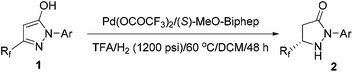Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd-catalyzed asymmetric hydrogenation of fluorinated aromatic pyrazol-5-ols via capture of active tautomers†
Zhang-Pei
Chen
a,
Mu-Wang
Chen
a,
Lei
Shi
a,
Chang-Bin
Yu
a and
Yong-Gui
Zhou
ab
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: ygzhou@dicp.ac.cn
bCollaborative Innovation Centre of Chemical Science and Engineering (Tianjin), Tianjin 300071, P. R. China
First published on 31st March 2015
Abstract
An efficient palladium-catalyzed asymmetric hydrogenation of fluorinated aromatic pyrazol-5-ols has been developed via capture of the active tautomers. A wide variety of 2,5-disubstituted and 2,4,5-trisubstituted pyrazolidinones have been synthesized with up to 96% and 95% ee, respectively. The hydrogenation pathway includes Brønsted acid promoted tautomerization of pyrazol-5-ols and Pd-catalyzed asymmetric hydrogenation of the active tautomer.
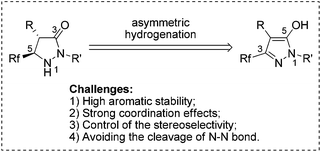
Pyrazolidinones and related 1,2-diaza-3-one heterocycles are highly desirable building blocks owing to the fact that they frequently set up the core framework of numerous pharmaceutically and agrochemically active compounds.1 Particularly, aside from drug development, optically pure pyrazolidinones have also shown great advantages in synthetic methodology.2 For example, the chiral pyrazolidinones could act as efficient catalysts to promote Diels–Alder reactions, and catalyze kinetic resolution of racemic secondary alcohols.3 Over the last decades, the introduction of fluorine into molecules has received increasing attention in the fields of medicinal, agricultural, and material chemistry, primarily because the isosteric replacement of hydrogen by fluorine enhanced the lipophilicity, metabolic stability, and bioavailability of the parent compounds.4 Consequently, reliable methods toward the facile generation of optically active fluorinated pyrazolidinones would be very desirable in organic synthesis and drug research. However, the methods to chiral pyrazolidinones were still limited to transformation from chiral materials,5 classical chemical resolution3a or kinetic resolution involving pyrazolidinone imides,6 and the synthesis of fluorinated pyrazolidinones was rarely explored. Considering the ready availability and easy preparation of fluorinated pyrazol-5-ols, asymmetric hydrogenation of these compounds would provide atom-economical and straightforward access to optically pure pyrazolidinones (Scheme 1).
Despite much progress having been achieved in the asymmetric hydrogenation of heteroaromatics7 including quinolines,8 iso-quinolines,9 quinoxalines,10 pyridines,11 indoles,12 pyrroles,13 (benzo)furans,14 (benzo)thiophenes,15 imidazoles,16 indolizines,17 pyrimidines,18 and naphthyridines,19 a great number of problems still remain unsettled in this field, such as the catalytic asymmetric hydrogenation of aromatic rings containing free hydroxyl, amido or other electron-enriched functional groups and heteroarenes containing two or more adjacent heteroatoms. The intrinsic problems are apparent: (i) the inherent stability resulting from aromaticity; (ii) the strong coordination effects endowed by the heteroatoms; (iii) the difficulty of controlling the stereoselectivity; (iv) the facile cleavage of the bond between the heteroatoms.20 Therefore, the hydrogenation of this kind of electron-enriched aromatic pyrazol-5-ol with a free hydroxyl and two adjacent nitrogen-atoms is a great and significant challenge. Herein, we wish to report our initial findings on the development of a Pd-catalyzed asymmetric hydrogenation of fluorinated pyrazol-5-ols with excellent enantioselectivities, yields, and diastereoselectivities.
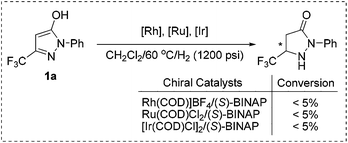
At the outset, the readily available 1-phenyl-3-(trifluoromethyl)-1H-pyrazol-5-ol 1a, which can be synthesized from the easily accessible starting materials ethyl 4,4,4-trifluoro-3-oxobutanoate and phenylhydrazine,21 was selected as the model substrate for investigation. To our disappointment, the hydrogenation failed to proceed in the presence of common Rh, Ru, and Ir catalysts (Scheme 2). This may be ascribed to the strong coordination effects and highly electron-enriched nature of fluorinated pyrazol-5-ols that impeded the hydrogenation.
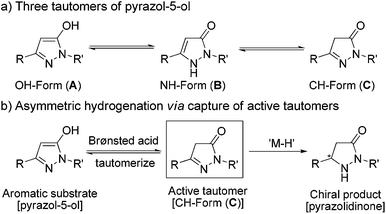
In principle, these kinds of substrates exist in three tautomeric forms, i.e. the OH– (form A), the NH– (form B) and the CH–isomer (form C) (Scheme 3).1e,22 A literature search23 and experimental data including X-ray crystal structure24 analysis demonstrated that form A is the most stable and dominant form. In view of the fact that the alkylation of pyrazol-5-ols would selectively lead to O-alkyl derivatives or N-alkyl derivatives as the alkylation products under specific requirements, we supposed that appropriate conditions would promote the tautomerization of the highly aromatic tautomer A to the more active tautomer C. Previous results have demonstrated that trifluoroacetic acid (TFA) could promote 1,4-dihydroxynaphthalene to form its stable tautomer tetralin-1,4-dione,25 and accelerate iminium–enamine isomerization to facilitate hydrogenation.26 On the basis of these analyses, we envisioned that the combination of a Pd catalyst which is excellently tolerant to acid27 and a Brønsted acid could be suitable for the asymmetric hydrogenation of fluorinated pyrazol-5-ols.
To our delight, the exposure of 1a to TFA in dichloromethane furnished the desired pyrazolidinone 2a with 91% ee and 54% conversion using Pd(OCOCF3)2/(S)-BINAP as the catalyst (Table 1, entry 1). When the reaction was carried out in 2,2,2-trifluoroethanol (TFE), the reactivity and enantioselectivity dropped slightly (entry 2). Subsequently, the effects of other acids were investigated, and TFA was the most suitable choice in view of the reactivity and enantioselectivity.
| Entry | Catalyst | Additive | Yield (%)b | Ee (%)c |
|---|---|---|---|---|
| a Reaction conditions: Pd(OCOCF3)2 (2 mol%), ligand (2.1 mol%), 1a (0.3 mmol), additive (0.3 mmol), H2 (1200 psi), DCM (2 mL), 60 °C, 36 h. b Isolated yields. c Determined by HPLC. d Using TFE as solvent. e 48 h. | ||||
| 1 | Pd(OCOCF3)2 + L1 | TFA | 54 | 91 |
| 2d | Pd(OCOCF3)2 + L1 | TFA | 52 | 90 |
| 3 | Pd(OCOCF3)2 + L1 | L-CSA | 46 | 90 |
| 4 | Pd(OCOCF3)2 + L1 | D-CSA | 39 | 90 |
| 5 | Pd(OCOCF3)2 + L1 | TsOH·H2O | 32 | 90 |
| 6 | Pd(OCOCF3)2 + L2 | TFA | 81 | 96 |
| 7 | Pd(OCOCF3)2 + L3 | TFA | 46 | 93 |
| 8 | Pd(OCOCF3)2 + L4 | TFA | 29 | 90 |
| 9 | Pd(OCOCF3)2 + L5 | TFA | 20 | 97 |
| 10e | Pd(OCOCF3)2 + L2 | TFA | 94 | 96 |
|
||||
Further examinations were focused on ligand screening. From the evaluation of the various commercially available chiral axial bisphosphine ligands, excellent enantioselectivity was obtained with ligand L2 (entry 6), providing the product in 96% ee and 81% isolated yield. When the reaction time was prolonged to 48 hours, the yield was further improved without loss of enantioselectivity (entry 10). Therefore, the optimal condition was established as: Pd(OCOCF3)2/L2/TFA in dichloromethane.
With the optimal conditions in hand, exploration of the substrate scope was carried out, and the results are summarized in Table 2. Gratifyingly, a variety of 1-aryl substituted substrates were smoothly converted to the corresponding pyrazolidinones with excellent enantioselectivities (82–96% ee). The electronic properties of the substituents on the phenyl ring had little effect on the activity and enantioselectivity (entry 5 vs. entries 6–8). However, the hydrogenation of 2-o-tolyl-substituted pyrazol-5-ol 1b gave a moderate 82% enantioselectivity and 67% yield (entry 2). When TangPhos L5, which was developed by Zhang's group in 2002,28 was employed and the temperature was elevated to 100 °C, the pyrazol-5-ol substrates (1i–1j) bearing a pentafluoroethyl substituent could also be hydrogenated with excellent enantioselectivities and yields (entries 9 and 10).
| Entry | R F | Ar | Yield (%)b | Ee (%)c |
|---|---|---|---|---|
| a Pd(OCOCF3)2 (2 mol%), (S)-MeO-Biphep (2.1 mol%), 1 (0.3 mmol), H2 (1200 psi), TFA (0.3 mmol), DCM (2 mL), 60 °C, 48 h. b Isolated yields. c Determined by HPLC. d Pd(OCOCF3)2 (4 mol%), (S)-MeO-Biphep (4.2 mol%), 1b(0.2 mmol), H2 (1200 psi), TFA (0.2 mmol), DCM (2 mL), 60 °C, 48 h. e Pd(OCOCF3)2 (4 mol%), (S,S′,R,R′)-TangPhos (5.2 mol%), 1 (0.2 mmol), H2 (1200 psi), L-CSA (0.2 mmol), TFE (2 mL), 100 °C, 48 h. | ||||
| 1 | CF3 | C6H5 | 94 (2a) | 96 (S) |
| 2d | CF3 | 2-MeC6H4 | 67 (2b) | 82 (+) |
| 3 | CF3 | 3-MeC6H4 | 93 (2c) | 95 (+) |
| 4 | CF3 | 4-MeC6H4 | 93 (2d) | 96 (+) |
| 5 | CF3 | 4-MeOC6H4 | 94 (2e) | 95 (+) |
| 6 | CF3 | 3-ClC6H4 | 89 (2f) | 95 (+) |
| 7 | CF3 | 3,4-Cl2C6H3 | 90 (2g) | 93 (+) |
| 8 | CF3 | 4-FC6H4 | 93 (2h) | 94 (+) |
| 9e | C2F5 | C6H5 | 95 (2i) | 94 (−) |
| 10e | C2F5 | 4-MeC6H4 | 92 (2j) | 95 (−) |
For the sake of further estimating the application possibility, a range of 4-substituted 3-(trifluoromethyl)-1H-pyrazol-5-ols (3a–3g) were also investigated (Table 3). The substrates with an alkyl-substituent at the 4-position could be hydrogenated smoothly, providing the corresponding the 2,4,5-trisubstituted pyrazolidinone derivatives with high enantioselectivity and diastereoselectivity. The high diastereoselectivity probably results from the thermodynamic stability of the trans products under these harsh acidic conditions. Substrates bearing long alkyl or bulky substituents at the C4 position gave slightly higher enantioselectivities (entry 1 vs. entries 3,6,7).
| Entry | R | Ar | Yield (%)b | Ee (%)c |
|---|---|---|---|---|
a Pd(OCOCF3)2 (4 mol%), (S,S′,R,R′)-TangPhos (5.2 mol%), 3 (0.2 mmol), H2 (1200 psi), L-CSA (0.2 mmol), TFE (2 mL), 100 °C, 48 h. In all cases dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
b Isolated yields.
c Determined by HPLC. 1.
b Isolated yields.
c Determined by HPLC.
|
||||
| 1 | Me | C6H5 | 92 (4a) | 89 (−) |
| 2 | Me | 4-MeC6H4 | 97 (4b) | 88 (+) |
| 3 | Et | C6H5 | 93 (4c) | 94 (+) |
| 4 | Et | 4-MeC6H4 | 92 (4d) | 93 (+) |
| 5 | Et | 3-MeC6H4 | 90 (4e) | 92 (+) |
| 6 | n Pr | C6H5 | 95 (4f) | 93 (+) |
| 7 | Bn | C6H5 | 94 (4g) | 95 (4S,5R) |
The absolute configurations of the hydrogenation products 2a and 4g were determined by X-ray diffraction analysis by recrystallization from the solvent mixture dichloromethane–n-hexane.29 The configurations of the other chiral products were assigned by analogy.
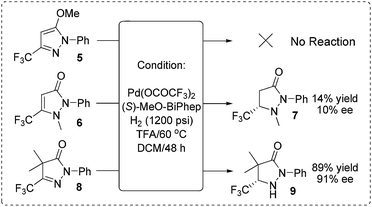
In order to verify our hypothesis that the hydrogenation was carried out via capture of the active tautomer, we synthesized three compounds (form A type 5, form B type 6 and form C type 8) and subject them to identical hydrogenation reactions (Scheme 4). As expected, no reaction was observed for the substrate 5; for substrate 6, a low 10% ee and 14% yield were obtained; the CH–form substrate 8 gave an excellent 91% ee with 89% yield. Based on the experimental results and stereochemistry of the products, we proposed that the reaction experienced the process of Brønsted acid promoted tautomerization to form CH–form tautomer C, followed by Pd-catalyzed asymmetric hydrogenation of the active tautomer C to give the optically active pyrazolidinones. This preliminary result demonstrated the practicability of our strategy of asymmetric hydrogenation of the inseparable active isomers to realize hydrogenation of the intractable isomerisable substrates.
Conclusions
An efficient palladium-catalyzed asymmetric hydrogenation of fluorinated aromatic pyrazol-5-ols has been developed via capture of the active tautomers. A wide variety of 2,5-disubstituted and 2,4,5-trisubstituted pyrazolidinone derivatives have been synthesized with up to 96% and 95% ee, respectively. The hydrogenation pathway includes Brønsted acid promoted tautomerization of the pyrazol-5-ols and palladium-catalyzed asymmetric hydrogenation of the active tautomer. This study provides some enlightenment of the application of asymmetric hydrogenation and useful information for the design of new reactions. Further study on applying this novel strategy to other aromatic compounds and exploration of the applications of the chiral pyrazolidinones are in progress in our laboratory.Acknowledgements
Financial support from the National Natural Science Foundation of China (21372220 and 21125208) is acknowledged.Notes and references
- (a) L. N. Jungheim and S. K. Sigmund, J. Org. Chem., 1987, 52, 4007 CrossRef CAS; (b) J. M. Indelicato and C. E. Pasini, J. Med. Chem., 1988, 31, 1227 CrossRef CAS; (c) R. E. Holmes and D. A. Neel, Tetrahedron Lett., 1990, 31, 5567 CrossRef CAS; (d) R. M. Claramunt and J. Elguero, Org. Prep. Proced. Int., 1991, 23, 273 CrossRef CAS; (e) E. Couloigner, D. Cartier and R. Labia, Bioorg. Med. Chem. Lett., 1999, 9, 2205 CrossRef CAS; (f) W. Chen, X. H. Yuan, R. Li, W. Du, Y. Wu, L. S. Ding and Y. C. Chen, Adv. Synth. Catal., 2006, 348, 1818 CrossRef CAS PubMed; (g) L. Pezdirc, U. Groselj, A. Meden, B. Stanovnik and J. Svete, Tetrahedron Lett., 2007, 48, 5205 CrossRef CAS PubMed; (h) L. Pezdirc, B. Stanovnik and J. Svete, Aust. J. Chem., 2009, 62, 1661 CrossRef CAS.
- C. M. R. Low, J. W. Black, H. B. Broughton, I. M. Buck, J. M. R. Davies, D. J. Dunstone, R. A. D. Hull, S. B. Kalindjian, I. M. McDonald, M. J. Pether, N. P. Shankley and K. I. M. Steel, J. Med. Chem., 2000, 43, 3505 CrossRef CAS PubMed.
- (a) E. Gould, T. Lebl, A. M. Z. Slawin, M. Reid and A. D. Smith, Tetrahedron, 2010, 66, 8992 CrossRef CAS PubMed; (b) G. Ma, J. Deng and M. P. Sibi, Angew. Chem., Int. Ed., 2014, 53, 11818 CrossRef CAS PubMed.
- (a) J.-A. Ma and D. Cahard, Chem. Rev., 2004, 104, 6119 CrossRef CAS PubMed; (b) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (c) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (d) J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef CAS; (e) D. Cahard, X. Xu, S. Couve-Bonnaire and X. Pannecoucke, Chem. Soc. Rev., 2010, 39, 558 RSC; (f) Y. Zheng and J.-A. Ma, Adv. Synth. Catal., 2010, 352, 2745 CrossRef CAS PubMed; (g) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS PubMed; (h) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed; (i) H.-R. He, Y.-Y. Huang and F. Verpoort, Acta Chim. Sin., 2013, 71, 700 CrossRef.
- C. Seki, M. Hirama, T. Sato, S. Takeda, Y. Kohari, K. Ishigaki, M. Ohuchi, K. Yokoi, H. Nakano, K. Uwai, N. Takano, K. Umemura and H. Matsuyama, Heterocycles, 2012, 85, 1045 CrossRef CAS.
- M. Wang, Z. Huang, J. Xu and Y.-R. Chi, J. Am. Chem. Soc., 2014, 136, 1214 CrossRef CAS PubMed.
- For recent reviews on asymmetric hydrogenation of heteroaromatic compounds, see: (a) F. Glorius, Org. Biomol. Chem., 2005, 3, 4171 RSC; (b) S.-M. Lu, X.-W. Han and Y.-G. Zhou, Chin. J. Org. Chem., 2005, 25, 634 CAS; (c) Y.-G. Zhou, Acc. Chem. Res., 2007, 40, 1357 CrossRef CAS PubMed; (d) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557 CrossRef CAS PubMed; (e) Y.-M. He, F.-T. Song and Q.-H. Fan, Top. Curr. Chem., 2014, 343, 145 CrossRef; (f) Z.-S. Ye, L. Shi and Y.-G. Zhou, Synlett, 2014, 25, 928 CrossRef CAS PubMed; (g) J. Xie and Q. Zhou, Acta Chim. Sin., 2012, 70, 1427 CrossRef CAS.
- For representative reports on asymmetric hydrogenation of quinolines, see: (a) W.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han and Y.-G. Zhou, J. Am. Chem. Soc., 2003, 125, 10536 CrossRef CAS PubMed; (b) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 3683 CrossRef CAS PubMed; (c) Q.-S. Guo, D.-M. Du and J. Xu, Angew. Chem., Int. Ed., 2008, 47, 759 CrossRef CAS PubMed; (d) H. Zhou, Z. Li, Z. Wang, T. Wang, L. Xu, Y. He, Q.-H. Fan, J. Pan, L. Gu and A. S. C. Chan, Angew. Chem., Int. Ed., 2008, 47, 8464 CrossRef CAS PubMed; (e) C. Wang, C. Li, X. Wu, A. Pettman and J. Xiao, Angew. Chem., Int. Ed., 2009, 48, 6524 CrossRef CAS PubMed; (f) T. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878 CrossRef CAS PubMed; (g) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed; (h) T. Wang, F. Chen, J. Qin, Y.-M. He and Q.-H. Fan, Angew. Chem., Int. Ed., 2013, 52, 7172 CrossRef CAS PubMed.
- For recent works on asymmetric reduction of isoquinolines, see: (a) S.-M. Lu, Y.-Q. Wang, X.-W. Han and Y.-G. Zhou, Angew. Chem., Int. Ed., 2006, 45, 2260 CrossRef CAS PubMed; (b) L. Shi, Z.-S. Ye, L.-L. Cao, R.-N. Guo, Y. Hu and Y.-G. Zhou, Angew. Chem., Int. Ed., 2012, 51, 8286 CrossRef CAS PubMed; (c) A. Iimuro, K. Yamaji, S. Kandula, T. Nagano, Y. Kita and K. Mashima, Angew. Chem., Int. Ed., 2013, 52, 2046 CrossRef CAS PubMed; (d) Z.-S. Ye, R.-N. Guo, X.-F. Cai, M.-W. Chen, L. Shi and Y.-G. Zhou, Angew. Chem., Int. Ed., 2013, 52, 3685 CrossRef CAS PubMed.
- For representative reports on asymmetric hydrogenation of quinoxalines, see: (a) C. Bianchini, P. Barbaro, G. Scapacci, E. Farnetti and M. Graziani, Organometallics, 1998, 17, 3308 CrossRef CAS; (b) W. Tang, L. Xu, Q.-H. Fan, J. Wang, B. Fan, Z. Zhou, K.-H. Lam and A. S. C. Chan, Angew. Chem., Int. Ed., 2009, 48, 9135 CrossRef CAS PubMed; (c) M. Rueping, F. Tato and F. R. Schoepke, Chem.–Eur. J., 2010, 16, 2688 CrossRef CAS PubMed; (d) Q.-A. Chen, D.-S. Wang, Y.-G. Zhou, Y. Duan, H.-J. Fan, Y. Yang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 6126 CrossRef CAS PubMed; (e) J. Qin, F. Chen, Z. Ding, Y.-M. He, L. Xu and Q.-H. Fan, Org. Lett., 2011, 13, 6568 CrossRef CAS PubMed; (f) Z. Zhang and H. Du, Angew. Chem., Int. Ed., 2015, 54, 623 CAS.
- For representative reports on asymmetric hydrogenation of pyridines, see: (a) M. Studer, C. Wedemeyer-Exl, F. Spindler and H.-U. Blaser, Monatsh. Chem., 2000, 131, 1335 CrossRef CAS; (b) F. Glorius, N. Spielkamp, S. Holle, R. Goddard and C. W. Lehmann, Angew. Chem., Int. Ed., 2004, 43, 2850 CrossRef CAS PubMed; (c) C. Y. Legault and A. B. Charette, J. Am. Chem. Soc., 2005, 127, 8966 CrossRef CAS PubMed; (d) M. Rueping and A. P. Antonchick, Angew. Chem., Int. Ed., 2007, 46, 4562 CrossRef CAS PubMed; (e) X.-B. Wang, W. Zeng and Y.-G. Zhou, Tetrahedron Lett., 2008, 49, 4922 CrossRef CAS PubMed; (f) W.-J. Tang, J. Tan, L.-J. Xu, K.-H. Lam, Q.-H. Fan and A. S. C. Chan, Adv. Synth. Catal., 2010, 352, 1055 CrossRef CAS PubMed; (g) Z.-S. Ye, M.-W. Chen, Q.-A. Chen, L. Shi, Y. Duan and Y.-G. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10181 CrossRef CAS PubMed; (h) Y. Liu and H. Du, J. Am. Chem. Soc., 2013, 135, 12968 CrossRef CAS PubMed; (i) M. Chang, Y. Huang, S. Liu, Y. Chen, S. W. Krska, I. W. Davies and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 12761 CrossRef CAS PubMed; (j) Y. Kita, A. Iimuro, S. Hida and K. Mashima, Chem. Lett., 2014, 43, 284 CrossRef CAS.
- For representative reports on asymmetric hydrogenation of indoles, see: (a) R. Kuwano, K. Sato, T. Kurokawa, D. Karube and Y. Ito, J. Am. Chem. Soc., 2000, 122, 7614 CrossRef CAS; (b) A. Baeza and A. Pfaltz, Chen.–Eur. J., 2010, 16, 2036 CrossRef CAS PubMed; (c) D.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou and X. Zhang, J. Am. Chem. Soc., 2010, 132, 8909 CrossRef CAS PubMed; (d) Y.-C. Xiao, C. Wang, Y. Yao, J. Sun and Y.-C. Chen, Angew. Chem., Int. Ed., 2011, 50, 10661 CrossRef CAS PubMed; (e) Y. Duan, L. Li, M.-W. Chen, C.-B. Yu, H.-J. Fan and Y.-G. Zhou, J. Am. Chem. Soc., 2014, 136, 7688 CrossRef CAS PubMed.
- For reports on asymmetric hydrogenation of pyrroles, see: (a) R. Kuwano, M. Kashiwabara, M. Ohsumi and H. Kusano, J. Am. Chem. Soc., 2008, 130, 808 CrossRef CAS PubMed; (b) D.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan and Y. Duan, J. Am. Chem. Soc., 2011, 133, 8866 CrossRef CAS PubMed.
- For reports on asymmetric hydrogenation of (benzo)furans, see: (a) S. Kaiser, S. P. Smidt and A. Pfaltz, Angew. Chem., Int. Ed., 2006, 45, 5194 CrossRef PubMed; (b) N. Ortega, S. Urban, B. Beiring and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 1710 CrossRef CAS PubMed; (c) J. Wysocki, N. Ortega and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 8751 CrossRef CAS PubMed.
- For a report on asymmetric hydrogenation of (benzo)thiophenes, see: S. Urban, B. Beiring, N. Ortega, D. Paul and F. Glorius, J. Am. Chem. Soc., 2012, 134, 15241 CrossRef CAS PubMed.
- For a report on asymmetric hydrogenation of imidazoles and oxazoles, see: R. Kuwano, N. Kameyama and R. Ikeda, J. Am. Chem. Soc., 2011, 133, 7312 CrossRef CAS PubMed.
- N. Ortega, D. D. Tang, S. Urban, D. Zhao and F. Glorius, Angew. Chem., Int. Ed., 2013, 52, 9500 CrossRef CAS PubMed.
- R. Kuwano, Y. Hashiguchi, R. Ikeda and K. Ishizuka, Angew. Chem., Int. Ed., 2015, 54, 2393 CrossRef CAS PubMed.
- J. Zhang, F. Chen, Y.-M. He and Q.-H. Fan, Angew. Chem., Int. Ed., 2015, 54, 4622 CrossRef CAS PubMed.
- For a report on asymmetric hydrogenation of benzisoxazoles with the cleavage of the bond between the heteroatoms, see: R. Ikeda and R. Kuwano, Molecules, 2012, 17, 6901 CrossRef CAS PubMed.
- J. Zhang, S. Yang, K. Zhang, J. Chen, H. Deng, M. Shao, H. Zhang and W. Cao, Tetrahedron, 2012, 9, 2121 CrossRef PubMed.
- W. Holzer, B. Plagens and K. Lorenz, Heterocycles, 1997, 45, 309 CrossRef CAS.
- S. Bieringer and W. Holzer, Heterocycles, 2006, 68, 1825 CrossRef CAS.
- ESI.†.
- (a) H. Laatsch, Liebigs Ann. Chem., 1980, 140 CrossRef CAS PubMed; (b) E. P. Kündig, A. Enriquez-Garcia, T. Lomberget and G. Bernardinelli, Angew. Chem., Int. Ed., 2006, 45, 98 CrossRef PubMed; (c) E. P. Kündig and A. Enriquez-Garcia, Beilstein J. Org. Chem., 2008, 4, 37 Search PubMed.
- (a) X.-Y. Zhou, M. Bao and Y.-G. Zhou, Adv. Synth. Catal., 2011, 353, 84 CrossRef CAS PubMed; (b) D.-S. Wang, J. Tang, Y.-G. Zhou, M.-W. Chen, C.-B. Yu, Y. Duan and G.-F. Jiang, Chem. Sci., 2011, 2, 803 RSC; (c) X.-Y. Zhou, D.-S. Wang, M. Bao and Y.-G. Zhou, Tetrahedron Lett., 2011, 52, 2826 CrossRef CAS PubMed; (d) C.-B. Yu, K. Gao, Q.-A. Chen, M. W. Chen and Y.-G. Zhou, Tetrahedron Lett., 2012, 53, 2560 CrossRef CAS PubMed; (e) Y. Duan, M.-W. Chen, Q.-A. Chen, C.-B. Yu and Y.-G. Zhou, Org. Biomol. Chem., 2012, 10, 1235 RSC.
- Y. Duan, L. Li, M.-W. Chen, C.-B. Yu, H.-J. Fan and Y.-G. Zhou, J. Am. Chem. Soc., 2014, 136, 7688 CrossRef CAS PubMed.
- W. Tang and X. Zhang, Angew. Chem., Int. Ed., 2002, 41, 1612 CrossRef CAS.
- ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1040656–1040658. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc00835b |
| This journal is © The Royal Society of Chemistry 2015 |