Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sugar silanes: versatile reagents for stereocontrolled glycosylation via intramolecular aglycone delivery†
Jordan T.
Walk‡
,
Zachary A.
Buchan‡§
and
John
Montgomery
*
Department of Chemistry, University of Michigan, 930 N. University Ave., Ann Arbor, MI 48109-1055, USA. E-mail: jmontg@umich.edu
First published on 14th April 2015
Abstract
A new method for the intramolecular glycosylation of alcohols is described. Utilizing carbohydrate-derived silanes, the catalytic dehydrogenative silylation of alcohols is followed by intramolecular glycosylation. Appropriate combinations of silane position and protecting groups allow highly selective access to β-manno, α-gluco, or β-gluco stereochemical relationships as well as 2-azido-2-deoxy-β-gluco- and 2-deoxy-β-glucosides. Intramolecular aglycone delivery from the C-2 or C-6 position provides 1,2-cis or 1,2-trans glycosides, respectively. Multifunctional acceptor substrates such as hydroxyketones and diols are tolerated and are glycosylated in a site-selective manner.
Introduction
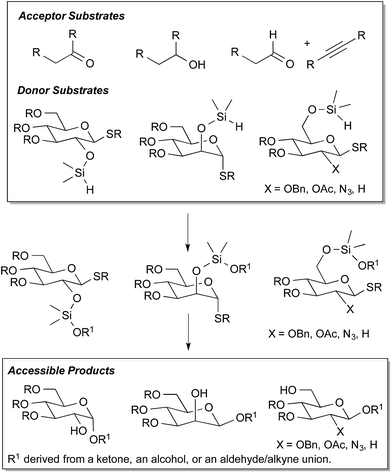
Despite the enormous progress that has been made in the development of chemical glycosylation methods, challenges remain in the efficiency and stereoselectivity of carbohydrate installation.1 Access to the various 1,2-stereochemical arrangements from a common carbohydrate donor is challenging, as careful matching of the anomeric leaving group, protecting groups that influence stereochemistry and reactivity, and reaction conditions is often required.2 The vast majority of methods require that only a single hydroxyl group is unmasked in the acceptor substrate. Furthermore, utilization of acceptor substrates other than alcohols and reactive electrophiles are virtually unexplored.3 Creative developments in intramolecular aglycone delivery,4 including the silicon-tethered version pioneered and developed by Stork5 and Bols,6 have served an important role in modern glycosylation technology. However, improved routes to the requisite tethered substrates and expansion of the range of accessible classes of glycoside products would significantly broaden the appeal and utility of these methods.To address the above challenges and limitations, our laboratory has focused on the development of “sugar silanes” as a versatile reagent class that enables an array of glycosylation processes, providing access to numerous 1,2-stereochemical relationships and utilizing several different types of donor substrates (Fig. 1). Our prior efforts have described the direct reductive glycosylation of carbonyl substrates and the three-component assembly of glycosylated products via the catalytic union of aldehydes, alkynes, and sugar silanes.7 In order to provide a more complete toolbox of glycosylation procedures from sugar silanes, and to address the essential issue of site-selectivity among various reactive functional groups, we now report the direct glycosylation of alcohol substrates by the dehydrogenative condensation with sugar silanes. In addition to identifying effective catalysts to promote this new transformation, utilization of C-25,6 or C-68 intramolecular delivery of the glycosyl acceptor enables highly stereoselective access to β-manno, α-gluco, or β-gluco configurations. The work overcomes challenges that plagued previous strategies for intramolecular aglycone delivery from the C-6 position. Stereocontrolled access to β-glucosides with benzyloxy or acyloxy C-2 substituents as well as 2-azido and 2-deoxyglycosides is made possible by the new procedures described herein.
Results and discussion
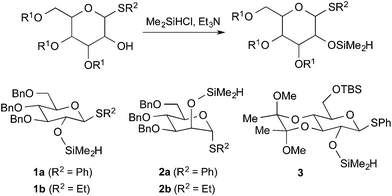
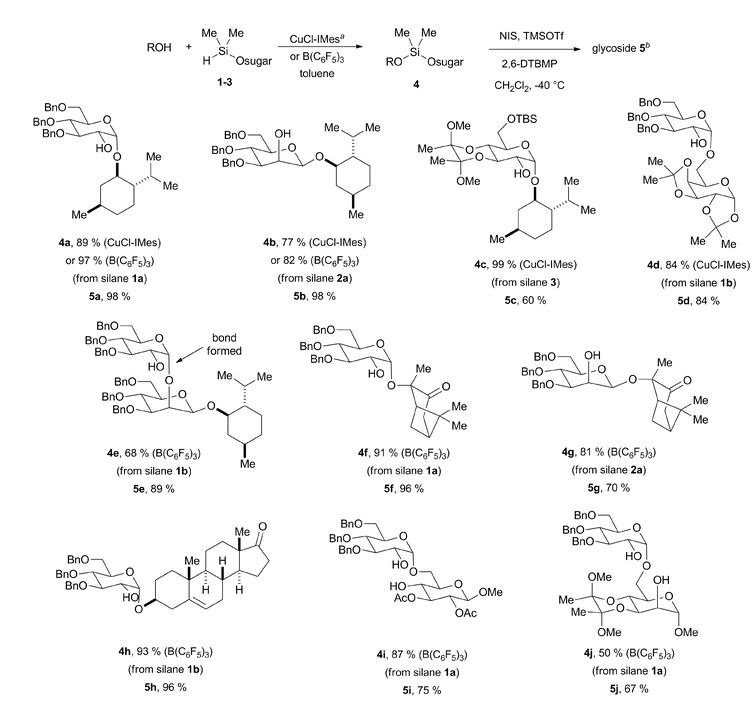
Given the precedent from Stork and Bols on intramolecular aglycone delivery from the C-2 position, our efforts first focused on the utility of mannose- and glucose-derived glycosyl donors bearing the requisite silane functionality at the C-2 position. These reagents are easily accessed in quantitative yield by C-2 protection with commercially available Me2SiHCl (Fig. 2). While bis-electrophilic reagents are most commonly used in the assembly of silyl linkages between two hydroxyls,5,6 the use of Me2SiHCl enjoys the advantage of high heterocoupling efficiency across a range of alcohol substrates.9 Following chloride displacement by the initially added alcohol, a second alcohol then condenses with the resulting silyl hydride (losing H2) in the presence of a transition metal or Lewis acid catalyst, thus effectively preventing homocoupling across a range of substrate combinations.Upon screening numerous catalyst systems to promote the dehydrogenative coupling with alcohol acceptors, two catalyst systems were identified as most robust and exhibiting complementary behavior. While the methods were often interchangeable with similar results, the use of B(C6F5)3 was most effective with more hindered 2° and 3° alcohol substrates,10 whereas a copper–IMes catalyst was most effective with 1° alcohols.11 As the following examples illustrate, a range of hindered and unhindered glycosidic linkages may be created by this method (Table 1). Couplings of menthol with glucose-derived silane 1a are effective using either CuCl–IMes or B(C6F5)3 as catalyst, with the latter allowing dehydrogenative coupling to afford silane intermediate 4a in near quantitative yield. The silyl-linked intermediates were stable to silica gel chromatography and were purified prior to glycosylation. Intramolecular glycosylation with N-iodosuccinimide, trimethylsilyl triflate, and 2,6-di-(t-butyl)-4-methylpyridine cleanly afforded α-glucoside 5a as a single stereoisomer in 98% isolated yield.3b Alternatively, mannose-derived silane 2a allowed the production of β-mannoside 5b in excellent overall yield as a single stereoisomer using either CuCl–IMes or B(C6F5)3 in the silylation step. Tolerance of acetal and silyl protecting groups was demonstrated through the formation of α-glucoside 5c as a single stereoisomer. The method may be applied to the synthesis of disaccharides as demonstrated by the formation of α-glucoside 5d as a single stereoisomer. Additionally, the iterative potential of the method is demonstrated by the synthesis of β-mannoside 5e. In this example, the product 5b is directly converted with sugar silane 1b to intermediate 4e. Intramolecular glycosylation then directly affords product 5e as a single stereoisomer. Given that the C-2 hydroxyl is deprotected during glycosylation, this method may be especially attractive for the synthesis of oligosaccharides that possess repeating C-2 glycosidic linkages. While this method allows glycosylations of the C-2 hydroxyl of mannose as example 5e illustrates, glycosylations of more hindered secondary hydroxyl sugar acceptors were not effective. While the above method builds upon the seminal contributions from Stork and Bols, the streamlined catalytic access to the silicon-tethered intermediates using readily accessible sugar silane reagents advances the practicality of the approach.
Given the range of acceptor substrates that are amenable to glycosylation with sugar silanes (Fig. 1), the chemoselective or regioselective derivatization of a single functional group in a multifunctional substrate becomes a significant question to address.12 As entropic factors govern the intramolecular glycosylation, the chemoselectivity of the initial attachment of the sugar silane to a single functional group introduces a strategy for site-selective glycosylation of complex multifunctional substrates. Since both ketones and alcohols are competent substrates for the catalytic addition of sugar silanes, chemo- and regioselective additions to both hydroxyketones and to diols would provide important advances towards site-selective glycosylation. To address this question, the dehydrogenative silylation of 2-hydroxypinanone was examined. For this substrate, highly chemoselective dehydrogenative silylation of the hydroxyl group was observed with both the glucose and mannose-derived sugar silane to enable the production of α-glucoside 5f or β-mannoside 5g as single stereoisomers through the intermediacy of 4f and 4g. To examine a spatial separation of the ketone and hydroxyl functionalities, the dehydrogenative silylation of a steroid framework was examined, with both the silylation and glycosylation proceeding in excellent yield. In this case, an A-ring hydroxyl was silylated without affecting the D-ring ketone, enabling the production of β-mannoside 5h as a single stereoisomer through the intermediacy of 4h. Finally, two examples involving the C-6 selective glycosylation of a sugar diol were illustrated. The reaction of a 4,6-diol derived from glucose underwent selective C-6 silylation and α-glucosylation to afford 5i. Similarly, reaction of a 2,6-diol derived from mannose underwent selective C-6 silylation and α-glucosylation to afford 5j.
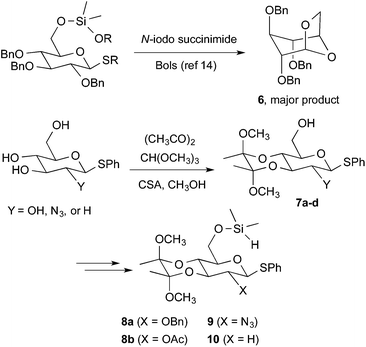
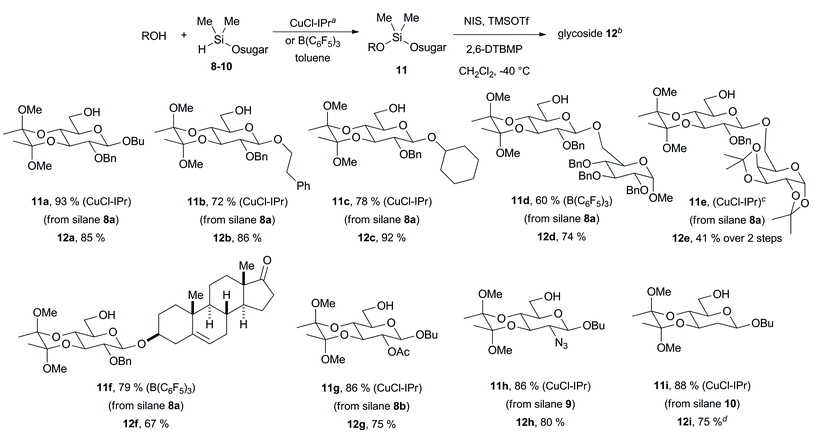
Whereas the synthesis of β-manno and α-gluco stereochemical relationships has been achieved through alternative intramolecular methods, an efficient strategy for β-gluco stereochemical relationships by intramolecular aglycone delivery has not been previously developed.13 Early efforts from Bols demonstrated that C-6 delivery is unsuccessful since bridged bicyclic product 6 is the major product via intramolecular delivery of the internal C-6 oxygen rather than the desired tethered aglycone (Fig. 3).14 While successes were seen with ribose frameworks,14 long-range intramolecular delivery with pyranosides remains an unsolved problem. We reasoned that installation of a conformational bias that prevents chair–chair interconversion should inhibit delivery of the undesired C-6 oxygenation. To evaluate this hypothesis, we utilized the strategy pioneered by Ley to protect the C-3/C-4 trans-diol via the cyclic bisacetal 7 (Fig. 3).15 This protecting group serves the two useful purposes of (1) providing streamlined access to the desired protecting group array, wherein late-stage installation of the C-6 silane is easily allowed, and (2) removing the conformational flexibility that allows the undesired [3.2.1]-oxabicyclic product 6 to form. This strategy allows straightforward access to a range of thiophenyl sugar silanes 8–10 bearing a C-6 silane, a C-3/C-4 trans-diol protected as the bis-acetal, and a range of C-2 substituents including benzyloxy, acetoxy, azido, and hydrogen substituents.
Silylations of alcohols using sugar silanes 8–10 were typically conducted with a CuCl–IPr catalyst derived from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cu
2 Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPr ratio (Table 2).16 Reactions of these silanes were most efficiently accomplished with this hindered catalyst, compared with the CuCl–IMes catalyst that was utilized with silylations of the more hindered C-2 sugar silanes 1–3 (Table 1). As a first pair of examples of the C-6 delivery strategy, the intramolecular glycosylations of butanol and phenethyl alcohol were conducted using sugar silane 8a. In both cases, β-glucoside products 12a and 12b were obtained as single stereoisomers (Table 2). Simple secondary alcohols such as cyclohexanol were effective participants as evidenced by the production of 12c in good yield as a single stereoisomer. Unlike the C-2 delivery procedures that tolerate hindered alcohols, secondary alcohols more hindered than cyclohexanol were poor substrates for C-6 delivery. More hindered substrates such as menthol and C-2 hydroxyl acceptors derived from glucose underwent glycosylation in moderate to low yield. Given this limitation, the CuCl–IPr catalyst system was used in the majority of the C-6 delivery examples as this method is most effective with the primary acceptor alcohols employed.
IPr ratio (Table 2).16 Reactions of these silanes were most efficiently accomplished with this hindered catalyst, compared with the CuCl–IMes catalyst that was utilized with silylations of the more hindered C-2 sugar silanes 1–3 (Table 1). As a first pair of examples of the C-6 delivery strategy, the intramolecular glycosylations of butanol and phenethyl alcohol were conducted using sugar silane 8a. In both cases, β-glucoside products 12a and 12b were obtained as single stereoisomers (Table 2). Simple secondary alcohols such as cyclohexanol were effective participants as evidenced by the production of 12c in good yield as a single stereoisomer. Unlike the C-2 delivery procedures that tolerate hindered alcohols, secondary alcohols more hindered than cyclohexanol were poor substrates for C-6 delivery. More hindered substrates such as menthol and C-2 hydroxyl acceptors derived from glucose underwent glycosylation in moderate to low yield. Given this limitation, the CuCl–IPr catalyst system was used in the majority of the C-6 delivery examples as this method is most effective with the primary acceptor alcohols employed.
The formation of C-6 linked disaccharides proceeded efficiently, as evidenced by the formation of products 12d and 12e. In the former case (12d), coupling using B(C6F5)3 avoided the formation of a homocoupling product derived from partial hydrolysis of the sugar silane reagent. In the latter case (12e), a small amount of an impurity derived from dimerization of the sugar donor 8a was inseparable from intermediate 11e. Nonetheless, subjecting the mixture to glycosylation conditions led to the clean production of product 12e, which was easily purified. Additionally, an example demonstrated the chemoselectivity for hydroxyls over ketones in the production of 12f. In this case, the site-selective alcohol silylation is best accomplished with B(C6F5)3 as the catalyst.
In addition to C-2 benzyloxy examples 12a–f, sugar silanes possessing C-2 acetoxy substituents, C-2 azido substituents, and those lacking C-2 substitution were cleanly tolerated in the production of 12g–i. It should be noted that the directing influence of C-2 acetyl and C-2 benzoyl protecting groups is commonly employed in the facile synthesis of β-glucosides. However, β-selective glycosylation of donors lacking C-2 acyloxy substituents, such as benzyloxy, 2-azido-2-deoxy,17 and 2-deoxyglycosides,2a,18 present much more challenging substrates for controlled β-selective glycosylation. Interestingly, recent studies have shown that 3,4-trans-cyclic protecting groups with 2-deoxyglycosides favor α-selective intermolecular glycosylations, which are thus fully complementary to the β-selective intramolecular process illustrated herein.18b With the exception of one example (12g), each of the examples in Table 2 involves the more challenging classes of substrates that lack a stereochemistry-directing C-2 substituent.
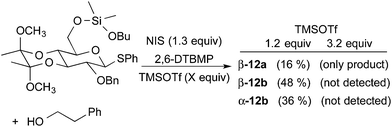
Given the difficulties noted above in prior strategies for C-6 delivery with alternate protecting groups (Fig. 3), control experiments were conducted to ensure that glycoside formation proceeds by an intramolecular process. Repeating the conversion of silyl intermediate 11a to glycoside 12a in the presence of exogenous phenethyl alcohol resulted in the formation of 12a (16%), 12b (48%), and the α-anomer of 12b (36%) as judged by analysis of the crude reaction mixture. However, increasing the amount of TMSOTf (3.2 equiv.) to enable complete silylation of the exogenous alcohol produced only 12a (Fig. 4). These results illustrate that silyl cleavage followed by intermolecular glycosylation is not occurring under the standard method reported (Table 2) since only the intramolecular delivery is β-selective. Furthermore, addition of excess TMSOTf effectively suppresses the glycosylation of free alcohols and enables the β-selective intramolecular delivery to exclusively proceed when an exogenous alcohol is present.
Conclusions
In summary, this work demonstrates a versatile new method for stereoselective glycosylation, utilizing the catalytic dehydrogenative coupling of sugar silane reagents with simple alcohols followed by silicon-tethered intramolecular aglycone delivery. The current work builds upon the known intramolecular glycosylation by C-2 delivery, and a new strategy enabling the stereochemically complementary and previously inaccessible delivery from C-6 has been developed by utilizing conformational constraints placed in the sugar donor. Put together, these methods provide great flexibility in the construction of glycosidic bonds with the available linkages including those of the β-manno, α-gluco, or β-gluco type. Additionally, challenging substrate classes including 2-benzyloxy, 2-azido, and 2-deoxy sugars are tolerated by the method, with the current procedure allowing glycosylation of primary hydroxyls. The site-selective glycosylation of hydroxyketones and sugar diols is enabled through this approach with proper selection of the dehydrogenative silylation catalyst. Future work will focus on the utilization of this work in combination with previously developed methods7 in increasingly complex illustrations of site-selective glycosylation of polyfunctional substrates.Acknowledgements
We thank the National Institutes of Health (GM57014) for support of this research.Notes and references
- (a) K. Toshima and K. Tatsuta, Chem. Rev., 1993, 93, 1503–1531 CrossRef CAS; (b) X. M. Zhu and R. R. Schmidt, Angew. Chem., Int. Ed., 2009, 48, 1900–1934 CrossRef CAS PubMed; (c) J. D. C. Codee, R. Litjens, L. J. van den Bos, H. S. Overkleeft and G. A. van der Marel, Chem. Soc. Rev., 2005, 34, 769–782 RSC; (d) M. Shimizu, H. Togo and M. Yokoyama, Synthesis, 1998, 799–822 CrossRef CAS PubMed; (e) A. Z. Aljahdali, P. Shi, Y. S. Zhong and G. A. O'Doherty, Adv. Carbohydr. Chem. Biochem., 2013, 69, 55–123 CrossRef CAS PubMed.
- For representative state-of-the-art methods: (a) J. P. Issa and C. S. Bennett, J. Am. Chem. Soc., 2014, 136, 5740–5744 CrossRef CAS PubMed; (b) T. Kimura, M. Sekine, D. Takahashi and K. Toshima, Angew. Chem., Int. Ed., 2013, 52, 12131–12134 CrossRef CAS PubMed; (c) Y. Okada, N. Asakura, M. Bando, Y. Ashikaga and H. Yamada, J. Am. Chem. Soc., 2012, 134, 6940–6943 CrossRef CAS PubMed; (d) L. Krock, D. Esposito, B. Castagner, C.-C. Wang, P. Bindschadler and P. H. Seeberger, Chem. Sci., 2012, 3, 1617–1622 RSC; (e) D. Kahne, S. Walker, Y. Cheng and D. Vanengen, J. Am. Chem. Soc., 1989, 111, 6881–6882 CrossRef CAS; (f) D. Crich and S. X. Sun, J. Am. Chem. Soc., 1998, 120, 435–436 CrossRef CAS.
- (a) D. Y. Zhu, K. N. Baryal, S. Adhikari and J. L. Zhu, J. Am. Chem. Soc., 2014, 136, 3172–3175 CrossRef CAS PubMed; (b) R. R. Schmidt, Angew. Chem., Int. Ed. Engl., 1986, 25, 212–235 CrossRef PubMed; (c) D. A. Ryan and D. Y. Gin, J. Am. Chem. Soc., 2008, 130, 15228–15229 CrossRef CAS PubMed.
- (a) K. H. Jung, M. Muller and R. R. Schmidt, Chem. Rev., 2000, 100, 4423–4442 CrossRef CAS PubMed; (b) I. Cumpstey, Carbohydr. Res., 2008, 343, 1553–1573 CrossRef CAS PubMed; (c) A. Ishiwata, Y. J. Lee and Y. Ito, Org. Biomol. Chem., 2010, 8, 3596–3608 RSC; (d) F. Barresi and O. Hindsgaul, J. Am. Chem. Soc., 1991, 113, 9376–9377 CrossRef CAS.
- (a) G. Stork and J. J. LaClair, J. Am. Chem. Soc., 1996, 118, 247–248 CrossRef CAS; (b) G. Stork and G. Kim, J. Am. Chem. Soc., 1992, 114, 1087–1088 CrossRef CAS; (c) See also: G. K. Packard and S. D. Rychnovsky, Org. Lett., 2001, 3, 3393–3396 CrossRef CAS PubMed.
- (a) M. Bols, Acta Chem. Scand., 1996, 50, 931–937 CrossRef CAS PubMed; (b) M. Bols, J. Chem. Soc., Chem. Commun., 1993, 791–792 RSC; (c) M. Bols, J. Chem. Soc., Chem. Commun., 1992, 913–914 RSC.
- (a) Z. A. Buchan, S. J. Bader and J. Montgomery, Angew. Chem., Int. Ed., 2009, 48, 4840–4844 CrossRef CAS PubMed; (b) K. M. Partridge, S. J. Bader, Z. A. Buchan, C. E. Taylor and J. Montgomery, Angew. Chem., Int. Ed., 2013, 52, 13647–13650 CrossRef CAS PubMed.
- H. Sugimura and R. Watanabe, Chem. Lett., 2008, 37, 1038–1039 CrossRef CAS.
- (a) M. Petit, G. Chouraqui, C. Aubert and M. Malacria, Org. Lett., 2003, 5, 2037–2040 CrossRef CAS PubMed; (b) S. Bracegirdle and E. A. Anderson, Chem. Soc. Rev., 2010, 39, 4114–4129 RSC.
- J. M. Blackwell, K. L. Foster, V. H. Beck and W. E. Piers, J. Org. Chem., 1999, 64, 4887–4892 CrossRef CAS PubMed.
- (a) H. Kaur, F. K. Zinn, E. D. Stevens and S. P. Nolan, Organometallics, 2004, 23, 1157–1160 CrossRef CAS; (b) S. Diez-Gonzalez, H. Kaur, F. K. Zinn, E. D. Stevens and S. P. Nolan, J. Org. Chem., 2005, 70, 4784–4796 CrossRef CAS PubMed.
- For advances in site-selective derivatization of sugar diols: (a) D. Lee and M. S. Taylor, Synthesis, 2012, 44, 3421–3431 CrossRef CAS PubMed; (b) D. Lee and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 3724–3727 CrossRef CAS PubMed; (c) E. Mensah, N. Camasso, W. Kaplan and P. Nagorny, Angew. Chem., Int. Ed., 2013, 52, 12932–12936 CrossRef CAS PubMed; (d) I. H. Chen, K. G. M. Kou, D. N. Le, C. M. Rathbun and V. M. Dong, Chem. – Eur. J., 2014, 20, 5013–5018 CrossRef CAS PubMed; (e) C. L. Allen and S. J. Miller, Org. Lett., 2013, 15, 6178–6181 CrossRef CAS PubMed.
- For advances using hydrogen-bonding interactions: (a) J. P. Yasomanee and A. V. Demchenko, Angew. Chem., Int. Ed., 2014, 53, 10453–10456 CrossRef CAS PubMed; (b) S. G. Pistorio, J. P. Yasomanee and A. V. Demchenko, Org. Lett., 2014, 16, 716–719 CrossRef CAS PubMed.
- M. Bols and H. C. Hansen, Chem. Lett., 1994, 1049–1052 CrossRef CAS.
- (a) S. V. Ley, D. K. Baeschlin, D. J. Dixon, A. C. Foster, S. J. Ince, H. W. M. Priepke and D. J. Reynolds, Chem. Rev., 2001, 101, 53–80 CrossRef CAS PubMed; (b) A. Hense, S. V. Ley, H. M. I. Osborn, D. R. Owen, J. F. Poisson, S. L. Warriner and K. E. Wesson, J. Chem. Soc., Perkin Trans. 1, 1997, 2023–2031 RSC.
- S. Díez-González, E. D. Stevens, N. M. Scott, J. L. Petersen and S. P. Nolan, Chem.–Eur. J., 2008, 14, 158–168 CrossRef PubMed.
- (a) J. Park, S. Kawatkar, J. H. Kim and G. J. Boons, Org. Lett., 2007, 9, 1959–1962 CrossRef CAS PubMed; (b) Y.-P. Hu, Y.-Q. Zhong, Z.-G. Chen, C.-Y. Chen, Z. Shi, M. M. L. Zulueta, C.-C. Ku, P.-Y. Lee, C.-C. Wang and S.-C. Hung, J. Am. Chem. Soc., 2012, 134, 20722–20727 CrossRef CAS PubMed.
- (a) A. Borovika and P. Nagorny, J. Carbohydr. Chem., 2012, 31, 255–283 CrossRef CAS; (b) E. I. Balmond, D. Benito-Alifonso, D. M. Coe, R. W. Alder, E. M. McGarrigle and M. C. Galan, Angew. Chem., Int. Ed., 2014, 53, 8190–8194 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and copies of NMR spectra. See DOI: 10.1039/c5sc00810g |
| ‡ J.T.W. and Z.A.B. contributed equally. |
| § Current address: Discovery Chemistry, R&D, Dow AgroSciences LLC, 9330 Zionsville Rd, Indianapolis, IN 46268. |
| This journal is © The Royal Society of Chemistry 2015 |