Open Access Article
Open Access ArticleCu-catalyzed transannulation reaction of pyridotriazoles with terminal alkynes under aerobic conditions: efficient synthesis of indolizines†
V.
Helan
,
A. V.
Gulevich
and
V.
Gevorgyan
*
Department of Chemistry, University of Illinois at Chicago, 845 W Taylor St., Room 4500, Chicago, Illinois 60607, USA. E-mail: vlad@uic.edu
First published on 5th January 2015
Abstract
A Cu(I)-catalyzed denitrogenative transannulation reaction of pyridotriazoles with terminal alkynes en route to indolizines was developed. Compared to the previously reported Rh-catalyzed transannulation reaction, this Cu-catalyzed method features aerobic conditions and a much broader scope of pyridotriazoles and alkynes.
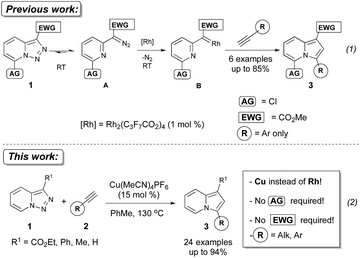
The transition-metal-catalyzed denitrogenative transannulation of pyridotriazoles represents an efficient method for the synthesis of fused nitrogen-containing heterocycles.1 This method is based on the ability of pyridotriazole to exist in an equilibrium with diazo-form A,2,3 which can be trapped with Rh(II) to form the reactive pyridyl carbene intermediate B, capable of reacting with terminal alkynes1a to produce valuable indolizines 3 (Scheme 1).4,5 However, this transannulation reaction has several shortcomings.
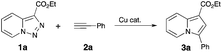
Thus, a Cl substituent at the C-7 position (AG, activating group) and an electron withdrawing ester group (EWG) at the C-3 position of the pyridotriazoles were requisite to facilitate the formation of a sufficient amount of the open form of triazole A even at room temperature and subsequently generate indolizines 3.2,3,6 In addition, the reaction was limited to aryl alkynes only (eqn (1)).1a Herein, we report the first general and efficient Cu-catalyzed transannulation of pyridotriazoles 1 with terminal alkynes 2 to form indolizines 3 (eqn (2)). This newly developed method features several important advantages over the previously reported Rh-catalyzed protocol.1a Thus, it is highly practical as it employs a cheap Cu-catalyst and efficiently operates under aerobic conditions. It is also more general demonstrating a much broader reaction scope, as unactivated pyridotriazoles 1 and aliphatic alkynes 2 now become competent reaction partners (eqn (2)).
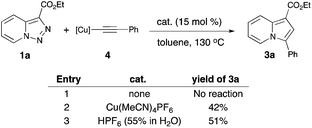
The above-mentioned transannulation reaction of pyridotriazoles 1 (eqn (1)),1 as well as the further developed and widely used transannulation reactions of N-sulfonyl 1,2,3-triazoles,7 require the use of a Rh-catalyst,8 which is one of the most expensive and rare metals used in catalysis. Naturally, the development of alternative catalysts for transannulation reactions of triazoles would dramatically increase the synthetic applicability of this methodology.9 Accordingly, aiming at the discovery of a cheaper catalyst and at expanding the scope of transannulation reactions of pyridotriazoles, we turned our attention to the potential employment of copper catalysts.10 To ensure sufficient amounts of the open form A of the unactivated pyridotriazole, we tested the potential transannulation reaction at elevated temperatures.3 Thus, we tested various copper catalysts in the reaction of unactivated pyridotriazole 1a with phenylacetylene 2a (Table 1). While CuCl was found to be inefficient (entry 1), the use of Cu(I) and Cu(II) triflates led to the formation of the corresponding indolizine 3a in moderate yields (entries 2 and 3).11 Delightfully, the more electrophilic Cu(MeCN)4PF6 catalyst turned out to be even more efficient in the formation of 3a (entry 4). Finally, after optimization of the temperature (entries 5, 6), a virtually quantitative yield of 3a was achieved (entry 6). Moreover, we were pleased to find that this reaction works equally efficiently under aerobic conditions (entry 7). As expected, under thermal conditions no reaction occurred (entry 8). Moreover, it was found that Rh2(hfb)4 is not a capable catalyst for this reaction (entry 9).
| Entry | Catalyst, mol% | T (°C) | Yieldb |
|---|---|---|---|
| a Triazole (1 equiv.), alkyne (3 equiv.), Cu cat. (15 mol%), toluene (1 M) in a Wheaton V-vial capped with a Mininert syringe valve. b GC/MS yields are given. c 1.2 equiv. of alkyne was used. d In air with 1.2 equiv. of alkyne. e Lower catalyst loading led to decreased reaction yields.11 f Polymerization of the alkyne was observed; hfb = heptafluorobutyrate. | |||
| 1 | CuCl, 15% | 100 | N.R. |
| 2 | CuOTf·0.5C6H6, 15% | 100 | 38% |
| 3 | Cu(OTf)2, 15% | 100 | 25% |
| 4 | Cu(MeCN)4PF6, 15% | 100 | 50% |
| 5c | Cu(MeCN)4PF6, 15% | 120 | 96% |
| 6c | Cu(MeCN)4PF6, 15% | 130 | 99% |
| 7d,e | Cu(MeCN)4PF6, 15% | 130 | 99% |
| 8 | No catalyst | 100 | N.R. |
| 9 | Rh2(hfb)4, 1% | 100 | N.R.f |
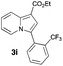
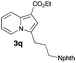
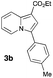
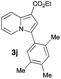
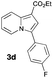
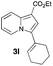
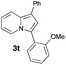
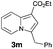
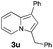
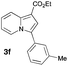
Having the optimized conditions in hand, we investigated the scope of this Cu-catalyzed transannulation reaction of pyridotriazoles with terminal alkynes (Table 2). A variety of aryl alkynes bearing electron-neutral, electron withdrawing and electron donating substituents at ortho-, meta- and para-positions produced the corresponding indolizines 3 in high yields upon reaction with pyridotriazole 1a (Table 2, entries 1–10).12 Heteroaromatic alkynes such as 3-thienyl acetylene and enyne led to the indolizines 3k, l in reasonable yields (entries 11 and 12). We were pleased to find that in contrast to the previously reported Rh-catalyzed reaction, aliphatic alkynes were also competent reactants. Thus, benzyl-, n-butyl, and c-hexyl acetylenes reacted smoothly to produce the corresponding indolizines in good yields (entries 13–15). To our delight, functional groups including benzyloxy- and N-phthalimido were perfectly tolerated under the reaction conditions (entries 16 and 17). Moreover, while our group previously reported the Rh-catalyzed transannulation reaction of pyridotriazoles with nitriles,1a the Cu-catalyzed transannulation showed a strong preference for the alkyne over the nitrile group. Thus, the reaction of pyridotriazole 1a with 5-hexynenitrile furnished indolizine 3r with the nitrile group staying intact (entry 18). Notably, pyridotriazoles which did not contain electron withdrawing groups at the C-3 position were found to be reactive substrates as well. Hence, the indolizines derived from 3-phenyl and 3-methyl pyridotriazoles were produced in reasonable yields (entries 19–23). Remarkably, even a non-substituted pyridotriazole (R1 = H) reacted with phenylacetylene to form indolizine 3x in a moderate yield. Noteworthily, trialkylsilyl-substituted alkynes were either unstable (TMS, TES) or stayed intact (TIPS) under the reaction conditions.
| Entry | Product | Yield, % | Entry | Product | Yield, % | Entry | Product | Yield, % |
|---|---|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||||
| 1 |
|
70 | 9 |
|
78 | 17 |
|
82 |
| 2 |
|
74 | 10 |
|
75 | 18 |
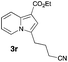
|
66 |
| 3 |
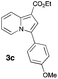
|
65 | 11 |
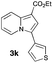
|
33 | 19 |
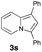
|
77 |
| 4 |
|
70 | 12 |
|
67 | 20 |
|
80 |
| 5 |
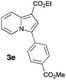
|
48 | 13 |
|
68 | 21 |
|
67 |
| 6 |
|
57 | 14 |
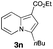
|
82 | 22 |
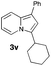
|
50 |
| 7 |
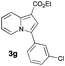
|
60 | 15 |
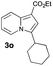
|
83 | 23 |
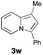
|
41 |
| 8 |
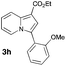
|
94 | 16 |
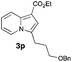
|
53 | 24 |
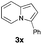
|
54 |
We envisioned two alternative pathways for this Cu-catalyzed transannulation reaction (Scheme 2). First, the copper catalyst can react with the terminal alkyne 2 to form copper acetylide 4, which would react with the α-imino diazo compound A to generate the Cu–carbene complex C (path a). Alternatively, the copper–carbene C can be formed via the reaction of alkyne 2 with copper–carbene B, which is produced from the diazo compound A and the Cu-catalyst (path b). Next, migratory insertion of the alkynyl group at the carbene C-atom of C would form the propargyl intermediate D.13 The latter would undergo cyclization via a nucleophilic attack of the pyridine nitrogen at the triple bond activated by the electrophilic Cu-species14 to produce the triazolyl-copper intermediate G. Also, one cannot exclude the formation of propargylic (E) or allenic (F) intermediates upon protiodemetalation of D. Cycloisomerization of E and F would form intermediate G.15 A subsequent protiodemetalation of G would lead to the indolizine 3.
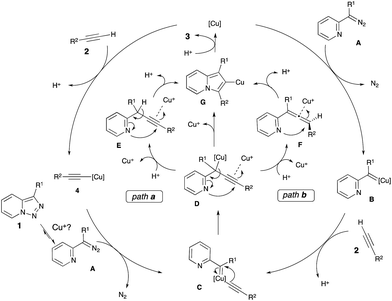 | ||
| Scheme 2 Proposed mechanism for the Cu-catalyzed transannulation reaction of pyridotriazoles with alkynes. | ||
In order to verify a potential involvement of Cu-acetylide 4 in this transformation, we performed several test experiments. First, it was found that the reaction of pyridotriazole 1a with 4 did not produce indolizine 3a (Scheme 3, entry 1). However, the reaction of 1a with 4 can be catalyzed by both Cu(MeCN)4PF6 (entry 2)16 and HPF6(aq.) (entry 3). This observation suggests that the presence of an electrophilic Cu-species is required to activate the alkyne during the cyclization of D into G,17,18 and potentially to shift the equilibrium of the pyridotriazole towards the reactive α-imino diazo compound A.19 Although more detailed studies are required to elucidate the exact mechanism of this transformation, based on literature data20,21 and the above-mentioned observations, it is believed that the reaction most likely proceeds via path a (Scheme 2).
Conclusions
We have developed a practical and efficient copper-catalyzed denitrogenative transannulation reaction of pyridotriazoles with terminal alkynes into indolizines. Compared to the known Rh-catalyzed transannulation reaction, this newly developed method features not only the use of a cheap Cu-catalyst and aerobic conditions, but also a much broader scope of multisubstituted indolizines that now can be accessed from unactivated pyridotriazoles and diverse terminal alkynes.Acknowledgements
The support of the National Institutes of Health (GM 64444) and National Science Foundation (CHE-1401722) is gratefully acknowledged. We also thank Dr S. Chuprakov for initial experiments.Notes and references
- (a) S. Chuprakov, F. W. Hwang and V. Gevorgyan, Angew. Chem., Int. Ed., 2007, 46, 4757 CrossRef CAS PubMed; (b) S. Chuprakov and V. Gevorgyan, Org. Lett., 2007, 9, 4463 CrossRef CAS PubMed.
- For general reviews on pyridotriazoles, see: (a) G. Jones, Adv. Heterocycl. Chem., 2002, 83, 1 CrossRef CAS; (b) B. Abarca-Gonzalez, J. Enzyme Inhib. Med. Chem., 2002, 17, 359 CrossRef CAS PubMed; (c) G. Jones and B. Abarca, Adv. Heterocycl. Chem., 2010, 100, 195 CrossRef CAS.
- For a recent review on rearrangement of 1,2,3-triazoles, see: V. Bakulev, W. Dehaen and T. B. Beryozkina, Thermal Rearrangements and Transformations of 1,2,3-Triazoles, Top. Heterocycl. Chem., 2015, 40, 1 Search PubMed.
- For selected recent reviews on bioactive indolizines, see: (a) V. Sharma and V. Kumar, Med. Chem. Res., 2014, 23, 3593 CrossRef CAS; (b) G. S. Singh and E. E. Mmatli, Eur. J. Med. Chem., 2011, 46, 5237 CrossRef CAS PubMed.
- For selected methods towards indolizines, see: (a) A. E. Tschitschibabin, Ber. Dtsch. Chem. Ges., 1927, 60, 1607 CrossRef; (b) A. R. Katritzky, G. Qiu, B. Yang and H.-Y. He, J. Org. Chem., 1999, 64, 7618 CrossRef CAS; (c) D. Basavaiah and A. J. Rao, Chem. Commun., 2003, 604 RSC; (d) I. Seregin and V. Gevorgyan, J. Am. Chem. Soc., 2006, 128, 12050 CrossRef CAS PubMed; (e) A. R. Hardin and R. Sarpong, Org. Lett., 2007, 9, 4547 CrossRef CAS PubMed; (f) J. Barluenga, G. Lonzi, L. Riesgo, L. A. López and M. Tomás, J. Am. Chem. Soc., 2010, 132, 13200 CrossRef CAS PubMed; (g) D. Chernyak, C. Skontos and V. Gevorgyan, Org. Lett., 2010, 12, 3242 CrossRef CAS PubMed; (h) D. Chernyak and V. Gevorgyan, Org. Lett., 2010, 12, 5558 CrossRef CAS PubMed; (i) Z. Li, D. Chernyak and V. Gevorgyan, Org. Lett., 2012, 14, 6056 CrossRef CAS PubMed; (j) X. Meng, P. Liao, J. Liua and X. Bi, Chem. Commun., 2014, 50, 11837 RSC; (k) M. Gao, J. Tian and A. Lei, Chem.– Asian J., 2014, 9, 2068 CrossRef CAS PubMed; (l) C. Feng, Y. Yan, Z. Zhang, K. Xu and Z. Wang, Org. Biomol. Chem., 2014, 12, 4837 RSC; (m) J. Sun, F. Wang, H. Hu, X. Wang, H. Wu and Y. Liu, J. Org. Chem., 2014, 79, 3992 CrossRef CAS PubMed; (n) J. Liu, L. Zhou, W. Ye and C. Wang, Chem. Commun., 2014, 50, 9068 RSC; (o) R. R. Jha, A. K. Danodia and A. K. Verma, Tetrahedron Lett., 2014, 55, 4724 CrossRef CAS PubMed and ref. 1 and 15.
- For formation of rhodium carbene from unactivated pyridotriazole, see: Y. Shi, A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2014, 53, 14191 CrossRef CAS PubMed.
- For reviews on reactions of iminocarbenes derived from N-sulfonyl 1,2,3-triazoles, see: (a) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC; (b) A. V. Gulevich and V. Gevorgyan, Angew. Chem., Int. Ed., 2013, 52, 1371 CrossRef CAS PubMed; (c) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed.
- For rare examples of Ni-catalyzed transannulation of N-sulfonyl 1,2,3-triazoles, see: (a) T. Miura, M. Yamauchi and M. Murakami, Chem. Commun., 2009, 1470 RSC; (b) T. Miura, K. Hiraga, T. Biyajima, T. Nakamuro and M. Murakami, Org. Lett., 2013, 15, 3298 CrossRef CAS PubMed.
- For selected recent reviews on reactions of metal–carbenes, see: (a) F. Z. Dörwald, in Metal Carbenes in Organic Synthesis, Wiley-VCH, Weinheim, 1999 Search PubMed; (b) Metal Carbenes in Organic Synthesis, ed. K. H. Dotz, Springer-Verlag, Berlin, Heidelberg, 2004 Search PubMed; (c) J. Egger and E. M. Carreira, Nat. Prod. Rep., 2014, 31, 449 RSC; (d) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236 CrossRef CAS PubMed.
- For a recent review on Cu-catalyzed reactions of diazocompounds, see: (a) X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162 RSC . For relevant examples, see; (b) E. Lourdusamy, L. Yao and C.-M. Park, Angew. Chem., Int. Ed., 2010, 49, 7963 CrossRef CAS PubMed; (c) R. Liu, M. Zhang, G. Winston-McPherson and W. Tang, Chem. Commun., 2013, 49, 4376 RSC.
- See ESI† for detailed optimization studies.
- The 7-Cl-substituted analog of 1a produced a complex mixture of products even at a lower temperature.
- For a review on the metal carbene migratory insertion, see: Y. Xia, Y. Zhang and J. Wang, ACS Catal., 2013, 3, 2586 CrossRef CAS.
- For activation of copper acetylide with electrophilic copper species, see: B. T. Worrell, J. A. Malik and V. V. Fokin, Science, 2013, 340, 457 CrossRef CAS PubMed.
- For selected examples of Cu-catalyzed cycloisomerization reactions of propargyl and allenyl pyridines into indolizines, see: (a) B. Yan, Y. Zhou, H. Zhang, J. Chen and Y. Liu, J. Org. Chem., 2007, 72, 7783 CrossRef CAS PubMed; (b) D. Chernyak, S. B. Gadamsetty and V. Gevorgyan, Org. Lett., 2008, 10, 2307 CrossRef CAS PubMed; (c) M. J. Albaladejo, F. Alonso and M. Yus, Chem.– Eur. J., 2013, 19, 5242 CrossRef CAS PubMed.
- Apparently, in this case, intermediate G was quenched by an eventual proton source, which was supported by deuterium labeling studies. See ESI† for details.
- It is believed that HPF6 liberates catalytic amounts of an electrophilic Cu-species by protonation of copper acetylide 4 (Scheme 3, entry 3).
- For selected recent reviews on the formation of heterocycles via attack of nucleophiles at the metal-activated triple bond, see: (a) B. Godoi, R. E. Schumacher and G. Zeni, Chem. Rev., 2011, 111, 2937 CrossRef CAS PubMed; (b) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed , and references therein. For a selected recent example, see; (c) C. He, J. Hao, H. Xu, Y. Mo, H. Liu, J. Han and A. Lei, Chem. Commun., 2012, 48, 11073 RSC.
- B. Chattopadhyay and V. Gevorgyan, Org. Lett., 2011, 13, 3746 CrossRef CAS PubMed.
- For the Cu-catalyzed reactions of diazo compounds with alkynes, see: (a) A. Suárez and G. Fu, Angew. Chem., Int. Ed., 2004, 43, 3580 CrossRef PubMed; (b) L. Zhou, J. Ma, Y. Zhang and J. Wang, Tetrahedron Lett., 2011, 52, 5484 CrossRef CAS PubMed; (c) Q. Xiao, Y. Xia, H. Li, Y. Zhamg and J. Wang, Angew. Chem., Int. Ed., 2011, 50, 1114 CrossRef CAS PubMed; (d) L. Zhou, Y. Shi, Q. Xiao, Y. Liu, F. Ye, Y. Zhang and J. Wang, Org. Lett., 2011, 13, 968 CrossRef CAS PubMed; (e) F. Ye, Y. Shi, L. Zhou, Q. Xiao, Y. Zhang and J. Wang, Org. Lett., 2011, 13, 5020 CrossRef CAS PubMed; (f) F. Ye, X. Ma, Q. Xiao, H. Li, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2012, 134, 5742 CrossRef CAS PubMed; (g) F. Ye, M. L. Hossain, Y. Xu, X. Ma, Q. Xiao, Y. Zhang and J. Wang, Chem.– Asian J., 2013, 8, 1404 CrossRef CAS PubMed; (h) M. L. Hossain, F. Ye, Y. Zhang and J. Wang, Tetrahedron, 2014, 70, 6957 CrossRef CAS PubMed; (i) M. L. Hossain, F. Ye, Z. Liu, Y. Xia, L. Zhou, Y. Zhang and J. Wang, J. Org. Chem., 2014, 79, 8689 CrossRef CAS PubMed.
- For computational studies of the Cu-catalyzed cross-coupling reaction of diazo compounds with alkynes, see: T. Wang, M. Wang, S. Fang and J. Liu, Organometallics, 2014, 33, 3941 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization for new compounds are provided. See DOI: 10.1039/c4sc03358b |
| This journal is © The Royal Society of Chemistry 2015 |