Open Access Article
Open Access ArticleBidirectional photoswitching of magnetic properties at room temperature: ligand-driven light-induced valence tautomerism†
Alexander
Witt
,
Frank W.
Heinemann
and
Marat M.
Khusniyarov
*
Friedrich-Alexander-University of Erlangen-Nuremberg, Department of Chemistry and Pharmacy, Egerlandstr. 1, 91058 Erlangen, Germany. E-mail: marat.khusniyarov@fau.de; Fax: +49 9131 8527367; Tel: +49 9131 8527464
First published on 22nd May 2015
Abstract
Valence tautomeric (VT) metal complexes are highly promising bistable molecular compounds for applications as molecular switches in molecular electronics and spintronics. Although VT species can be switched with light, the photoswitching in all reported systems requires very low temperatures (usually below 20 K) because photoinduced states are highly unstable at room temperature. The thermal instability hinders any practical application of these complexes in genuine devices. In this report, for the first time we demonstrate photoswitching of VT species and associated magnetic properties at room temperature. The bidirectional photoswitching in solution is due to cis–trans photoisomerizable 4-styrylpyridine ligands deliberately integrated into cobalt dioxolene molecular complexes. The novel type of photoswitching has been coined Ligand-Driven Light-Induced Valence Tautomerism (LD-LIVT). The photoconversion of VT states of 28% has been achieved in solution at room temperature. The photoinduced states show extraordinary thermal stability for hours at room temperature, as compared to common nanoseconds reported previously. The switching proceeds at molecular level with the effective photoswitching rate of 3 × 1013 molecules per s under our conditions. Consequently, this work may open new horizons in applications of molecular switches based on VT metal complexes in molecular devices functioning at room temperature.
Introduction
Valence tautomeric (VT) metal complexes are well known systems showing molecular bistability.1,2 The close energies of metal- and ligand-based redox-active orbitals allow a reversible intramolecular electron transfer to occur, which gives rise to distinctly different electronic states (redox isomers). Particularly interesting are VT cobalt dioxolenes because intramolecular electron transfer is accompanied by a spin state change at the cobalt center resulting in reversible switching between diamagnetic low-spin (ls) cobalt(III) (S = 0) and paramagnetic high-spin (hs) cobalt(II) (S = 3/2) ions. Greatly differing magnetic properties of the two states and the opportunity to switch reversibly between them by external stimuli render VT cobalt complexes highly attractive for applications as molecular switches, sensors and molecule-based memory units.3–6The switching in VT metal complexes can be triggered by temperature, pressure, magnetic field, soft X-rays or by light.7 Most of the research in the field is dedicated to the development of thermally switchable VT species. However, switching with light is much more attractive for prospective applications due to high speed of addressing, superior resolution, and high selectivity. It is known that photoswitching in VT metal complexes can be achieved by irradiation into appropriate charge transfer (CT) absorption bands.8 However, the lifetime of such photoinduced metastable states is in the order of nanoseconds at room temperature (RT). The metastable states can be stabilized by cooling which allows the photoswitching to be performed only at very low temperatures, usually below 20 K.9–12 Shultz et al. were able to stabilize photoinduced states at higher temperatures up to 90 K using crystal lattice effects.13,14 However, the photoswitching becomes impossible at molecular level in such a case. Thus, all previously reported VT photoswitches operate only at very low temperatures, which results in serious limitations for their use in molecular devices.
Nonetheless, a photoinduced state might be stabilized by introducing photoisomerizable ligands into VT systems15 in such a way that a ligand-based photoreaction affects the energy of redox-active orbitals and thus triggers intramolecular electron transfer. The stability of such photoinduced state is dictated by the stability of the photoisomer of the ligand which can exceed years at RT.16 The very first step in this direction has been attempted recently by Frank et al.17 Although their spirooxazine-derived ligand maintained photoactivity within a VT cobalt complex, no solid evidence for the photoswitching of VT states at RT has been provided. Thus, VT metal complexes that can be switched with light at RT remained unknown to date.
Our group works on stabilization of photoinduced states in VT and spin-crossover metal complexes by integration of photoisomerizable ligands into bistable species.18–20 Very recently, we have developed a VT cobalt dioxolene system featuring two photoactive trans-4-phenylazopyridine ligands and introduced a new concept for switching VT states called “Coordination-Induced Valence Tautomerism” (CIVT).20 Unfortunately, low thermal stability of photogenerated cis-4-phenylazopyridine precluded a detailed examination of photoswitching in this system. To improve photophysical properties of the system we decided to substitute 4-phenylazopyridine with 4-styrylpyridine (4-stypy) ligands that show high thermal stability of both cis- and trans-isomers.21
Here, we report two VT cobalt complexes trans-6 and cis-6 containing photoactive trans-4-stypy and cis-4-stypy ligands, respectively (Scheme 1). Their molecular and electronic structures are thoroughly investigated by variable-temperature X-ray crystallography, magnetic susceptibility measurements, NMR, EPR and electronic absorption spectroscopy, electrochemistry and titration experiments. For the first time, the electronic states of VT metal complexes are switched with light at room temperature. Consequently, the switching of VT states allows to control magnetic properties with light at ambient temperatures. This opens new horizons for application of molecular switches based on VT metal complexes in functioning molecular devices.
Experimental section
Materials
All starting materials and solvents were used as received without further purification unless otherwise noted. Pure anhydrous solvents, required for work under inert atmosphere, were collected from a solid state solvent purification system (Glass Contour System, Irvine, CA) and stored over activated molecular sieves.Syntheses
Precursor cobalt tetramer was obtained as a benzene solvate [Co(tbdiox)2]4·(C6H6)2.75 by a synthetic procedure described by Pierpont et al.22trans-4-Styrylpyridine (trans-4-stypy) was prepared according to a method described by Chiang and Hartung.23cis-4-Styrylpyridine (cis-4-stypy) was obtained in a Wittig reaction as reported by Williams et al.21[Co(tbdiox)2(trans-4-stypy)2] (trans-6) was prepared according to a common procedure for bis(o-dioxolene) cobalt complexes:1 under inert atmosphere, trans-4-styrylpyridine (200 mg, 1.10 mmol) dissolved in toluene (10 mL) was added dropwise to [Co(tbdiox)2]4·(C6H6)2.75 (300 mg, 0.14 mmol) dissolved in hot toluene (50 mL, 80 °C). The reaction mixture was stirred overnight at 80 °C and then stored at −35 °C for 3 days before a grey-green precipitate was collected and dried in vacuo (274 mg, yield: 58%). Elemental analysis calcd (%) for C54H62CoN2O4: C 75.24, H 7.25, N 3.25; found: C 74.99, H 7.24, N 3.32.
[Co(tbdiox)2(cis-4-stypy)2] (cis-6) was obtained following a similar synthetic protocol: under inert atmosphere, cis-4-styrylpyridine (295 mg, 1.63 mmol) was added to a hot toluene solution (70 mL, 80 °C) of [Co(tbdiox)2]4·(C6H6)2.75 (448 mg, 0.20 mmol). The reaction mixture was stirred for 2 days at 80 °C and then stored at −35 °C for several days before a black crystalline precipitate was collected and dried in vacuo (503 mg, yield: 73%). Elemental analysis calcd (%) for C54H62CoN2O4: C 75.24, H 7.25, N 3.25; found: C 75.83, H 7.13, N 3.36.
Instrumentation and physical measurements
Elemental analyses were carried out with a EURO EA analyser from Euro Vector. Magnetic susceptibility data on solid samples were collected using a Quantum Design MPMS-XL SQUID magnetometer. The data were obtained for microcrystalline samples restrained within a polycarbonate gel capsule. DC susceptibility data were collected in the temperature range 2–400 K at applied magnetic field of 1 T. The program JulX was used for the simulation and analysis of magnetic data.24 Electrochemical measurements were performed under nitrogen atmosphere at RT using a standard three-electrode setup with glassy carbon working electrode and platinum rods as counter and reference electrodes. The potentiostat was a μAutolab Type-III. Analyte solutions were prepared in CH2Cl2 containing 0.1 M nBu4NPF6 as supporting electrolyte. All potentials are referenced to the Fc+/0 redox couple measured after adding ferrocene to the analyte solution. EPR spectra were recorded on a Jeol CW spectrometer JES-FA200 equipped with an X-band Gunn diode oscillator bridge and a cylindrical mode cavity. Simulations were performed using the program W95EPR written by F. Neese.25 Irradiation experiments were conducted in situ through a quartz window in the cavity. NMR spectra of trans-6 in solution were recorded in rotating 5 mm o.d. tubes with Jeol JNM-LA 400 FT NMR spectrometer and processed with Delta V4.0 software provided by Jeol Ltd. NMR spectra of cis-6 in solution were obtained without rotating, in 5 mm o.d. tubes on a Bruker Avance DRX 400 WB spectrometer and processed with TopSpin 1.3 software. Magnetic susceptibility in solutions were determined by the Evans NMR method.26 During the variable temperature and titration experiments the outer tube (standard 5 mm o.d. NMR tube with a PTFE spindle valve) contained a reference solvent mixture C7H8/C7D8/TMS (10/2/1), while the inner tube (capillary tube with 1.5 mm diameter sealed with inert wax) contained the paramagnetic complex in the same solvent mixture. The irradiation experiments were performed using a rotating 5 mm o.d. quartz NMR tube with a PTFE spindle valve with the complex solution in the outer tube and the reference solvent mixture in the inner capillary tube. Electronic absorption spectra were recorded with a Shimadzu UV 3600 spectrophotometer. The samples were prepared under anaerobic conditions and sealed in QS Quartz Suprasil cells (10 mm light path) with PTFE spindle valves. Variable-temperature spectra were recorded with an Analytik Jena SPECORD S600 spectrophotometer. The samples were prepared under anaerobic conditions and measurements were conducted inside a glove box using QS Quartz Suprasil cells (10 mm light path). The solutions were continuously stirred with a magnet bar and the temperature inside the cells was monitored. An LOT-Oriel Xe(OF) arc lamp (1 kW) equipped with an Omni-λ 300 monochromator was used as a wavelength-variable light source in all irradiation experiments except the in situ irradiation followed by EPR spectroscopy. For the latter, an LOT-Oriel Xe(OF) arc lamp (150 W) equipped with an Andover bandpass filter (CWL: 322.9 nm, Tmax: 28.0%, FWHM: 10.6 nm) were employed.X-ray crystallographic data collection and structure refinement
Suitable crystals were embedded in protective perfluoropolyalkyl ether oil and transferred to the cold nitrogen gas stream of the diffractometer. Intensity data for trans-6 were collected at 120, 295 and 305 K, and for cis-6 at 100 K on a Bruker Kappa Smart APEX2 (Mo Kα radiation, λ = 0.71073 Å, graphite monochromator). Data were corrected for Lorentz and polarization effects; semi empirical absorption corrections were applied on the basis of multiple scans using SADABS.27 The structures were solved by direct methods and refined by full-matrix least squares procedures on F2 using SHELXTL NT 6.12.28 All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were placed in position of optimized geometry and their isotropic displacement parameters were tied to those of their corresponding carrier atoms by a factor of 1.2 or 1.5. SIMU and ISOR restraints were applied in the refinement of the disorder. The crystallographic data, data collection and structure refinement details are summarized in Table 1 (ESI†).| trans-6 | trans-6 | trans-6 | cis-6·4(C7H8) | |
|---|---|---|---|---|
| Temperature, K | 120 | 295 | 305 | 100 |
| Chemical formula | C54H62CoN2O4 | C54H62CoN2O4 | C54H62CoN2O4 | C54H62CoN2O4·4(C7H8) |
| F w | 861.99 | 861.99 | 861.99 | 1230.52 |
| Cryst size, mm | 0.30 × 0.26 × 0.16 | 0.30 × 0.26 × 0.16 | 0.30 × 0.26 × 0.16 | 0.50 × 0.34 × 0.24 |
| Cryst sys | Triclinic | Triclinic | Triclinic | Monoclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
| a, Å | 10.6748(4) | 10.7956(11) | 9.8997(9) | 16.5308(6) |
| b, Å | 11.9906(5) | 12.0991(13) | 10.8120(10) | 11.7257(4) |
| c, Å | 19.6875(8) | 19.834(2) | 12.1278(11) | 18.9821(7) |
| α, deg | 101.316(2) | 102.162(6) | 104.002(5) | 90 |
| β, deg | 101.9532(17) | 101.446(5) | 102.270(5) | 107.445(2) |
| γ, deg | 103.7626(17) | 104.036(5) | 101.408(5) | 90 |
| V, Å3 | 2313.24(16) | 2370.3(4) | 1187.49(19) | 3510.2(2) |
| Z | 2 | 2 | 1 | 2 |
| ρ calcd, g cm−3 | 1.238 | 1.208 | 1.205 | 1.164 |
| Reflns collected/2θmax, deg | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 386/56.1 386/56.1 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 041/55.32 041/55.32 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490/56.18 490/56.18 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5055/56.0 5055/56.0 |
| Unique reflns/I > 2σ(I) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179/9566 179/9566 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996/6741 996/6741 |
5742/4688 | 7474 |
| No. of param/restraints | 550/0 | 832/900 | 441/93 | 476/225 |
| λ, Å/μ(Kα), mm−1 | 0.71073/0.418 | 0.71073/0.408 | 0.71073/0.407 | 0.71073/0.295 |
| R 1 [I > 2σ(I)] | 0.0348 | 0.0422 | 0.0354 | 0.0394 |
| wR2/goodness of fit | 0.0921/1.042 | 0.1315/1.026 | 0.0917/1.033 | 0.0998 |
| Residual density, e Å−3 | +0.480/−0.368 | +0.340/−0.284 | +0.338/−0.279 | +0.487/−0.367 |
At temperatures of 120 K and 295 K trans-6 was situated on a general position of space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . While at 120 K the molecule was well ordered, disorder was observed at higher temperatures. At 295 K the two pyridyl ligands were subjected to orientational disorder affecting the whole ligand moieties. Two alternative orientations of the pyridyl ligand featuring inverted orientations of the central C
. While at 120 K the molecule was well ordered, disorder was observed at higher temperatures. At 295 K the two pyridyl ligands were subjected to orientational disorder affecting the whole ligand moieties. Two alternative orientations of the pyridyl ligand featuring inverted orientations of the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond were refined resulting in site occupancies of 85.4(3) and 14.6(3)% of the 50 involved atoms (for a graphical representation of the disorder see ESI†). Furthermore, one of the four tBu groups was disordered. Here, also two different orientations were refined giving site occupancies of 52(1) and 48(1)% for the atoms C22–C24 and C22A–C24A, respectively.
C double bond were refined resulting in site occupancies of 85.4(3) and 14.6(3)% of the 50 involved atoms (for a graphical representation of the disorder see ESI†). Furthermore, one of the four tBu groups was disordered. Here, also two different orientations were refined giving site occupancies of 52(1) and 48(1)% for the atoms C22–C24 and C22A–C24A, respectively.
The situation changed at temperatures above 295 K. At 305 K the complex molecule was now located on a crystallographic inversion center and exhibited crystallographically imposed Ci symmetry. As in the 295 K structure the pyridyl ligands were subjected to disorder with different orientations of the central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond and were refined resulting in site occupancies of 83.6(6) and 16.4(6)% of the involved atoms. Furthermore, one of the crystallographically independent tBu groups was disordered. Here, again two different orientations were refined giving site occupancies of 69(2) and 31(2)% for the atoms C8–C10 and C8A–C10A, respectively. All three crystal structures of trans-6 point to a ls-CoIII(Cat)(SQ) electronic configuration (vide infra). However, owing to Ci symmetry at 305 K one cannot distinguish between a mixed-valent delocalized ls-CoIII(Cat)(SQ) ↔ ls-CoIII(SQ)(Cat) electronic structure and a positional disorder in the localized ls-CoIII(Cat)(SQ) form.14,20 The observation of a crystallographic inversion center at higher temperatures may be due to increasing thermal vibration of atoms accompanied by the incipient valence tautomeric transition as confirmed by magnetic measurements.
C double bond and were refined resulting in site occupancies of 83.6(6) and 16.4(6)% of the involved atoms. Furthermore, one of the crystallographically independent tBu groups was disordered. Here, again two different orientations were refined giving site occupancies of 69(2) and 31(2)% for the atoms C8–C10 and C8A–C10A, respectively. All three crystal structures of trans-6 point to a ls-CoIII(Cat)(SQ) electronic configuration (vide infra). However, owing to Ci symmetry at 305 K one cannot distinguish between a mixed-valent delocalized ls-CoIII(Cat)(SQ) ↔ ls-CoIII(SQ)(Cat) electronic structure and a positional disorder in the localized ls-CoIII(Cat)(SQ) form.14,20 The observation of a crystallographic inversion center at higher temperatures may be due to increasing thermal vibration of atoms accompanied by the incipient valence tautomeric transition as confirmed by magnetic measurements.
Molecules of cis-6 at 100 K are located on a crystallographic inversion center and exhibit Ci symmetry. The compound crystallized with four molecules of toluene per formula unit. One of the two independent toluene molecules was disordered. Two alternative orientations were refined resulting in site occupancies of 61(1) and 39(1)% for the atoms C201–C207 and C211–C217. SAME and SIMU restraints were applied in the refinement of the disordered toluene.
Results and discussion
Syntheses and crystal structures
The neutral complexes trans-6 and cis-6 were synthesized according to a common procedure for VT cobalt dioxolenes.1 Slow precipitation from toluene solutions afforded crystals suitable for X-ray structure determination.29 At 120 K trans-6 reveals a distorted octahedral geometry featuring two equatorial bidentate o-dioxolene and two axial monodentate trans-4-stypy ligands (Fig. 1). Short Co–O (1.854(1)⋯1.916(1) Å) and Co–N (1.938(1) and 1.945(1) Å) distances are characteristic for a low-spin (ls) cobalt(III) ion. Intraligand C–O and (O)C–C(O) bond lengths, generally diagnostic of the oxidation level of o-dioxolenes,30 indicate the presence of a catecholate dianion (Cat2−) and a semi-quinone monoanion π-radical (SQ−). The SQ state for one of the ligands is further confirmed by quinoid-type distortion with alternating long-short bonds. Thus, the ligand mixed valency is localized in the solid state at low temperatures. At T = 305 K all cobalt–ligand bonds remain short, but the quinoid-type distortion is observed in both dioxolenes.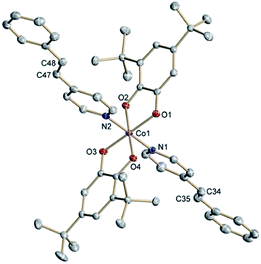 | ||
| Fig. 1 Molecular structure of trans-6 determined at 120 K. Thermal ellipsoids are drawn at 50% probability. Hydrogen atoms are omitted for clarity. | ||
The structurally related cis-6 features two axial cis-4-stypy ligands (Fig. 2). Due to steric factors the pyridyl and phenyl rings of cis-4-stypy cannot be coplanar. Thus, the two rings form a dihedral angle of 50.1° confining a twisted ethylene bridge. Similar twisted geometry has been observed previously.31,32 Short Co–N (1.940(1)) and Co–O (1.885(1) and 1.878(1) Å) distances point to a ls-CoIII center at 100 K. Similar to trans-6 at 305 K, intraligand bonds of both o-dioxolenes in cis-6 are equally subjected to quinoid type distortion corroborating Cat2−/SQ− ligand mixed valency.
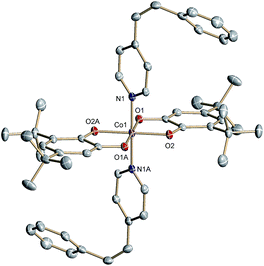 | ||
| Fig. 2 Molecular structure of cis-6 determined at 100 K. Thermal ellipsoids are drawn at 50% probability. Hydrogen atoms are omitted for clarity. | ||
Magnetic properties in solid state
The ls-CoIII(Cat)(SQ) ground state for trans-6 is corroborated by magnetic susceptibility measurements on a microcrystalline sample revealing an effective magnetic moment μeff = 1.79μB invariant in the temperature range 8–250 K (see ESI†). The data could be fitted for a spin doublet S = 1/2 system to afford g = 2.064 and a Weiss constant θ = −0.15 K. Hence, based on both, bond length analysis and magnetic measurements, the electronic structure of solid trans-6 below 250 K is unambiguously assigned as [ls-CoIII(Cat)(SQ)(trans-4-stypy)2] (trans-6LS). At temperatures above 250 K magnetic moment gradually increased to 4.00μB at 400 K revealing a thermally induced trans-6LS → [hs-CoII(SQ)2(trans-4-stypy)2] (trans-6HS; hs = high-spin) transition. The data were fitted with the van't Hoff equation (see ESI†): with the high-temperature limit of magnetic moment fixed at 5.0μB as a common value for hs-CoII(SQ)2 isomers,13 our best fit provides an enthalpy change ΔH = 41(1) kJ mol−1 and an entropy change ΔS = 104(3) J mol−1 K−1.33Similar to trans-6, magnetic data for solid cis-6 below 250 K were fitted for an S = 1/2 spin system with g = 2.073 and θ = −0.62 K (see ESI†); and the electronic structure was assigned as [ls-CoIII(Cat)(SQ)(cis-4-stypy)2] (cis-6LS). The thermal transition cis-6LS → [hs-CoII(SQ)2(cis-4-stypy)2] (cis-6HS), indicated by the increase in μeff at higher temperatures, was fitted to yield ΔH = 52(2) kJ mol−1 and ΔS = 131(3) J mol−1 K−1 (see ESI†). The thermodynamic parameters obtained for both trans-6 and cis-6 in solid state are in agreement with typical thermodynamic parameters for thermally induced VT transitions.34 The evolution of μeff with temperature was fully reversible without detectable hysteresis in both cases. Transition temperatures T1/2 in solid state were estimated at 394 and 397 K for trans-6 and cis-6, respectively.
Electrochemistry
Cyclic voltammograms obtained for a CH2Cl2 solution of trans-6 at RT display one oxidation wave with a half-wave potential E1/2 = −0.33 V and two reduction events at E1/2 = −0.67 and −1.12 V versus Fc+/0 (see ESI†). The ratio between normalized oxidative and reductive peak currents for all three waves are near unity pointing to reversible processes. However, the peak separations ΔE for reduction events significantly exceed the ideal value of 58 mV expected for a diffusion-controlled reversible one-electron transfer. Very similar cyclic voltammograms measured for cis-6 show one oxidation at E1/2 = −0.29 V and two reductions at E1/2 = −0.69 and −1.11 V (see ESI†). All observed redox events were assigned to the redox-active bis(dioxolene)cobalt core. Yet, a detailed assignment is difficult due to partial dissociation of the complexes in solution (vide infra).Magnetic properties in solution
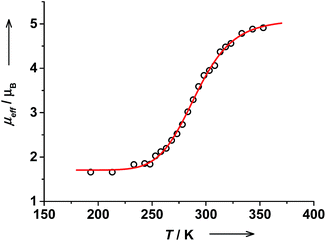
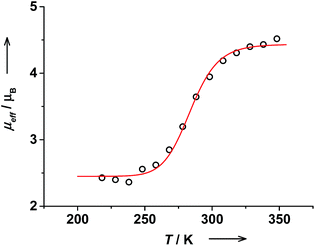
The effective magnetic moment of trans-6 in solution determined by the Evans NMR method26 remained nearly constant at 1.7μB below 215 K, which is in agreement with a pure ls-CoIII(Cat)(SQ) state. With rising temperature, μeff gradually increased reaching 4.91(5)μB at 353 K (Fig. 3). These data were fitted using the van't Hoff equation to yield ΔH = 43(5) kJ mol−1, ΔS = 144(16) J mol−1 K−1 and the high-temperature limit of magnetic moment of 5.1(1)μB that is characteristic for a pure hs-CoII(SQ)2 state. The transition temperature T1/2 was estimated at 299 K. At RT this solution contains 44% of hs-CoII(SQ)2 and 56% of ls-CoIII(Cat)(SQ) species as estimated from RT magnetic moment of 3.59(6)μB.Magnetic moment of cis-6 in solution changes gradually from 2.42(6)μB at 218 K to 4.52(6)μB at 348 K (Fig. 4). The data was fitted to give ΔH = 67(9) kJ mol−1 and ΔS = 232(33) J mol−1 K−1. The high temperature limit for magnetic moment is 4.44(3)μB that is within the range of typical values for a pure hs-CoII(SQ)2 state.35 However, the low temperature limit of 2.45(3)μB is higher than expected for a pure ls-CoIII(Cat)(SQ) state. This might be due to the presence of some paramagnetic impurities in solution of cis-6.20 The transition temperature T1/2 was estimated at 287 K. Note, that the T1/2 value for cis-6 is slightly lower than that for trans-6 in solution (Table 2).
| trans-6 | cis-6 | |||||
|---|---|---|---|---|---|---|
| ΔH, kJ mol−1 | ΔS, J mol−1 K−1 | T 1/2, K | ΔH, kJ mol−1 | ΔS, J mol−1 K−1 | T 1/2, K | |
| Solid state (SQUID) | 41(1) | 104(3) | 394 | 52(2) | 131(3) | 397 |
| Solution (Evans NMR) | 43(5) | 144(16) | 299 | 67(9) | 232(33) | 287 |
| Solution (UV-vis) | 56(1) | 190(5) | 295 | 52(3) | 184(11) | 283 |
| Solution (UV-vis, excess 4-stypy) | 39(2) | 108(9) | 361 | 37(2) | 110(7) | 336 |
Electronic absorption spectra in solution
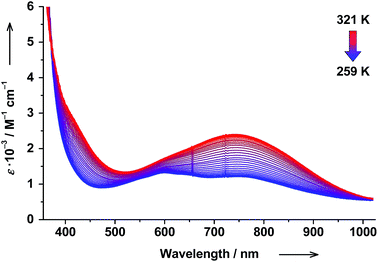
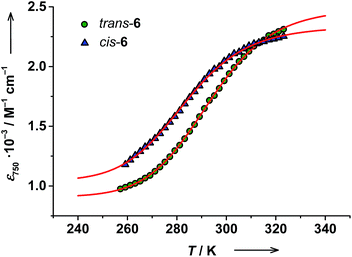
The RT electronic absorption spectrum of trans-6 dissolved in toluene is dominated by a strong band at 307 nm (ε = 6.1 × 104 M−1 cm−1) arising from π → π* transitions of trans-4-stypy, which overlap with intraligand dioxolene bands (see ESI†).36,37 A relatively weak broad band at 750 nm (ε = 1.8 × 103 M−1 cm−1) with a shoulder at ∼600 nm are characteristic CT transitions in VT cobalt dioxolenes.2 The broad band at ∼2650 nm, blighted by solvent overtones in the short-wavelength infrared (SWIR) region, was assigned as an intervalence ligand-to-ligand charge transfer (IVLLCT) transition, which is a spectral fingerprint of mixed-valent ls-CoIII(Cat)(SQ) species (Fig. 7).35 The half width Δνexp of this band was estimated at 860 cm−1. This value is smaller than the half width calculated using the Hush equation Δνcalcd = (2310 × νmax)1/2 = 2981 cm−1.38 Consequently, trans-6LS behaves as a class-III fully delocalized ligand mixed-valent system in solution.At 320 K the solution of trans-6 appeared green colored and the 600 nm absorption was nearly buried beneath the broad 750 nm band. Upon cooling to 259 K, the solution turned bluish-green, the 750 nm band decreased in intensity, and the absorption at 600 nm became more prominent (Fig. 5), which indicates a hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) transition.20 Since the available region of our spectrophotometer for variable-temperature measurements is limited to 1020 nm, the spectral evolution in the SWIR region could not be recorded. The temperature dependence of the 750 nm band was fitted according to the van't Hoff equation to yield ΔH = 56(1) kJ mol−1 and ΔS = 190(5) J mol−1 K−1 (Fig. 6). Consequently, the estimated T1/2 value for trans-6 is 295 K that is in very good agreement with the value determined by NMR spectroscopy (299 K).
Similar to trans-6, a strong band at 306 nm (ε = 4.2 × 104 M−1 cm−1) caused by cis-4-stypy and dioxolene intraligand transitions was observed for cis-6 solution at RT (see ESI†). As compared to trans-6, the shoulder at ∼600 nm is less developed and the IVLLCT transition at ∼2650 nm is weaker for cis-6 (see ESI†). These two features indicate that at RT the ls-CoIII(Cat)(SQ) fraction in cis-6 solution is lower than in trans-6 solution. Thus, the VT equilibrium is more shifted toward the hs-CoII(SQ)2 species in case of cis-6, which is very likely due to weaker coordination of cis-4-stypy (vide infra).
Similar to trans-complex, a color change was observed upon cooling a cis-6 solution. The temperature dependence of absorption at 750 nm was fitted to give ΔH = 52(3) kJ mol−1 and ΔS = 184(11) J mol−1 K−1. The estimated T1/2 value of 283 K is in very good agreement with the value obtained from the Evans method (287 K) (Fig. 6). Again note, that T1/2 values for cis-6 are slightly lower than those of trans-6 in solution (Table 2).
Titration with 4-stypy ligands
The thermodynamic parameters obtained for both trans-6 and cis-6 in solution are larger than those in the solid state and clearly exceed typical values for a thermally induced VT transition, which points to partial dissociation.20 The presence of a five-coordinate hs-CoII(SQ)2 species trans-5 (Scheme 1) was verified by titrating a solution of trans-6 with trans-4-stypy ligand at RT. Upon addition of the ligand, the green colored solution became bluish-green, the 750 nm band decreased and absorption at 600 nm became more developed (Fig. 7). These changes closely resemble those in the variable-temperature experiments (vide supra) and point to a hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) transition. The formation of ls-CoIII(Cat)(SQ) species upon titration is further corroborated by the growing IVLLCT band at ∼2650 nm. An isosbestic point was observed at 1060 nm. Very similar color and spectral changes were observed upon addition of cis-4-stypy ligand to a solution of cis-6 (see ESI†). Thus, the solution of cis-6 contains some five-coordinate cis-5 species.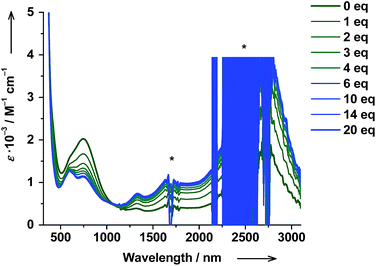 | ||
| Fig. 7 Changes in absorption spectrum of trans-6 dissolved in toluene upon titration with trans-4-stypy at RT. Signals marked with asterix (*) are due to solvent or change of detector. | ||
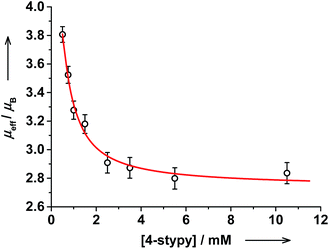
Magnetic properties of trans-6 in solution changed on titration as well: the RT magnetic moment decreased gradually from 3.81(6)μB (prior to trans-4-stypy addition) to 2.84(7)μB after addition of trans-4-stypy (10 eq.). This is in agreement with the suggested hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) switching. Similarly to trans-6, a decrease in μeff from 4.1(1) to 3.1(1)μB was observed upon addition of cis-4-stypy (16 eq.) to a solution of cis-6 (see ESI†). Thus, the VT states in both trans- and cis-complexes can be switched at RT via addition of corresponding pyridine ligands, as we previously documented in Coordination-Induced Valence Tautomerism (CIVT) effect.20
In order to obtain thermodynamic parameters for pure VT transitions between the ls-CoIII(Cat)(SQ) and hs-CoII(SQ)2 redox isomers, we recorded variable-temperature electronic spectra on solutions of trans-6 and cis-6 containing an excess of respective 4-stypy ligand. Thus, the dissociation and formation of five-coordinate species in these solutions must be suppressed. The corresponding van't Hoff fits provided ΔH = 39(2) kJ mol−1 and ΔS = 108(9) J mol−1 K−1 for trans-6 and ΔH = 37(2) kJ mol−1 and ΔS = 110(7) J mol−1 K−1 for cis-6 (see ESI†). Note, that these values are smaller than those obtained on solutions without excess of 4-stypy ligands and now they are in agreement with typical values reported for VT isomers in solution.35
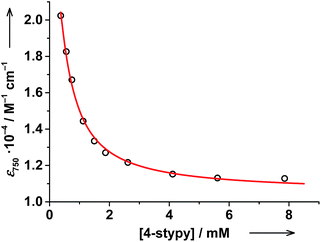
The evolution of the electronic spectrum of trans-6 and the decrease of magnetic moment on titration were both fitted using a non-linear regression approach (see ESI†).39 The intensity of the 750 nm absorption band as a function of trans-4-stypy concentration (Fig. 8) was fitted to yield an association constant Ka = 4(1) × 103 L mol−1 (eqn (1)). In excellent agreement with this value, a similar fitting for magnetic moment gave Ka = 6(2) × 103 L mol−1 (Fig. 9). Given the values of Ka, the toluene solution of the complex must contain ∼45% of the five-coordinate trans-5 and ∼55% of the six-coordinate trans-6 at given concentrations at RT.
Similarly, the changes of electronic spectrum and magnetic moment of cis-6 upon addition of cis-4-stypy gave Ka values of 1.1(3) × 103 and 0.3(1) × 103 L mol−1, respectively (see ESI†). Although there is some discrepancy in the values obtained by two methods, it is more important that the Ka values for cis-6 are significantly smaller than those for trans-6. Thus, cis-4-stypy is a weaker ligand than trans-4-stypy. This is corroborated by X-ray crystallography, showing that trans-4-stypy ligands in trans-6 are planar thus better π-acceptors, than non-planar cis-4-stypy ligands in cis-6.
Note, that the RT magnetic moment of dissolved trans-6 decreased to only 2.8μB in the presence of a large excess of trans-4-stypy (20 eq.), but not to the low temperature limit of 1.7μB. This seeming inconsistency can be readily resolved by considering that the parent trans-6 exists as a mixture of VT isomers trans-6LS ↔ trans-6HS at RT in solution (eqn (1)). Thus, at low temperatures only trans-6LS is present in solution in agreement with the observed magnetic moment of 1.7μB. At RT 45% of trans-6 dissociates to form trans-5 (vide supra). In the presence of a large excess of trans-4-stypy the dissociation is suppressed leaving a mixture of trans-6LS and trans-6HS at RT. The estimations from magnetic data yield 78% of trans-6LS and 22% of trans-6HS present in this solution at RT. The same applies to cis-6: in the presence of a large excess of cis-4-stypy the equilibrium in solution at RT is virtually shifted to six-coordinate species cis-6HS and cis-6LS (eqn (1)).
 | (1) |
Photochemistry
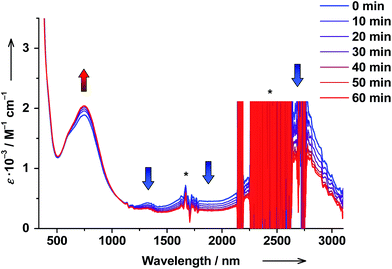
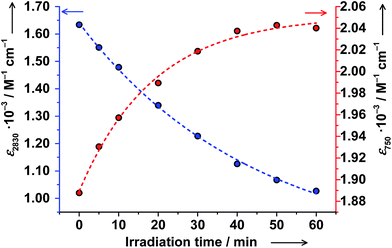
Solutions of trans-6 and cis-6 differ in transition temperatures T1/2 and association constants Ka. Consequently, the amount of ls-CoIII(Cat)(SQ) and hs-CoII(SQ)2 species in the two solutions is different. Thus, trans ↔ cis photoisomerization of 4-stypy ligands offers the opportunity to induce a shift in VT equilibrium and to switch magnetic properties at RT.When trans-6 solution was irradiated at λ = 320 nm, the absorption band at 307 nm decreased in intensity confirming trans → cis isomerization of 4-stypy (see ESI†). Upon irradiation, the IVLLCT band at ∼2650 nm decreased and the CT band at 750 nm increased revealing an isosbestic point at 1060 nm (Fig. 10). The photostationary state (PSS) was nearly reached within 60 min (Fig. 11). It is worth mentioning that very similar spectral changes were observed for ls-CoIII(Cat)(SQ) → hs-CoII(SQ)2 transition in variable-temperature and CIVT experiments (vide supra). Thus, the trans → cis photoisomerization of 4-stypy did induce a VT transition in solution at RT.
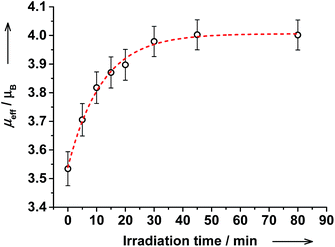
These results are strongly supported by NMR spectroscopy. Upon irradiation, the magnetic moment increased gradually from 3.53(6) to 4.00(6)μB and the PSS was reached within 45 min (Fig. 12). Upon further irradiation, no changes in NMR and electronic spectra were detected verifying stability of the PSS. The amount of ls-CoIII(Cat)(SQ) species decreased from 60% before irradiation to 43% after irradiation. Thus, the ls-CoIII(Cat)(SQ) → hs-CoII(SQ)2 photoconversion of 28% ((60–43)/60 × 100) was achieved at RT. Importantly, the electronic spectrum and magnetic moment remained unchanged upon storing the irradiated solution at RT for at least 20 hours! Thus, the photogenerated VT state reveals extraordinary thermal stability at RT, as compared to common nanoseconds.8 This is to expect, since cis-4-stypy shows exceptional high thermal stability.21
The ls-CoIII(Cat)(SQ) → hs-CoII(SQ)2 photoswitching in trans-6 solution was further confirmed by EPR spectroscopy. The hs-CoII(SQ)2 isomers are usually EPR silent, whereas the ls-CoIII(Cat)(SQ) state can be readily detected both at low and RT. Thus, a solution of trans-6 at RT shows a typical isotropic spectrum with giso = 1.9980 and a 59Co super-hyperfine coupling constant Aiso = 10.7 × 10−4 cm−1, which is synonymous with a ligand-based radical ls-CoIII(Cat)(SQ).40 Upon in situ irradiation at λ = 323 nm at RT, the signal of ls-CoIII(Cat)(SQ) species gradually decreased, whereas no new signals appeared (Fig. 13). The PSS was reached within 60 min.
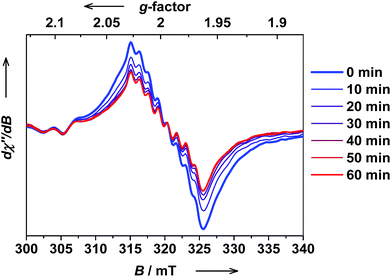 | ||
| Fig. 13 The evolution of the X-band EPR spectrum of trans-6 dissolved in benzene upon UV irradiation at RT (c = 1.0 × 10−4 M, λ = 323 ± 5 nm, 150 W Xe lamp, frequency: 8.9410 GHz, modulation: 0.7 mT, power: 20 mW). Note, that the signal is due to ls-CoIII(Cat)(SQ), whereas hs-CoII(SQ)2 species are not detected. Relatively noisy signal is due to low concentration of trans-6, required to speed up bulk photolysis, and consequently very small amount of EPR active ls-CoIII(Cat)(SQ) species present in solution. The spectra of a more concentrated solution and at low temperatures with increased ls-CoIII(Cat)(SQ) content are given in ESI.† | ||
We examined the possibility to perform trans → cis photoisomerization by low-energy excitation in visible as well.41 Whereas irradiation at λ = 420 and 750 nm did not induce desired photoreaction, irradiation at λ = 600 nm did induce trans → cis isomerization. Unfortunately, the latter photoreaction was accompanied by some unidentified side reactions. Thus, the irradiation at λ = 320 nm is the most efficient and clear way to perform trans → cis isomerization in trans-6 solution.
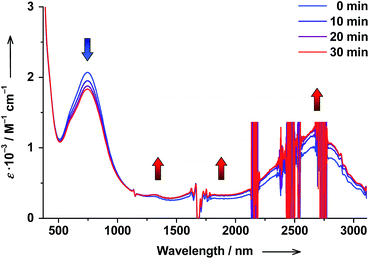
The reverse hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) photoswitching could be accomplished as well starting from cis-6 complex and performing cis → trans ligand-based photoisomerization. When cis-6 solution was irradiated at λ = 272 nm, the cis → trans photoisomerization of 4-stypy could be unambiguously confirmed by increasing 306 nm band in electronic spectrum (see ESI†). Simultaneously, the 750 nm CT band decreased in intensity, a shoulder at 600 nm developed, and the LLIVCT band at ∼2650 nm increased before the PSS was reached within 30 min (Fig. 14). The observed spectral changes show exactly opposite trends compared to the trans → cis photoisomerization experiment. Thus, a cis → trans photoisomerization of 4-stypy induces hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) conversion at RT.
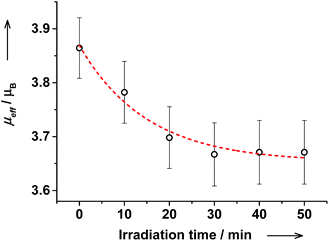
The reverse photoswitching of VT states in cis-6 solution was further corroborated by the decrease of magnetic moment from 3.9(1) to 3.7(1)μB upon irradiation at λ = 272 nm (Fig. 15). After irradiation the electronic spectrum and magnetic moment remained virtually unchanged during at least 3 hours demonstrating high stability of photogenerated species.
Two mechanisms for the observed photoswitching of VT states should be considered. Upon irradiation at λ = 320 nm, trans-4-stypy is converted to cis-4-stypy (coordinated and non-coordinated). The non-planar geometry of cis-4-stypy renders it a weaker π-acceptor compared to the trans-form. Thus, the ligand field is reduced upon trans → cis isomerization, which should stabilize the hs-CoII(SQ)2 state in six-coordinate species 6. At the same time, cis-4-stypy is a weaker coordinating ligand than the trans-isomer,42 as was confirmed by differing associate constants (vide supra). Thus, the equilibrium between five-coordinate 5 and six-coordinate 6 species is expected to shift upon trans → cis isomerization. In this case some {6LS ↔ 6HS} species is converted to 5 increasing the hs-CoII(SQ)2 content (eqn (1)).
Similar considerations apply to reverse reaction, when cis-4-stypy is converted to trans-4-stypy by irradiating at λ = 272 nm. The observed hs-CoII(SQ)2 → ls-CoIII(Cat)(SQ) photoconversion may be due to increased ligand field splitting accompanying the cis → trans isomerization. Alternatively, the equilibrium between 5 and {6LS ↔ 6HS} may be shifted toward the six-coordinate species (eqn (1)) due to stronger coordinating trans-4-stypy, thus leading to increased ls-CoIII(Cat)(SQ) content.
To gain a better mechanistic understanding, we performed an irradiation experiment on a trans-6 solution containing a large excess of trans-4-stypy (50 eq.), in which the dissociation is virtually suppressed. Upon extended exposure to UV light the absorption spectrum did not show any detectable changes in visible and SWIR regions characteristic for VT conversion. Therefore, we concluded, that the successful photoswitching of VT states in pristine solutions of trans-6 and cis-6 is unlikely due to variation of the ligand field strength, but to great extent due to the light-induced shift in equilibrium between five- and six-coordinate species.
It is important to note that the photoswitching of electronic states in trans-6 and cis-6 solutions is of a pure molecular origin. However, the reported irradiation times (typically 30–60 min) correspond the switching of huge number of molecules (bulk photolysis), collectively detected by spectroscopic methods. Since we know how many molecules are switched from ls-CoIII(Cat)(SQ) to hs-CoII(SQ)2 state upon UV irradiation (appr. 1017), the effective photoswitching rate under our conditions can be estimated as 1017 molecules ÷ 3600 s = 3 × 1013 molecules per s.
In unison with early works by Boillot15 and recent works of Herges and Tuczek42,43 on bistable spin-crossover metal complexes, we coin the observed effect Ligand-Driven Light-Induced Valence Tautomerism (LD-LIVT).
Conclusions
In spite of great efforts on the development of molecular photoswitches on the base of valence tautomeric metal complexes during the last decade, the switching with light has been restricted to very low temperatures (usually below 20 K). Here, for the first time we have demonstrated light-induced switching in valence tautomeric cobalt complexes at room temperature. The driving force for the photoswitching is bidirectional trans ↔ cis photoisomerization of 4-styrylpyridine ligands deliberately integrated into bistable magnetic molecules. The photoswitching results in modulation of magnetic properties in solution at room temperature. The novel effect has been coined Ligand-Driven Light-Induced Valence Tautomerism (LD-LIVT) and proceeds at molecular level with very high effective photoswitching rates. Extensive spectroscopic studies reveal photoconversion of 28% and extraordinary thermal stability of photoinduced states at RT for hours, as compared to common nanoseconds. Consequently, this work may open new horizons in applications of magnetic switches based on valence tautomeric metal complexes in molecular devices functioning at room temperature.Acknowledgements
We thank the Fonds der Chemischen Industrie (Liebig Fellowship for MMK) and Deutsche Forschungsgemeinschaft (research grant KH 279/2) for financial support. Prof. Karsten Meyer (Friedrich-Alexander-University of Erlangen-Nuremberg) is acknowledged for general support. We thank Dr Andreas Scheurer and Dr Achim Zahl for assistance with NMR spectroscopy. Fabian Waidhas and Felix Ruf are acknowledged for preliminary photoexperiments with cis-6.Notes and references
- R. M. Buchanan and C. G. Pierpont, J. Am. Chem. Soc., 1980, 102, 4951–4957 CrossRef CAS
.
- D. N. Hendrickson and C. G. Pierpont, Top. Curr. Chem., 2004, 234, 63–95 CrossRef CAS
.
- J. Sedo, J. Saiz-Poseu, F. Busque and D. Ruiz-Molina, Adv. Mater., 2013, 25, 653–701 CrossRef CAS PubMed
.
- V. I. Minkin, Russ. Chem. Bull., 2008, 57, 687–717 CrossRef CAS
.
- O. Sato, J. Tao and Y.-Z. Zhang, Angew. Chem., Int. Ed., 2007, 46, 2152–2187 CrossRef CAS PubMed
.
- A. Dei, D. Gatteschi, C. Sangregorio and L. Sorace, Acc. Chem. Res., 2004, 37, 827–835 CrossRef CAS PubMed
.
- T. Tezgerevska, K. G. Alley and C. Boskovic, Coord. Chem. Rev., 2014, 268, 23–40 CrossRef CAS
.
- D. M. Adams, B. L. Li, J. D. Simon and D. N. Hendrickson, Angew. Chem., Int. Ed., 1995, 34, 1481–1483 CrossRef CAS
.
- O. Sato, S. Hayami, Z.-Z. Gu, K. Seki, R. Nakajima and A. Fujishima, Chem. Lett., 2001, 30, 874–875 CrossRef
.
-
F. Varret, M. Nogues and A. Goujon, in Magnetism: Molecules to Materials, ed. J. S. Miller and M. Drillon, Wiley-VCH Verlag, New York, 2001, pp. 257–295 Search PubMed
.
- O. Sato, A. L. Cui, R. Matsuda, J. Tao and S. Hayami, Acc. Chem. Res., 2007, 40, 361–369 CrossRef CAS PubMed
.
- A. Beni, C. Carbonera, A. Dei, J. F. Letard, R. Righini, C. Sangregorio and L. Sorace, J. Braz. Chem. Soc., 2006, 17, 1522–1533 CrossRef CAS
.
- R. D. Schmidt, D. A. Shultz, J. D. Martin and P. D. Boyle, J. Am. Chem. Soc., 2010, 132, 6261–6273 CrossRef CAS PubMed
.
- R. D. Schmidt, D. A. Shultz and J. D. Martin, Inorg. Chem., 2010, 49, 3162–3168 CrossRef CAS PubMed
.
- M.-L. Boillot, J. Zarembowitch and A. Sour, Top. Curr. Chem., 2004, 234, 261–276 CrossRef CAS
.
-
Molecular Switches, ed. B. L. Feringa and W. R. Browne, WILEY-VCH Verlag & Co. KGaA, Weinheim, Germany, 2011 Search PubMed
.
- M. M. Paquette, R. A. Kopelman, E. Beitler and N. L. Frank, Chem. Commun., 2009, 5424–5426 RSC
.
- M. Milek, A. Witt, C. Streb, F. W. Heinemann and M. M. Khusniyarov, Dalton Trans., 2013, 42, 5237–5241 RSC
.
- M. Milek, F. W. Heinemann and M. M. Khusniyarov, Inorg. Chem., 2013, 52, 11585–11592 CrossRef CAS PubMed
.
- A. Witt, F. W. Heinemann, S. Sproules and M. M. Khusniyarov, Chem.–Eur. J., 2014, 20, 11149–11162 CrossRef CAS PubMed
.
- J. L. R. Williams, R. E. Adel, J. M. Carlson, G. A. Reynolds, D. G. Borden and J. A. Ford, J. Org. Chem., 1963, 28, 387–390 CrossRef CAS
.
- R. M. Buchanan, B. Fitzgerald and C. G. Pierpont, Inorg. Chem., 1979, 18, 3439–3444 CrossRef CAS
.
- M.-C. Chiang and W. Hartung, J. Org. Chem., 1945, 10, 21–25 CrossRef CAS
.
-
E. Bill and JulX, version 1.5, MPI for Bioinorganic Chemistry, Muelheim/Ruhr, Germany, 2008 Search PubMed
.
-
F. Neese, Diploma thesis, University of Konstanz, Konstanz, Germany, 1993
.
- D. F. Evans, J. Chem. Soc., 1959, 2003–2005 RSC
.
-
SADABS 2008/1, Bruker AXS, Inc., Madison, WI, 2009 Search PubMed
.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed
.
- CCDC 1015850–1015852 and 1058866 contain the supplementary crystallographic data for this paper.
- C. G. Pierpont, Coord. Chem. Rev., 2001, 216, 99–125 CrossRef
.
- C. Roux, J. Zarembowitch, B. Gallois, T. Granier and R. Claude, Inorg. Chem., 1994, 33, 2273–2279 CrossRef CAS
.
- M. L. Boillot, S. Pillet, A. Tissot, E. Rivière, N. Claiser and C. Lecomte, Inorg. Chem., 2009, 48, 4729–4736 CrossRef CAS PubMed
.
- Variation of the high-temperature limit of the effective magnetic moment between 5.0 and 6.0 μB does not change the fit parameters significantly (see ESI†).
- Y. Mulyana, G. Poneti, B. Moubaraki, K. S. Murray, B. F. Abrahams, L. Sorace and C. Boskovic, Dalton Trans., 2010, 39, 4757–4767 RSC
.
- D. M. Adams and D. N. Hendrickson, J. Am. Chem. Soc., 1996, 118, 11515–11528 CrossRef CAS
.
-
H. Rau, in Photochromism. Molecules and Systems, ed. H. Dürr and H. Bouas-Laurent, Elsevier, Amsterdam, 2003, pp. 64–164 Search PubMed
.
- N. A. Pavlova, A. I. Poddel'sky, A. S. Bogomyakov, G. K. Fukin, V. K. Cherkasov and G. A. Abakumov, Inorg. Chem. Commun., 2011, 14, 1661–1664 CrossRef CAS
.
-
N. S. Hush, in Progress in Inorganic Chemistry, John Wiley & Sons, Inc., 1967, pp. 391–444 Search PubMed
.
- Note, that the non-linear regression fit used to obtain association constants implies only two species involved into equilibrium, whereas actually three species (5, 6LT and 6HT) are present in solution. However, the employed model is still valid, if the temperature is kept constant and thus the ratio between 6LT and 6HT remains constant too. Hence, the model describing the equilibrium between 5 and a quasi-species {6LT↔ 6HT} is correct.
- D. M. Adams, L. Noodleman and D. N. Hendrickson, Inorg. Chem., 1997, 36, 3966–3984 CrossRef CAS
.
- A. Tissot, M.-L. Boillot, S. Pillet, E. Codjovi, K. Boukheddaden and L. M. L. Daku, J. Phys. Chem. C, 2010, 114, 21715–21722 CAS
.
- S. Thies, H. Sell, C. Schütt, C. Bornholdt, C. Näther, F. Tuczek and R. Herges, J. Am. Chem. Soc., 2011, 133, 16243–16250 CrossRef CAS PubMed
.
- S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sonnichsen, F. Tuczek and R. Herges, Science, 2011, 331, 445–448 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Derivation of the non-linear regression fitting functions, crystallographic details, magnetization and electrochemical data, EPR and electronic absorption spectra. CCDC 1015850–1015852 and 1058866. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc00130g |
| This journal is © The Royal Society of Chemistry 2015 |