Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biphen[n]arenes†
Huanqing
Chen‡
a,
Jiazeng
Fan‡
a,
Xiaoshi
Hu
b,
Junwei
Ma
a,
Shilu
Wang
a,
Jian
Li
a,
Yihua
Yu
b,
Xueshun
Jia
a and
Chunju
Li
*ac
aDepartment of Chemistry, Shanghai University, Shanghai, 200444, P. R. China. E-mail: cjli@shu.edu.cn
bShanghai Key Laboratory of Magnetic Resonance, Department of Physics, East China Normal University, Shanghai, 200062, P. R. China
cBeijing National Laboratory for Molecular Sciences (BNLMS), Beijing, 100190, P. R. China
First published on 17th September 2014
Abstract
To design and exploit novel macrocyclic synthetic receptors is a permanent and challenging topic in supramolecular chemistry. Here we describe the one-pot synthesis, unique geometries and intriguing host–guest properties of a new class of supramolecular macrocycles – biphen[n]arenes (n = 3, 4), which are made up of 4,4′-biphenol or 4,4′-biphenol ether units linked by methylene bridges at the 3- and 3′- positions. The biphenarene macrocycles are conveniently accessible/modifiable and extremely guest-friendly. Particularly, biphen[4]arene is capable of forming inclusion complexes with not only organic cationic guests but also neutral π-electron deficient molecules. Compared with calixarenes, resorcinarenes, cyclotriveratrylenes and pillararenes with substituted mono-benzene units, the biphen[n]arenes reported here possess significantly different characteristics in both their topologic structures and their recognition properties, and thus can find broad applications in supramolecular chemistry and other areas.
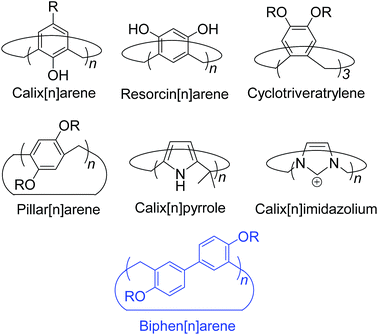
Introduction
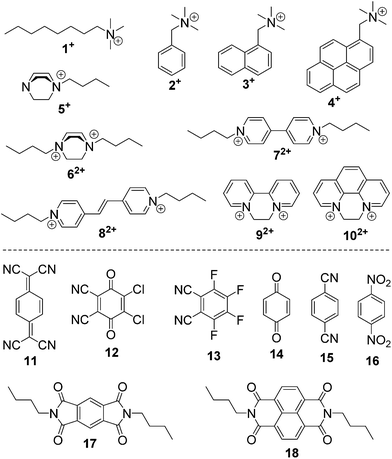
Macrocyclic synthetic receptors bearing preorganized cavities and multivalent binding sites have played a vital role in the birth of modern supramolecular chemistry and its rapid development.1,2 To design and exploit novel macrocyclic hosts with unique structures and good host–guest properties is a permanent and challenging topic in this area. Some new molecular containers recently reported include Sessler's “Texas-sized” box,3 Chun and Singh's calix[4]imidazolium,4 Ogoshi's pillar[5]arene,5 Stoddart's Ex-box,6 Sindelar's bambus[6]uril,7 Flood's cyanostar,8 and others.9,10 Among the supramolecular hosts, macrocyclic arenes based on methylene linked aromatic rings have been the focus of considerable recent research. Starting from calixarenes, the third generation of supramolecular hosts, a series of their structurally similar scaffolds have also been developed, displaying different geometries and molecular recognition/self-assembly behaviors (Scheme 1).4,5,11–13 For example, calixpyrroles, calixpyridines and the recently reported caliximidazoliums show considerable promise in the area of anion complexation and sensing.4,11 Cyclotriveratrylenes have been utilized for the complexation and separation of fullerenes.12,14 Pillararenes with symmetrical pillar architectures have exhibited novel binding abilities towards neutral guests.15 Furthermore, these macrocycles have also seeded many potential applications in biology, and materials and environmental science such as drug delivery,16 extraction and separation,14,17 stimuli responsive materials,18 and artificial transmembrane channels.19Hitherto, most of the macrocyclic arenes have been based on mono-benzene and mono-heterocycle units (Scheme 1). Typical supramolecular macrocycles consisting of substituted benzene monomers include calixarene from phenol, resorcinarene from resorcinol, cyclotriveratrylene from veratrole, and pillararene from hydroquinone. Additionally, calixnaphthalenes20 have also been demonstrated, but they have not gained as much attention because (i) they do not show good cavity host–guest properties; (ii) their structures are not novel and are similar to calixarenes; and (iii) their synthesis is not easy and usually needs multi-step reactions.
Herein, we report the synthesis, structures, and molecular binding behavior of a new family of macrocyclic arenes, which are made up of 4,4′-biphenol or 4,4′-biphenol ether units linked by methylene bridges at the 3- and 3′- positions (Scheme 1). According to the naming convention of resorcin[4]arenes (based on resorcinol monomers), this new family of supramolecular macrocycles is named as biphen[n]arenes. The biphenarene hosts designed here could be conveniently achieved by a one-pot Lewis acid-catalyzed condensation from commercial reagents, and they are expected, and have been found, to have extraordinary architectures and intriguing binding properties.
Results and discussion
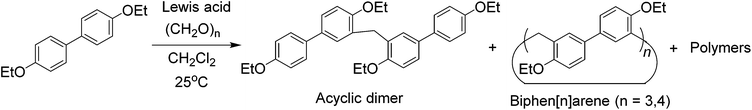
For the purpose of easy preparation, a direct cyclization strategy but not a fragment coupling approach was preferentially adopted in the present studies. It was found that the reactions of 4,4′-biphenol diethyl ether and paraformaldehyde (or formaldehyde aqueous solution) in the presence of a strong base (e.g. NaOH and KOH) or strong acid (e.g. HCl, H2SO4 and CF3COOH) could not give any cyclic oligomers. Nevertheless, when using a Lewis acid, such as FeCl3, BF3·O(Et)2, and trifluoromethanesulfonic acid (TfOH), as the catalyst, the reactions proceeded smoothly, and one acyclic dimer and two cyclic oligomers containing 3 and 4 biphenol diethyl ether units were successfully obtained (Scheme 2). After several attempts, BF3·O(Et)2 proved to be a little more efficient than FeCl3 and TfOH. After optimisation of the reaction conditions in the presence of BF3·O(Et)2 with respect to reaction solvent, temperature, and catalyst amount, the acyclic dimer (BPD), per-ethylated biphen[3]arene (EtBP3) and biphen[4]arene (EtBP4) were prepared in 9%, 22% and 8% yields, respectively. Furthermore, another larger macrocycle with a m/z value corresponding to the cyclic pentamer was also detected in the high-resolution mass spectrometry (HRMS) experiments of the reaction mixture. However, its yield was so poor that it was not successfully isolated for further characterization. The reaction time significantly affects the product yields. It was found that the best reaction time was 1.5–2 hours; further extending the time decreased the yields of both EtBP3 and EtBP4 and increased the yield of the polymeric product. For example, after 24 hours, the yields of EtBP3 and EtBP4 were only 5% and 1%. Furthermore, the condensation of 4,4′-biphenol dimethyl ether was also examined. Similarly, the acyclic dimer (yield: 12%), cyclic trimer (per-methylated biphen[3]arene, MeBP3, yield: 24%) and cyclic tetramer (per-methylated biphen[4]arene, MeBP4, yield: 5%) were successfully prepared. It should be pointed out that the distributions of the cyclic trimer and the tetramer were totally different in the syntheses of the ethylated and methylated biphenarenes, with yield ratios for trimer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tetramer of 2.8
tetramer of 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4.8
1 and 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
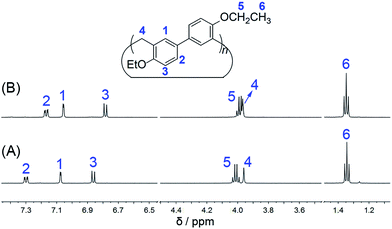
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively. EtBP3 and EtBP4 were characterized by 1H NMR, 13C NMR, and HRMS, as well as by their melting points. They exhibit similar 1H NMR and 13C NMR spectra, as shown in Fig. 1 and S1–S4.†
1 respectively. EtBP3 and EtBP4 were characterized by 1H NMR, 13C NMR, and HRMS, as well as by their melting points. They exhibit similar 1H NMR and 13C NMR spectra, as shown in Fig. 1 and S1–S4.†
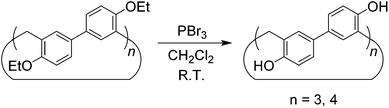
Although the cyclotrimer and cyclotetramer were formed in only moderate yields (30% overall yield), the biphenarene hosts are intrinsically easy to prepare since they can be obtained by a one-step condensation reaction using commercial reagents. It is well documented that the modification of supramolecular hosts by attaching various peripheral functional groups can provide further interesting properties and functionalities. As depicted in Scheme 3, the cleavage of the ether groups in EtBP3 and EtBP4 by reaction with excess BBr3 in CH2Cl2 could quantitatively produce per-hydroxylated biphen[3,4]arenes (OHBP3 and OHBP4). Therefore, it will be straightforward to prepare functionalized biphenarene derivatives through nucleophilic substitution reactions between OHBP3/OHBP4 with alkylating agents in the presence of a suitable base. Besides the hydroxyl groups, the benzene rings of biphenarenes should also be reactive sites. That is to say, biphenarene hosts can be not only easy to prepare, but also facilely chemically modified. This is certainly significant for the further construction of efficient recognition/assembly systems and extending the applications of this new family of macrocycles.
Single crystals of EtBP3 and EtBP4 suitable for X-ray analysis were grown by slow evaporation of their CH2Cl2–n-hexane solutions at room temperature. As can be seen from Fig. 2, their structures are completely different. EtBP3 exhibits a distorted triangular-prism structure and does not have an effective cavity in the solid state (Fig. 2B and C). EtBP4 has a cuboid-like structure and exists in the form of a ‘partial chair’ topology, which is similar to that of the “Texas-sized” box.3 The biphen[4]arene molecular container can be regarded as a new type of neutral molecular box with π-electron rich cavities, which could complement electron-deficient tetracationic boxes such as Stoddart's “blue box”21 and Sessler's “Texas-sized” box.3 It is also interesting to note that the biphenyl units in biphenarenes could exist in two conformations, i.e., a cis- and trans-conformation according to the relative position of the two methylene linkers (Fig. 2A). While all of the three biphenyl units in EtBP3 are in the cis-conformation (Fig. 2B and C), in EtBP4 two of the biphenyl units are in the cis-conformation and two are in the trans-conformation which are positioned in an alternating manner (Fig. 2D and E).
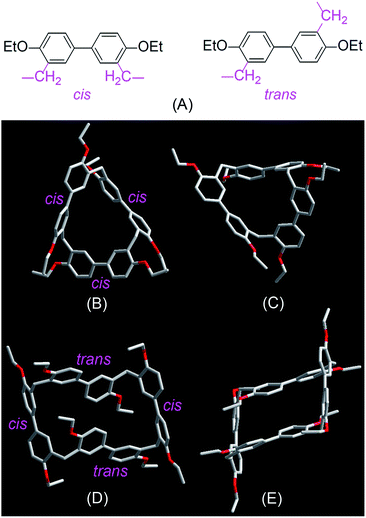 | ||
| Fig. 2 Cis- and trans-conformation of biphenyl monomers in biphenarenes (A) and crystal structures of EtBP3 (B and C) and EtBP4 (D and E). | ||
The host–guest chemistry of EtBP3 and EtBP4 was then investigated. Due to their π-electron rich characteristics, a series of organic cationic molecules (1+–102+) and neutral π-electron deficient molecules (11–18) were chosen as guests (Scheme 4). Fig. 3 shows the 1H NMR spectra of n-octyltrimethyl ammonium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate (1·BArF) in CDCl3 recorded in the absence and in the presence of approximately 1.0 equiv. of the EtBP3/EtBP4 hosts. It is found that in the presence of EtBP4, the proton signals of 1+ derived from the methyl Ha and methylenes exhibit very pronounced upfield displacements (for example, Δδ = −0.99 and −1.11 ppm for Ha and Hb) and broadening as a consequence of inclusion-induced shielding effects (Fig. 3D). Meanwhile, the signal corresponding to the tail methyl (Hi) shifts slightly downfield (Δδ = 0.02 ppm), which is characteristic of the protons being located just outside the host's cavity portal.22 On the other hand, the host is deshielded by the presence of the guest, since the proton signals of EtBP4 display downfield displacement (Δδ = 0.01–0.04 ppm). The binding induced NMR changes are consistent with the formation of an interpenetrated complex. In contrast, upon the addition of EtBP3, no obvious signal changes can be observed for Hc−i of 1·BArF, and the head Ha and Hb show relatively small upfield shifts (−0.05 ppm). These results indicate the formation of a shallow inclusion complex with the guest's “+NMe3” site. This is reasonable since EtBP3 does not possess an effective cavity (Fig. 2B and C). The formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1·BArF⊂EtBP3/EtBP4 complexes was further confirmed by electrospray ionization (ESI) mass spectroscopy experiments (Fig. S38†) and Job plots (Fig. S39†). In the ESI mass spectrum of an equimolar mixture of 1·BArF and EtBP3 (or EtBP4), only one intense peak for the 1
1 1·BArF⊂EtBP3/EtBP4 complexes was further confirmed by electrospray ionization (ESI) mass spectroscopy experiments (Fig. S38†) and Job plots (Fig. S39†). In the ESI mass spectrum of an equimolar mixture of 1·BArF and EtBP3 (or EtBP4), only one intense peak for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex [1⊂EtBP3]+ with m/z 934.6 (or [1⊂EtBP4]+ with m/z 1188.7) was observed. By employing 1H NMR titration experiments, the association constants (Ka) for these two complexes were determined. As expected, the Ka value of 1·BArF⊂EtBP4 ((1.3 ± 0.1) × 103 M−1) is much larger than that for EtBP3 (28 ± 2 M−1) due to their completely different binding characteristics.
1 complex [1⊂EtBP3]+ with m/z 934.6 (or [1⊂EtBP4]+ with m/z 1188.7) was observed. By employing 1H NMR titration experiments, the association constants (Ka) for these two complexes were determined. As expected, the Ka value of 1·BArF⊂EtBP4 ((1.3 ± 0.1) × 103 M−1) is much larger than that for EtBP3 (28 ± 2 M−1) due to their completely different binding characteristics.
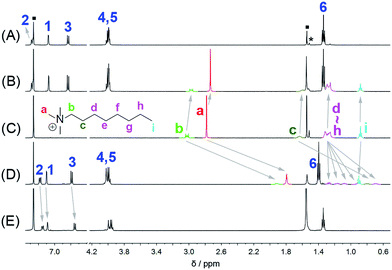 | ||
| Fig. 3 1H NMR spectra (500 MHz, 298 K) of (A) EtBP3, (B) 1·BArF + EtBP3, (C) 1·BArF, (D) 1·BArF + EtBP4, and (E) EtBP4 in CDCl3 at 2.9–3.2 mM. “■” = solvent/water; “*” = solvent impurities. | ||
For another three quaternary ammonium salts 2·BArF–4·BArF, similar complexation modes were found, i.e., they formed interpenetrated [2]pseudorotaxane-type complexes with EtBP4, but shallow inclusion complexes with EtBP3. All four quaternary ammoniums give similar Ka values upon complexation with EtBP3 because their binding sites are “+NMe3” moieties and the substituents do not affect the host–guest affinities. However, for EtBP4, the binding abilities are closely related to the substituted groups since they are engulfed by the host. The Ka values of EtBP4 with 3·BArF and 4·BArF with larger naphthyl and pyrenyl moieties are 3.9 and 3.7 times larger than that for 2·BArF with a phenyl group. This may be attributed to the size-fit effects between the guests and EtBP4; larger naphthyl and pyrenyl groups are relatively suitable for the EtBP4 cavity, leading to large association constants. Meanwhile, 1+ with an n-octyl group exhibits a stronger affinity than 2+, possibly because the flexible octyl group could twist to fit the host cavity.
Among the cationic guests, 5·BArF and 6·2BArF bearing 1,4-diazabicyclo [2.2.2] octane (DBO) cations exhibit the largest binding constants with EtBP4 (of the magnitude of 104 M−1, Table 1), suggesting that spherical DBO moieties are excellent matches in size and shape with EtBP4's cavity (Fig. S40 and S41†). EtBP4 can also form [2]pseudorotaxane-type complexes with pyridinium-based dicationic guests 7·2BArF–10·2BArF (Fig. S43 and S46†); the larger guests 92+ and 102+ show stronger binding strengths than 72+ and 82+ (Table 1). Although EtBP3 cannot bind with 72+ and 82+ (Fig. S42†), it can form shallow inclusion complexes with 92+ and 102+ in such a way that the main binding site for the host is the “+N(CH2)2N+” part (Fig. S44†), which are similar to the complexes formed with the quaternary ammonium guests 1·BArF–4·BArF.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation of the guests with EtBP3/EtBP4 at 298 K
1 complexation of the guests with EtBP3/EtBP4 at 298 K
| Guest | Solventb | EtBP3 | EtBP4 |
|---|---|---|---|
| a The Ka values were determined by NMR titration methods. b Dicationic guests 62+–102+ are not soluble in CDCl3, so their Ka values with the hosts were determined in CD2Cl2. c No interactions were found or at least the association constants were too small (<10 M−1) to be accurately calculated. | |||
| 1+ | CDCl3 | 28 ± 2 | (1.3 ± 0.1) × 103 |
| 2+ | CDCl3 | 29 ± 1 | 570 ± 40 |
| 3+ | CDCl3 | 28 ± 2 | (2.2 ± 0.3) × 103 |
| 4+ | CDCl3 | 26 ± 3 | (2.1 ± 0.2) × 103 |
| 5+ | CDCl3 | (1.5 ± 0.3) × 104 | |
| 62+ | CD2Cl2 | (3.1 ± 0.4) × 104 | |
| 72+ | CD2Cl2 | 92 ± 5 | |
| 82+ | CD2Cl2 | 41 ± 6 | |
| 92+ | CD2Cl2 | 34 ± 4 | 320 ± 30 |
| 102+ | CD2Cl2 | 39 ± 2 | 390 ± 10 |
| 11 | CDCl3 | 61 ± 12 | |
| 12 | CDCl3 | 100 ± 20 | |
| 13–18 | CDCl3 | ||
The complexation of EtBP3 and EtBP4 towards a series of neutral π-electron deficient molecules, 11–18 (Scheme 4), was then examined. In the presence of EtBP4, the peak for the aromatic protons (Ha) of 7,7,8,8-tetracyanoquinodimethane (TCNQ, 11) displays substantial upfield shifts (Δδ = −0.20 ppm) and broadening effects compared to the free guest as a consequence of inclusion-induced shielding effects (Fig. S47†). In contrast, no obvious NMR changes were observed when mixing 11 and EtBP3 (Fig. S46†). These observations reveal that 11 could form an inclusion complex with the larger EtBP4, but cannot bind with the smaller EtBP3. Although 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ, 12) does not have proton signals, its complexation with EtBP4 can be detected according to the signal changes of the host (Fig. S48A†). For the other six neutral guests, 13–18, no effective host–guest interactions with either EtBP3 or EtBP4 were found (Fig. S48–S50†). Although the corresponding Ka values are relatively low, 61 ± 12 M−1 for 11⊂EtBP4 and 100 ± 20 M−1 for 12⊂EtBP4, the complexation of neutral guests are interesting since calixarenes usually cannot form such complexes in organic solutions.
Conclusions
In summary, we have presented a new family of macrocyclic receptors, biphen[n]arenes (n = 3,4). They are made up of 4,4′-biphenol or 4,4′-biphenol ether units linked by methylene bridges at the 3- and 3′- positions. The biphenarene hosts reported here have the following intrinsic characteristics and properties:(i) easy accessibility: per-ethylated biphen[3,4]arenes were prepared through a one-step condensation using commercial reagents; the deprotection of the ethoxy moieties in per-ethylated biphen[3,4]arenes quantitatively produced per-hydroxylated biphen[3,4]arenes;
(ii) convenient modification: it should be possible to conveniently chemically modify them due to their reactive hydroxyl groups and benzene rings;
(iii) unique geometries: their topologic structures are completely different to macrocyclic arenes based on mono-benzene units such as calixarenes, resorcinarenes, cyclotriveratrylenes and pillararenes;
(iv) excellent cavity host–guest properties: biphen[4]arene is extremely guest-friendly, and is capable of binding both cationic guests and neutral molecules to form inclusion complexes.
Considering the above aspects, this new family of hosts is expected to be applicable in a variety of supramolecular systems such as rotaxanes, catenanes and polymeric aggregates. Furthermore, it is easy to afford their functionalized derivatives, making them promising candidates for applications in chemosensors, nanomaterials, ion and molecule transport, supramolecular amphiphiles, and etc. We believe that biphenarene chemistry will be quite prosperous in the near future.
Acknowledgements
This work was supported by the NNSFC (no. 21472122, 21272155 and 21272147), the Innovation Program of Shanghai Municipal Education Commission (no. 13YZ010) and the Beijing National Laboratory for Molecular Sciences.Notes and references
- (a) C. J. Pedersen, Angew. Chem., Int. Ed., 1988, 27, 1021 CrossRef; (b) J.-M. Lehn, Science, 1993, 260, 1762 CAS; (c) D. J. Cram, T. Kaneda, R. C. Helgeson and G. M. Lein, J. Am. Chem. Soc., 1979, 101, 6752 CrossRef CAS; (d) P. R. Ashton, B. Odell, M. V. Reddington, A. M. Z. Slawin, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed., 1988, 27, 1550 CrossRef; (e) W. A. Freeman, W. L. Mock and N. Y. Shih, J. Am. Chem. Soc., 1981, 103, 7367 CrossRef CAS; (f) J. W. Lee, S. Samal, N. Selvapalam, H.-J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS PubMed; (g) D. R. Stewart and C. D. Gutsche, J. Am. Chem. Soc., 1999, 121, 4136 CrossRef CAS; (h) N. Yamaguchi and H. W. Gibson, Angew. Chem., Int. Ed., 1999, 38, 143 CrossRef CAS; (i) G. W. Gokel, W. M. Leevy and M. E. Weber, Chem. Rev., 2004, 104, 2723 CrossRef CAS PubMed; (j) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed.
- (a) A. van Quaethem, P. Lussis, D. A. Leigh, A.-S. Duwez and C.-A. Fustin, Chem. Sci., 2014, 5, 1449 RSC; (b) M. von Delius, E. M. Geertsema and D. A. Leigh, Nat. Chem., 2010, 2, 96 CrossRef CAS PubMed; (c) D. Ma, G. Hettiarachchi, D. Nguyen, B. Zhang, J. B. Wittenberg, P. Y. Zavalij, V. Briken and L. Isaacs, Nat. Chem., 2012, 4, 503 CrossRef CAS PubMed; (d) H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume and A. Harada, Nat. Commun., 2012, 3, 603 CrossRef PubMed; (e) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed; (f) W. M. Nau, Nat. Chem., 2010, 2, 248 CrossRef CAS PubMed; (g) A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603 CrossRef CAS PubMed; (h) X. Yan, S. Li, J. B. Pollock, T. R. Cook, J. Chen, Y. Zhang, X. Ji, Y. Yu, F. Huang and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 15585 CrossRef CAS PubMed; (i) W. Jiang, H. D. F. Winkler and C. A. Schalley, J. Am. Chem. Soc., 2008, 130, 13852 CrossRef CAS PubMed; (j) R. Sun, C. Xue, X. Ma, M. Gao, H. Tian and Q. Li, J. Am. Chem. Soc., 2013, 135, 5990 CrossRef CAS PubMed; (k) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 134, 15712 CrossRef CAS PubMed; (l) D.-S. Guo, V. D. Uzunova, X. Su, Y. Liu and W. M. Nau, Chem. Sci., 2011, 2, 1722 RSC; (m) P. A. Gale and C. Caltagirone, Chem. Soc. Rev. 10.1039/c4cs00179f; (n) S. Hu, J. Li, J. Xiang, J. Pan, S. Luo and J.-P. Cheng, J. Am. Chem. Soc., 2010, 132, 7216 CrossRef CAS PubMed; (o) H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853 CrossRef CAS PubMed; (p) N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426 CrossRef CAS PubMed; (q) G. Ghale, A. G. Lanctôt, H. T. Kreissl, M. H. Jacob, H. Weingart, M. Winterhalter and W. M. Nau, Angew. Chem., Int. Ed., 2014, 53, 2762 CrossRef CAS PubMed.
- (a) H.-Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch and J. L. Sessler, Nat. Chem., 2010, 2, 406 CrossRef CAS PubMed; (b) B. M. Rambo, H.-Y. Gong, M. Oh and J. L. Sessler, Acc. Chem. Res., 2012, 45, 1390 CrossRef CAS PubMed.
- Y. Chun, N. J. Singh, I.-C. Hwang, J. W. Lee, S. U. Yu and K. S. Kim, Nat. Commun., 2013, 4, 1797 CrossRef PubMed.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed.
- (a) J. C. Barnes, M. Juríček, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 183 CrossRef CAS PubMed; (b) M. Juríček, J. C. Barnes, E. J. Dale, W.-G. Liu, N. L. Strutt, C. J. Bruns, N. A. Vermeulen, K. Ghooray, A. A. Sarjeant, C. L. Stern, Y. Y. Botros, W. A. Goddard III and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 12736 CrossRef PubMed.
- J. Svec, M. Necas and V. Sindelar, Angew. Chem., Int. Ed., 2010, 49, 2378 CrossRef CAS PubMed.
- S. Lee, C.-H. Chen and A. H. Flood, Nat. Chem., 2013, 5, 704 CrossRef CAS PubMed.
- (a) C. Ke, H. Destecroix, M. P. Crump and A. P. Davis, Nat. Chem., 2012, 4, 718 CrossRef CAS PubMed; (b) Q. Gan, Y. Ferrand, C. Bao, B. Kauffmann, A. Grélard, H. Jiang and I. Huc, Science, 2011, 331, 1172 CrossRef CAS PubMed; (c) A. Brown, K. M. Mullen, J. Ryu, M. J. Chmielewski, S. M. Santos, V. Felix, A. L. Thompson, J. E. Warren, S. I. Pascu and P. D. Beer, J. Am. Chem. Soc., 2009, 131, 4937 CrossRef CAS PubMed; (d) B.-Y. Wang, X. Bao, Z. Yan, V. Maslak, C. M. Hadad and J. D. Badjić, J. Am. Chem. Soc., 2008, 130, 15127 CrossRef CAS PubMed; (e) S. Liu, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 16798 CrossRef CAS PubMed; (f) C. L. D. Gibb and B. C. Gibb, J. Am. Chem. Soc., 2004, 126, 11408 CrossRef CAS PubMed; (g) H. Zhou, Y. Zhao, G. Gao, S. Li, J. Lan and J. You, J. Am. Chem. Soc., 2013, 135, 14908 CrossRef CAS PubMed; (h) R. Ahmed, A. Altieri, D. M. D'Souza, D. A. Leigh, K. M. Mullen, M. Papmeyer, A. M. Z. Slawin, J. K. Y. Wong and J. D. Woollins, J. Am. Chem. Soc., 2011, 133, 12304 CrossRef CAS PubMed; (i) K. Kondo, A. Suzuki, M. Akita and M. Yoshizawa, Angew. Chem., Int. Ed, 2013, 52, 2308 CrossRef CAS PubMed.
- (a) K. Tashiro and T. Aida, Chem. Soc. Rev., 2007, 36, 189 RSC; (b) M. Hardouin-Lerouge, P. Hudhomme and M. Sallé, Chem. Soc. Rev., 2011, 40, 30 RSC; (c) C.-F. Chen, Chem. Commun., 2011, 47, 1674 RSC; (d) F. Huang, C. Slebodnick, K. A. Switek and H. W. Gibson, Chem. Commun., 2006, 1929 RSC; (e) S. T. Schneebeli, C. Cheng, K. J. Hartlieb, N. L. Strutt, A. A. Sarjeant, C. L. Stern and J. F. Stoddart, Chem.–Eur. J., 2013, 19, 3860 CrossRef CAS PubMed; (f) M. Lisbjerg, B. M. Jessen, B. Rasmussen, B. E. Nielsen, A. Ø. Madsen and M. Pittelkow, Chem. Sci., 2014, 5, 2647 RSC; (g) M.-X. Wang, X.-H. Zhang and Q.-Y. Zheng, Angew. Chem., Int. Ed., 2004, 43, 838 CrossRef CAS PubMed; (h) X.-J. Cheng, L.-L. Liang, K. Chen, N.-N. Ji, X. Xiao, J.-X. Zhang, Y.-Q. Zhang, S.-F. Xue, Q.-J. Zhu, X.-L. Ni and Z. Tao, Angew. Chem., Int. Ed., 2013, 52, 7252 CrossRef CAS PubMed; (i) M. Herm, O. Molt and T. Schrader, Angew. Chem., Int. Ed., 2001, 40, 3148 CrossRef CAS; (j) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS PubMed; (k) L.-Y. You, S.-G. Chen, X. Zhao, Y. Liu, W.-X. Lan, Y. Zhang, H.-J. Lu, C.-Y. Cao and Z.-T. Li, Angew. Chem., Int. Ed., 2012, 51, 1657 CrossRef CAS PubMed.
- (a) P. A. Gale, J. L. Sessler, V. Kral and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS; (b) S. K. Kim, J. L. Sessler, D. E. Gross, C.-H. Lee, J. S. Kim, V. M. Lynch, L. H. Delmau and B. P. Hay, J. Am. Chem. Soc., 2010, 132, 5827 CrossRef CAS PubMed.
- (a) M. J. Hardie, Chem. Soc. Rev., 2010, 39, 516 RSC; (b) Y. Y. Shi, J. T. Yu, Z. T. Huang and Q. Y. Zheng, Sci. China: Chem., 2009, 39, 329 Search PubMed.
- (a) W. Jiang and J. Rebek Jr, J. Am. Chem. Soc., 2012, 134, 17498 CrossRef CAS PubMed; (b) M. Yamanaka, M. Kawaharada, Y. Nito, H. Takaya and K. Kobayashi, J. Am. Chem. Soc., 2011, 133, 16650 CrossRef CAS PubMed; (c) G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS PubMed; (d) Z. Zhang, C. Han, G. Yu and F. Huang, Chem. Sci., 2012, 3, 3026 RSC.
- E. Huerta, G. A. Metselaar, A. Fragoso, E. Santos, C. Bo and J. de Mendoza, Angew. Chem., Int. Ed., 2007, 46, 202 CrossRef CAS PubMed.
- Recently, we reported the surprising host–guest properties of pillar[5]arenes towards neutral 1,4-disubstituted butane guests. See: (a) C. Li, Chem. Commun., 2014, 50, 12420 RSC; (b) C. Li, K. Han, J. Li, Y. Zhang, W. Chen, Y. Yu and X. Jia, Chem.–Eur. J., 2013, 19, 11892 CrossRef CAS PubMed; (c) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967 RSC; (d) C. Li, S. Chen, J. Li, K. Han, M. Xu, B. Hu, Y. Yu and X. Jia, Chem. Commun., 2011, 47, 11294 RSC.
- (a) D.-S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925 CrossRef CAS PubMed; (b) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed.
- M. P. Wintergerst, T. G. Levitskaia, B. A. Moyer, J. L. Sessler and L. H. Delmau, J. Am. Chem. Soc., 2008, 130, 4129 CrossRef CAS PubMed.
- (a) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397 CrossRef CAS PubMed; (b) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC.
- (a) J. L. Seganish, P. V. Santacroce, K. J. Salimian, J. C. Fettinger, P. Zavalij and J. T. Davis, Angew. Chem., Int. Ed., 2006, 45, 3334 CrossRef CAS PubMed; (b) W. Si, L. Chen, X.-B. Hu, G. Tang, Z. Chen and J.-L. Hou, Angew. Chem., Int. Ed., 2011, 50, 12564 CrossRef CAS PubMed.
- P. E. Georghiou, M. Ashram, Z. Li and S. G. Chaulk, J. Org. Chem., 1995, 60, 7284 CrossRef CAS.
- B. Odell, M. V. Reddington, A. M. Z. Slawin, N. Spencer, J. F. Stoddart and D. J. Williams, Angew. Chem., Int. Ed., 1988, 27, 1547 CrossRef.
- W. L. Mock, Top. Curr. Chem., 1995, 175, 1 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed synthetic procedures and characterization, crystal data for EtBP3 and EtBP4, ESI spectra, additional 1H NMR spectra, Job plots, and determination of the association constants. CCDC 1016929 and 1016930. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc02422b |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |