed as the intermediates. These gold(III) species undergo a bimolecular reductive elimination with arylboronic acid (devoid of transmetallation) by a concerted process wherein B–F and C–C bonds are formed simultaneously (Scheme 1, left).11a,11c,11e,12 In this work, we wish to establish well-defined aryl gold(III) models, to comprehensively understand their elementary reactivities with arylboronic acids and gain further insight into the mechanism of gold-catalyzed oxidative C(sp2)–H arylation, which will benefit the maturation of catalytic biaryl couplings involving a gold(I)/gold(III) redox cycle.
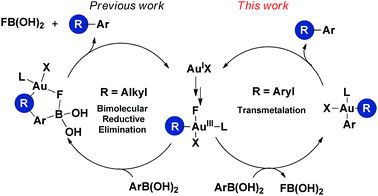 | ||
| Scheme 1 Reactivities of alkylgold(III) (left) and arylgold(III) (right) species with arylboronic acid. | ||
Gold actually has a rich history in organometallic chemistry. As early as 1931, a seminal work by Kharasch and Isbell illustrated the C–H bond activation of arenes with gold(III) chloride to form aryl gold(III) complexes.13 Thereafter, a variety of aryl gold(III) complexes were prepared by C–H bond activation14,15 and transmetallation reactions with Au(III) species.16 Inspired by chelation-assisted transition-metal catalyzed C–H bond arylation of arenes,17,18 we envisaged that the installation of a coordinating group on the aromatic ring would help us further understand the fundamental chemistry of aryl gold(III) reactivity, particularly involving redox cycling. The introduction of a coordinating unit would have the following advantages: (1) the extra chelation could help activate the C(sp2)–H bond to form a cyclometalated Au(III) complex and synchronously stabilize the resulting Au(III) species; and (2) more importantly, the fixed coordinating group may partially avoid associative ligand/anion exchange, facilitating discrimination of the active Au(III) species. Thus, the well-defined aryl cycloaurated complexes would allow us to gain more insights into the mechanism of transformations.
Results and discussion
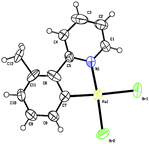
The gold-catalyzed aryl C–H bond functionalization via a chelation-assisted strategy has never been reported before. Given that the 2-phenylpyridine derivatives are the most commonly used scaffolds for investigating transition metal-catalyzed directed C–H activation of arenes,17a–c,e–f we herein took 2-(o-tolyl)pyridine 1a as a molecular model to illuminate both the stoichiometric and catalytic reactivities19 of aryl gold(III) species. Dihaloauracycles like [Au(py)X2] can be easily prepared by the cycloauration of the corresponding arenes with HAuCl4 or NaAuCl4 by Constable and Leese,20 and later by other researchers.21 By a modification of the above methods, stoichiometric AuX3 (X = Cl, Br) was first chosen to react with 1a in t-BuOH at ambient temperature. The resulting coordination compounds 2a and 2b were heated in a mixture of acetonitrile and water at 130 °C, affording the five-membered cis-aryl gold(III) complexes [Au(tpy)X2] 3a and 3b, respectively (eqn (1)). The cyclometalated Au(III) structure of 3b was demonstrated by X-ray crystallographic analysis (Fig. 1).† Similar to other cycloaurated [Au(C^N)Cl2] species,16a,22 complexes 3a and 3b are exceedingly stable towards water and air.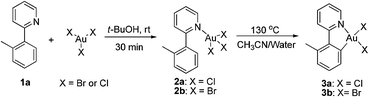 | (1) |
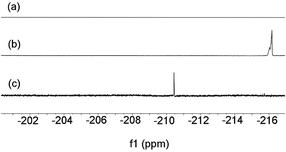
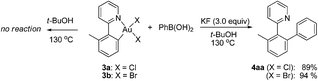
With the isolated monometallic cis-aryl gold(III) complexes 3a and 3b in hand, we wish to explore both stoichiometric and catalytic reactivities with arylboronic acids. A series of stoichiometric experiments was first conducted. The cis-aryl gold(III) complexes 3a and 3b proved to be unreactive upon treatment with phenylboronic acid in t-BuOH at 130 °C. Encouraged by the role of fluoride in the gold-catalyzed oxidative heteroarylation of alkenes,11 potassium fluoride was added to the reaction mixture. Fortunately, the desired product 4aa was obtained in excellent yields (for 3a, 89%; for 3b, 94%) (eqn (2)). To clarify the role of the fluoride anion, we subsequently investigated the reaction of 3a with phenylboronic acid in the presence of either t-BuOK or KOH instead of KF. The coupled product 4aa was obtained in 84% and 87% yields in t-BuOK and KOH, respectively. The 19F NMR spectra of a mixture of 3a and KF, either in the presence or absence of phenylboronic acid in DMSO-d6 solution, did not exhibit the presence of a Au(III)–F bond, which indicated that anion exchange between Cl− or Br− and F− did not occur (Fig. 2a). On the basis of the above observations, we rationalized that the fluoride anion might perform as a Lewis base to cooperatively activate the C–B bond and further promote the cleavage of the C–B bond in the transmetallation, which was similar to that of the transition metal-catalyzed oxidative coupling reactions involving arylboronic acids.23
 | (2) |
 | (3) |
 | (4) |
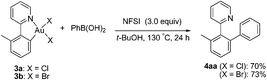
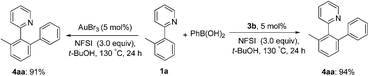
Next, we attempted to develop a catalytic version by using an external oxidant to access a gold(I)/gold(III) catalytic cycle. It is known that electrophilic fluorinating reagents and iodine(III) oxidants could efficiently accomplish the oxidation of Au(I) to Au(III). In combination with the role of the fluoride anion as a Lewis base mentioned above, we herein concentrated on the fluorine-containing oxidants. The stoichiometric gold(III) complexes 3a and 3b could react with phenylboronic acid (3.0 equiv.) to form the biaryl 4aa in 70% (X = Cl) and 73% (X = Br) yields in the presence of N-fluorobenzenesulfonimide (NFSI) (eqn (3)), whereas PhI(OAc)2 failed to promote the coupling reaction (Table S1,† entry 11). Treatment of 3b with phenylboronic acid and Selectfluor also produced 4aa in 72% yield. Importantly, both the cyclometalated gold(III) complex 3b (5 mol%) and AuBr3 (5 mol%) could catalyze the reaction between 1a and phenylboronic acid to form the cross-coupled 4aa in 94% and 91% yields, respectively, which hinted that the five-membered aryl gold(III) species might be a plausible intermediate in the catalytic cycle (eqn (4)). In addition, the yield of 4aa was reduced to 58% in the presence of AuBr3 (5 mol%) when the amounts of phenylboronic acid were decreased from 3.0 equiv. to 1.0 equiv. GC-MS analysis of the stoichiometric reaction of 3a with PhB(OH)2 in the presence of NFSI showed the formation of biphenyl. Further 19F NMR spectrum analysis of a mixture of NFSI and t-BuOH demonstrated a new signal at −39.3 ppm (19F NMR of NSFI: −169.2 ppm). These observations implied the fluoride equivalents might be produced from the reduction of NFSI by phenylboronic acid and/or t-BuOH in the reaction system.
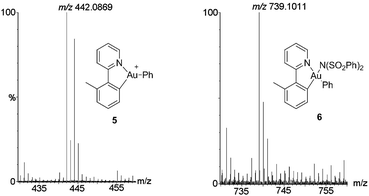
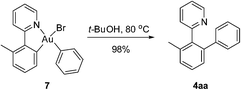
To further clarify the biaryl gold(III) intermediates, the ESI-HRMS analysis of the reactions of 3b or 1a/AuBr3 with phenylboronic acid was performed in the presence of NSFI. Fortunately, the peaks at m/z 442.0869 and 739.1011, respectively, corresponding to the characteristic patterns of the five-membered biaryl gold(III) species 5 ([M]+, MW 442.0870) and 6 ([M + H]+, MW 739.1000) were observed,24 which suggested a transmetallation process from boronic acid to gold(III) (Fig. 3). Despite this evidence, attempts to separate the intermediate from the reaction system failed, probably due to the rapid reductive elimination at 130 °C. As an alternative, we turned to the synthesis of the cyclometalated biaryl gold(III) species [AuBr(Ph)(tpy)] (7), allowing direct observation of the reaction intermediate. By a modified Tilset method,25 the sequential reaction of 1a with Au(OAc)3 and PhMgBr afforded complex 7, which was determined by single crystal X-ray diffraction (Fig. 4).† [AuBr(Ph)(tpy)] could give the hetero-coupled product 4aa in 98% yield in t-BuOH at 80 °C (eqn (5)), showing that the biaryl reductive elimination step was similar to those of traditional palladium and rhodium-catalyzed biaryl coupling reactions. This observation also clearly disclosed that the presence of the Au(III)–F bond was not indispensable in the reductive elimination step and was distinguished from the reactivity of alkylgold(III) species with arylboronic acid (Scheme 1, left). Considering that the catalytic reaction required a high reaction temperature of 130 °C (eqn (4)) and failed to give the arylated product 4aa at 80 °C, it was suggested that reductive elimination might not be the rate-determining step.
 | (5) |
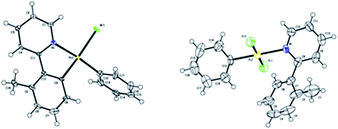 | ||
| Fig. 4 ORTEP diagrams of 7 (left) and 8 (right). Thermal ellipsoids are shown at the 50% probability level. | ||
As the gold-catalyzed cross-coupling reaction between an arene and an arylboronic acid was demonstrated, this raised the question of whether the arene C–H auration preceded the C–B auration or vice versa. The reaction of [AuCl2(Ph)(tpy)] (8) could not discriminate between the two. The gold(III) complex 8 could be prepared by the modified method of Fuchita et al.15d and Limbach et al.,26 involving the auration of benzene with AuCl3 and subsequent coordination with 1a. The structure of complex 8 was demonstrated by X-ray crystallographic analysis (Fig. 4).† As a result, the stoichiometric reaction of 8 in t-BuOH did not afford the cross-coupled product either in the presence or absence of NFSI (eqn (6)). These results not only indicated that the C–B auration occurred after cyclometallation, but also precluded the possibility of C–B activation before the Au(I) was oxidized to Au(III).
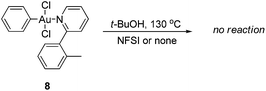 | (6) |
To elucidate whether a Au(III)–F bond was formed in the catalytic cycle, the 19F NMR spectra were investigated. The 19F NMR spectrum of a mixture of AuCl and NSFI in t-BuOH at 130 °C exhibited a signal of −216.2 ppm (Fig. 2b). Indeed, AuCl (5.0 mol%) as the pre-catalyst could promote the reaction of 1a with phenylboronic acid to produce 4aa albeit in a lower yield of 38%. The catalytic system of 1a and PhB(OH)2 showed a 19F NMR peak at 210.4 ppm (Fig. 2c). These results suggested that the Au(III)–F bond might be generated in the catalytic reaction.27
Subsequently, the H/D exchange experiments were performed. Exposure of 2-(o-tolyl)pyridine 1a to t-BuOD did not lead to the deuterated product, either in the presence or absence of phenylboronic acid, which indicated that the directed cleavage of the ortho C–H bond was actually an irreversible process (eqn (7)). A small H/D kinetic isotope effect (KIE) of 0.96 for two separate reactions with 1a and [D]-1a revealed that the C(sp2)–H bond cleavage was not involved in the rate-determining step (eqn (8)).28 The observed kH/kD value implied that the formation of a C–H(D) sigma–agostic complex might be rate limiting.29
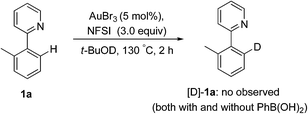 | (7) |
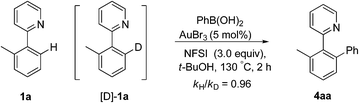 | (8) |
Based on the above observations, a plausible mechanistic pathway of the gold(III)-catalyzed directed C–H bond arylation of an arene with an arylboronic acid was proposed. First, the intermediate IM1 was formed by the rapid coordination of a gold(III) species with 1a, followed by an irreversible C–H bond auration to give the five-membered cycloaurated species IM2. The transmetallation from boronic acid to gold(III) center might involve both scenarios TS1 and TS2.16d,16e,30 In TS1, the Au(III)–F bond formed via an oxidation of Au(I)X by NFSI11a–c,12 activated the C–B bond through a four-membered transition state (Fig. 2, right). In TS2, the non-coordinated fluoride anion promoted the cleavage of the C–B bond. In the coupling reaction, NFSI not only served as a strong oxidant, but also offered a fluoride source to promote the transmetallation process. The biaryl gold(III) intermediate IM3 underwent a reductive elimination to generate the desired biaryl product. Finally, the released gold(I) species was reoxidized by NFSI to complete the catalytic cycle (Scheme 2).
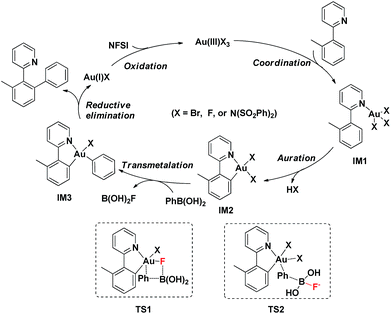 | ||
| Scheme 2 Proposed mechanism of gold(III)-catalyzed C(sp2)–H bond arylation with arylboronic acid via a chelation-assisted strategy. | ||
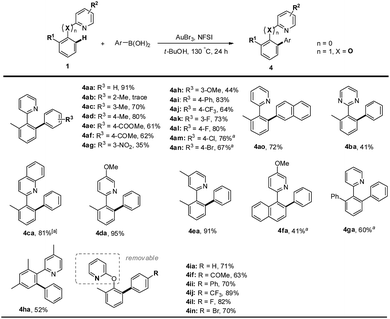
Finally, we sought to examine the scope of this protocol with respect to both arylboronic acids and arenes (Scheme 3).31 We were pleased to observe that a series of meta- or para-substituted phenylboronic acids smoothly reacted with 2-(o-tolyl)pyridine 1a to deliver the heterocoupled products in medium to excellent yields. However, the ortho-substituted phenylboronic acid gave a trace amount of product, presumably due to the steric effect (4ab). Furthermore, arylboronic acids bearing either electron-rich (4ac, 4ad and 4ah) or electron-deficient (4ae–ag and 4aj–al) substituents proceeded smoothly. Other biaryls containing N-heteroarenes such as pyridine, quinoline and pyrimidine also underwent cross-coupling reactions with phenylboronic acid. The gold(III)-catalyzed oxidative reaction was very tolerant of halogen groups such as Cl and Br (4am, 4an and 4in). In addition to heteroaryl-aryls proposed to form a five-membered auracycle, 2-(o-tolyloxy)pyridine also went through the arene C–H arylation with a variety of arylboronic acids (4ia–4in), which might involve a six-membered auracycle. The pyridyl group of the resulting biaryls could be removed to afford the 1,1′-biphenyl-2-ol derivatives.32
Conclusions
In summary, taking advantage of chelation-assisted C–H bond activation, we have established the well-defined five-membered aryl gold(III) complexes [Au(tpy)X2] (3a and 3b) and [AuBr(Ph)(tpy)] (7), as well as the aryl gold(III) complex [AuCl2(Ph)(tpy)] (8), as models for a comprehensive insight into the mechanism of gold-catalyzed oxidative C–H arylation of arenes. The stoichiometric behaviors of the complexes with arylboronic acids provide straightforward access to propel our nascent understanding of the immature redox chemistry of gold catalysts. Observable arene C–H activation, transmetallation and biaryl reductive elimination have provided direct evidence for a mechanistic hypothesis. In this work, the chelation-assisted C–H activation strategy has been used for the development of the gold(III)-catalyzed oxidative cross-coupling reactions between arenes and arylating reagents. Compared with the works of Lloyd-Jones and Russell,7,8 the present work demonstrates the possibility of a different catalytic cycle in which (1) the auration reaction step could be replaced by a cycloauration reaction if the arene has an appropriate ortho-substituent, (2) the arylboronic acids can be used instead of aryl silanes as transmetallating agents, and (3) the oxidant can be NFSI instead of iodine(III) oxidant.Acknowledgements
This work was supported by grants from the National Basic Research Program of China (973 Program, 2011CB808601), and the National NSF of China (nos 21432005, 21025205, 21202105, 21272160, 21321061 and J1103315/J0104).Notes and references
- For selected reviews on redox-neutral gold catalysis, see: (a) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (c) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS PubMed; (d) R. A. Widenhoefer, Chem. - Eur. J., 2008, 14, 5382 CrossRef CAS PubMed; (e) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (f) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS PubMed; (g) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (h) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208 RSC; (i) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed; (j) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (k) D.-H. Zhang, X.-Y. Tang and M. Shi, Acc. Chem. Res., 2014, 47, 913 CrossRef CAS PubMed.
- For selected reviews on gold-catalyzed oxidative couplings, see: (a) H. A. Wegner and M. Auzias, Angew. Chem., Int. Ed., 2011, 50, 8236 CrossRef CAS PubMed; (b) M. N. Hopkinson, A. D. Gee and V. Gouverneur, Chem. - Eur. J., 2011, 17, 8248 CrossRef CAS PubMed.
- For gold-catalyzed homo-coupling of simple arenes, see: (a) A. Kar, N. Mangu, H. M. Kaiser, M. Beller and M. K. Tse, Chem. Commun., 2008, 386 RSC; (b) A. Kar, N. Mangu, H. M. Kaiser and M. K. Tse, J. Organomet. Chem., 2009, 694, 524 CrossRef CAS PubMed.
- For stoichiometric gold salt mediated homo-coupling of heteroarenes, see: (a) E. C. Constable and L. R. Sousa, J. Organomet. Chem., 1992, 427, 125 CrossRef CAS; (b) A. K. Sahoo, Y. Nakamura, N. Aratani, K. S. Kim, S. B. Noh, H. Shinokubo, D. Kim and A. Osuka, Org. Lett., 2006, 8, 4141 CrossRef CAS PubMed.
- For stoichiometric hetero-coupling processes, see: (a) J. Vicente, M. D. Bermúdez, J. Escribano, M. P. Carrillo and P. G. Jones, J. Chem. Soc., Dalton Trans., 1990, 3083 RSC; (b) J. Vicente, M. D. Bermúdez and J. Escribano, Organometallics, 1991, 10, 3380 CrossRef CAS; (c) J. Vicente, M. D. Bermúdez and F. J. Carrión, Inorg. Chim. Acta, 1994, 220, 1 CrossRef CAS; (d) J. Vicente, M.-D. Bermúdez, F.-J. Carrión and P. G. Jones, Chem. Ber., 1996, 129, 1395 CrossRef CAS; (e) X. C. Cambeiro, T. C. Boorman, P. Lu and I. Larrosa, Angew. Chem., Int. Ed., 2013, 52, 1781 CrossRef CAS PubMed; (f) M. Hofer and C. Nevado, Tetrahedron, 2013, 69, 5751 CrossRef CAS PubMed.
- For exceptionally fast aryl–aryl bond reductive elimination from oxidized gold center to form homocoupled products, see: W. J. Wolf, M. S. Winston and F. D. Toste, Nat. Chem., 2014, 6, 159 CrossRef CAS PubMed.
- L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, Science, 2012, 337, 1644 CrossRef CAS PubMed.
- L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, J. Am. Chem. Soc., 2014, 136, 254 CrossRef CAS PubMed.
- S. G. Bratsch, J. Phys. Chem. Ref. Data, 1989, 18, 1 CrossRef CAS PubMed.
- (a) M. Schuler, F. Silva, C. Bobbio, A. Tessier and V. Gouverneur, Angew. Chem., Int. Ed., 2008, 47, 7927 CrossRef CAS PubMed; (b) M. N. Hopkinson, A. Tessier, A. Salisbury, G. T. Giuffredi, L. E. Combettes, A. D. Gee and V. Gouverneur, Chem. - Eur. J., 2010, 16, 4739 CrossRef CAS PubMed.
- For selected examples, see: (a) W. E. Brenzovich Jr, D. Benitez, A. D. Lackner, H. P. Shunatona, E. Tkatchouk, W. A. Goddard III and F. D. Toste, Angew. Chem., Int. Ed., 2010, 49, 5519 CrossRef PubMed; (b) G. Zhang, L. Cui, Y. Wang and L. Zhang, J. Am. Chem. Soc., 2010, 132, 1474 CrossRef CAS PubMed; (c) A. D. Melhado, W. E. Brenzovich Jr, A. D. Lackner and F. D. Toste, J. Am. Chem. Soc., 2010, 132, 8885 CrossRef CAS PubMed; (d) G. Zhang, Y. Luo, Y. Wang and L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 4450 CrossRef CAS PubMed; (e) E. Tkatchouk, N. P. Mankad, D. Benitez, W. A. Goddard III and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 14293 CrossRef CAS PubMed; (f) L. T. Ball, G. C. Lloyd-Jones and C. A. Russell, Chem. - Eur. J., 2012, 18, 2931 CrossRef CAS PubMed; (g) B. Sahoo, M. N. Hopkinson and F. Glorius, J. Am. Chem. Soc., 2013, 135, 5505 CrossRef CAS PubMed.
- N. P. Mankad and F. D. Toste, J. Am. Chem. Soc., 2010, 132, 12859 CrossRef CAS PubMed.
- M. S. Kharasch and H. S. Isbell, J. Am. Chem. Soc., 1931, 53, 3053 CrossRef CAS.
- T. C. Boorman and I. Larrosa, Chem. Soc. Rev., 2011, 40, 1910 RSC.
- For selected examples of stoichiometric intermolecular auration of (hetero)arenes with Au(III) by C–H bond activation, see: (a) K. S. Liddle and C. Parkin, J. Chem. Soc., Chem. Commun., 1972, 26a RSC; (b) P. W. J. de Graaf, J. Boersma and G. J. M. van der Kerk, J. Organomet. Chem., 1976, 105, 399 CrossRef CAS; (c) E. C. Constable, R. P. G. Henney, P. R. Raithby and L. R. Sousa, Angew. Chem., Int. Ed. Engl., 1991, 30, 1363 CrossRef; (d) Y. Fuchita, Y. Utsunomiya and M. Yasutake, J. Chem. Soc., Dalton Trans., 2001, 2330 RSC.
- For selected examples of the preparation of aryl Au(III) complexes by transmetallation reactions with Au(III) species, see: (a) J. Vicente and M. T. Chicote, Inorg. Chim. Acta, 1981, 54, L259 CrossRef; (b) J. Vicente, M. T. Chicote, A. Arcas, M. Artigao and R. Jimenez, J. Organomet. Chem., 1983, 247, 123 CrossRef CAS; (c) J. Vicente, M.-T. Chicote, M. D. Bermúdez, M. J. Sanchez-Santano, P. G. Jones, C. Fittschen and G. M. Sheldrick, J. Organomet. Chem., 1986, 310, 401 CrossRef CAS; (d) D.-A. Roşca, D. A. Smith and M. Bochmann, Chem. Commun., 2012, 48, 7247 RSC; (e) D. A. Smith, D.-A. Roşca and M. Bochmann, Organometallics, 2012, 31, 5998 CrossRef CAS.
- For selected reviews relative to the transition-metal catalyzed chelation-assisted C(sp2)–H bond arylation, see: (a) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS PubMed; (b) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677 RSC; (c) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (d) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (e) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (f) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed.
- For selected examples on the palladium-catalyzed C–H bond arylation of arenes with directing groups, see: (a) D. Kalyani, N. R. Deprez, L. V. Desai and M. S. Sanford, J. Am. Chem. Soc., 2005, 127, 7330 CrossRef CAS PubMed; (b) S. Yang, B. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 6066 CrossRef CAS PubMed; (c) D.-H. Wang, T.-S. Mei and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 17676 CrossRef CAS PubMed; (d) N. R. Deprez and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 11234 CrossRef CAS PubMed; (e) T. Nishikata, A. R. Abela and B. H. Lipshutz, Angew. Chem., Int. Ed., 2010, 49, 781 CrossRef CAS PubMed; (f) B. Xiao, Y. Fu, J. Xu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef CAS PubMed; (g) C. S. Yeung, X. Zhao, N. Borduas and V. M. Dong, Chem. Sci., 2010, 1, 331 RSC; (h) M. J. Tredwell, M. Gulias, N. G. Bremeyer, C. C. C. Johansson, B. S. L. Collins and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 1076 CrossRef CAS PubMed; (i) J.-H. Chu, H.-P. Huang, W.-T. Hsu, S.-T. Chen and M.-J. Wu, Organometallics, 2014, 33, 1190 CrossRef CAS.
- For an example of the five- and six-membered cycloaurated complex-catalyzed synthesis of propargylic amines, chiral allenes and isoxazoles, see: K. K.-Y. Kung, V. K.-Y. Lo, H.-M. Ko, G.-L. Li, P.-Y. Chan, K.-C. Leung, Z. Zhou, M.-Z. Wang, C.-M. Che and M.-K. Wong, Adv. Synth. Catal., 2013, 355, 2055 CrossRef CAS.
- E. C. Constable and T. A. Leese, J. Organomet. Chem., 1989, 363, 419 CrossRef CAS.
- (a) R. V. Parish, Met.-Based Drugs, 1999, 6, 271 CrossRef CAS PubMed; (b) W. Henderson, B. K. Nicholson, S. J. Faville, D. Fan and J. D. Ranford, J. Organomet. Chem., 2001, 631, 41 CrossRef CAS; (c) L. Pazderski, T. Pawlak, J. Sitkowski, L. Kozerski and E. Szłyka, Magn. Reson. Chem., 2009, 47, 932 CrossRef CAS PubMed.
- (a) J. Vicente, M. T. Chicote and M. D. Bermúdez, Inorg. Chim. Acta, 1982, 63, 35 CrossRef CAS; (b) J. Vicente, M. T. Chicote and M. D. Bermúdez, J. Organomet. Chem., 1984, 268, 191 CrossRef CAS; (c) J. Vicente, M.-D. Bermúdez, F.-J. Carrión and P. G. Jones, Chem. Ber., 1996, 129, 1301 CrossRef CAS.
- (a) S. Kirchberg, R. Fröhlich and A. Studer, Angew. Chem., Int. Ed., 2009, 48, 4235 CrossRef CAS PubMed; (b) L. Chu and F.-L. Qing, Org. Lett., 2010, 12, 5060 CrossRef CAS PubMed; (c) B. Liu, X. Qin, K. Li, X. Li, Q. Guo, J. Lan and J. You, Chem. - Eur. J., 2010, 16, 11836 CrossRef CAS PubMed; (d) J. Xu, B. Xiao, C.-Q. Xie, D.-F. Luo, L. Liu and Y. Fu, Angew. Chem., Int. Ed., 2012, 51, 12551 CrossRef CAS PubMed.
- (a) H. Zhang, W. Pu, T. Xiong, Y. Li, X. Zhou, K. Sun, Q. Liu and Q. Zhang, Angew. Chem., Int. Ed., 2013, 52, 2529 CrossRef CAS PubMed; (b) G. B. Boursalian, M.-Y. Ngai, K. N. Hojczyk and T. Ritter, J. Am. Chem. Soc., 2013, 135, 13278 CrossRef CAS PubMed.
- For the microwave synthesis of Au(OCOCF3)2(tpy), see: E. Langseth, C. H. Görbitz, R. H. Heyn and M. Tilset, Organometallics, 2012, 31, 6567 CrossRef CAS.
- S. Lavy, J. J. Miller, M. Paĕický, A.-S. Rodrigues, F. Rominger, C. Jäkel, D. Serra, N. Vinokurov and M. Limbach, Adv. Synth. Catal., 2010, 352, 2993 CrossRef CAS.
- E. Bernhardt, M. Finze and H. Willner, J. Fluorine Chem., 2004, 125, 967 CrossRef CAS PubMed.
- (a) M. Gómez-Gallego and M. A. Sierra, Chem. Rev., 2011, 111, 4857 CrossRef PubMed; (b) E. M. Simmons and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 3066 CrossRef CAS PubMed.
- For selected reviews, see: (a) A. D. Ryabov, Chem. Rev., 1990, 90, 403 CrossRef CAS; (b) W. D. Jones, Acc. Chem. Res., 2003, 36, 140 CrossRef CAS PubMed; (c) D. Balcells, E. Clot and O. Eisenstein, Chem. Rev., 2010, 110, 749 CrossRef CAS PubMed . For selected examples, see: ; (d) M. Lavin, E. M. Holt and R. H. Crabtree, Organometallics, 1989, 8, 99 CrossRef CAS; (e) A. Vigalok, O. Uzan, L. J. W. Shimon, Y. Ben-David, J. M. L. Martin and D. Milstein, J. Am. Chem. Soc., 1998, 120, 12539 CrossRef CAS; (f) B. Rybtchinski, R. Cohen, Y. Ben-David, J. M. L. Martin and D. Milstein, J. Am. Chem. Soc., 2003, 125, 11041 CrossRef CAS PubMed; (g) D. L. Davies, S. M. A. Donald and S. A. Macgregor, J. Am. Chem. Soc., 2005, 127, 13754 CrossRef CAS PubMed; (h) S. H. Crosby, G. J. Clarkson and J. P. Rourke, J. Am. Chem. Soc., 2009, 131, 14142 CrossRef CAS PubMed; (i) W.-B. Liu, C. Zheng, C.-X. Zhuo, L.-X. Dai and S.-L. You, J. Am. Chem. Soc., 2012, 134, 4812 CrossRef CAS PubMed.
- M. D. Levin and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 6211 CrossRef CAS PubMed.
- For optimization of reaction conditions, see Table S1.†.
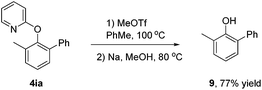
- The pyridyl group of 4ia could be removed to afford 9 in 77% yield. See: L. Ackermann, E. Diers and A. Manvar, Org. Lett., 2012, 14, 1154
.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, analytical data. CCDC 1000469, 1007212 and 1019879. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc02070g |
| This journal is © The Royal Society of Chemistry 2015 |