Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Is a polymer semiconductor having a “perfect” regular structure desirable for organic thin film transistors?†
Wei
Hong‡
a,
Shaoyun
Chen‡
abd,
Bin
Sun
a,
Mark A.
Arnould
c,
Yuezhong
Meng
b and
Yuning
Li
*a
aDepartment of Chemical Engineering and Waterloo Institute for Nanotechnology (WIN), University of Waterloo, 200 University Avenue West, Waterloo, Ontario N2L 3G1, Canada. E-mail: yuning.li@uwaterloo.ca
bThe Key Laboratory of Low-carbon Chemistry & Energy Conservation of Guangdong Province/State Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-Sen University, Guangzhou, 510275, P. R. China
cXerox Corporation, 800 Phillips Road, Bldg. 0139-64A, Webster, NY 14580, USA
dCollege of Chemistry and Life Science, Quanzhou Normal University, Quanzhou, Fujian 362000, P. R. China
First published on 31st March 2015
Abstract
This study utilized high temperature NMR and matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry to reveal that appreciable amounts of structural defects are present in the diketopyrrolopyrrole (DPP)–quaterthiophene copolymers (PDQT) synthesized by the Stille coupling polymerization with Pd(PPh3)2Cl2, Pd2(dba)3/P(o-tol)3, and Pd(PPh3)4 catalyst systems. It was proposed that these structural defects were produced via homocoupling side reactions of the C–Br bonds and the organostannane species. Model Stille coupling reactions further substantiated that the amount of structural defects are catalyst-dependent following the order of Pd(PPh3)2Cl2 > Pd2(dba)3/P(o-tol)3 > Pd(PPh3)4. To verify the structural assignments, “perfect” structurally regular PDQT polymers were prepared using Yamamoto coupling polymerization. When compared to the structurally regular polymers, the polymers containing defects exhibited notable redshifts in their absorption spectra. Surprisingly, the “perfect” structurally regular polymers showed poor molecular ordering in thin films and very low charge transport performance as channel semiconductors in organic thin film transistors (OTFTs). On the contrary, all the “defected” polymers exhibited much improved molecular ordering and significantly higher charge carrier mobility.
Introduction
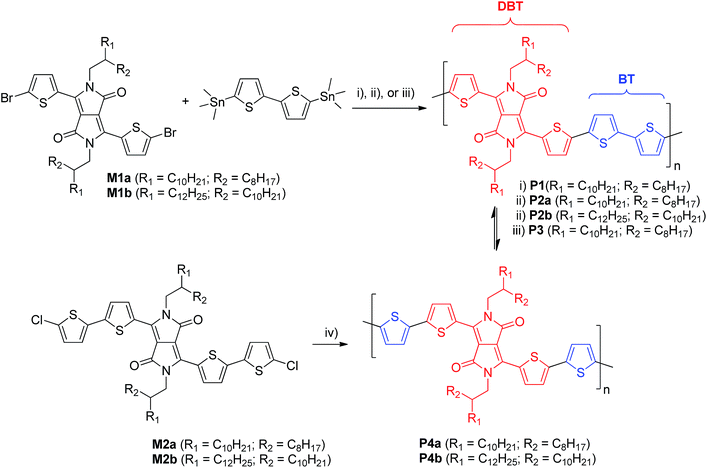
π-Conjugated polymers are considered to be promising semiconductors for organic thin film transistors (OTFTs), which have potential applications in flexible displays, radio frequency identification (RFID) tags, sensors, etc.1–4 Polymer semiconductors capable of forming highly ordered molecular organization are preferred and have been extensively pursued to achieve efficient charge transport performance,5–9 even though in some cases amorphous/disordered polymers could provide adequate performance.10–16 Conjugated polymers with well-defined repeat units have been considered desirable for OTFTs because they can self-assemble into a long-range chain packing order that would promote charge carrier transport. The best known example of this behavior is poly(3-hexylthiophene) (P3HT). The regio-irregular P3HT forms amorphous films that exhibit low charge carrier mobility (∼10−5 cm2 V−1 s−1),17 but the highly regio-regular, head-to-tail P3HT is crystalline and has demonstrated significantly improved mobility (up to ∼0.1 cm2 V−1 s−1)5,18 in OTFTs. Very few random copolymers have been reported for OTFTs19,20 since the molecular ordering of random copolymers would be hindered, resulting in lower mobility compared to the regular alternating copolymers.19In recent years, polymer semiconductors having alternating electron donor (D) and acceptor (A) units on their backbone have received escalating attention due to their remarkable performance as channel semiconductors in OTFTs.21–26 Charge carrier mobility as high as ∼10 cm2 V−1 s−1 has recently been achieved.27–30 The excellent charge transport performance observed for these D–A polymers is believed to be brought about by the strong intermolecular D–A interaction that could shorten the co-facial π–π stacking distance between conjugated polymer main chains allowing for more efficient charge hopping.23 One of the most commonly used synthetic approaches to D–A polymers is the Stille coupling reaction,31,32 where an organoditin (or organodistannane) and an organodielectrophile (often a dihalo compound) are used as comonomers in the presence of a Pd catalyst to form a long chain polymer.
Previously we reported a D–A polymer, PDQT (Scheme 1)33–35 having a diketopyrrolopyrrole (DPP) and a quaterthiophene in the repeat unit, which showed high hole mobility up to 6.9 cm2 V−1 s−1 in OTFTs. Initially this polymer (P1) was synthesized using the Stille coupling reaction of 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione (M1a) and 5,5′-bis(trimethylstannyl)-2,2′-bithiophene with Pd(PPh3)Cl2 as a catalyst.33 In subsequent studies, we also used another catalyst system consisting of Pd2(dba)3/P(o-tol)3 (P2) in order to increase the molecular weight of the polymer to improve mobility.27 Interestingly, we noticed that the PDQT polymers synthesized with these two different catalyst systems showed dramatically different UV-Vis absorption spectra in both solution and the solid state. This suggests that they may have different backbone structures and that at least one of these two catalysts resulted in structural defects, prompting us to investigate their structural differences in more detail. The Suzuki coupling reaction36 is another frequently used cross-coupling polymerization technique for synthesizing conjugated polymers. A recent paper by Janssen and coworkers37 reported that homocoupling side reactions of the bromo compounds were observed in the Suzuki coupling reaction with a catalyst system Pd2(dba)3/PPh3 (with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The presence of these structural defects had a detrimental effect on the photovoltaic performance of the polymers and should be avoided. The authors also suggested that the Stille coupling reaction with the same catalyst system might involve similar side reactions.
1). The presence of these structural defects had a detrimental effect on the photovoltaic performance of the polymers and should be avoided. The authors also suggested that the Stille coupling reaction with the same catalyst system might involve similar side reactions.
In this study, we investigated the structures and properties of PDQT polymers synthesized by the Stille coupling polymerization using three common catalyst systems: Pd(PPh3)Cl2, Pd2(dba)3/P(o-tol)3, and Pd(PPh3)4. Our results unambiguously demonstrate that significant amounts of structural defects were produced during the Stille coupling polymerization. It was found that these structural defects have significant impacts on the UV-Vis absorption, molecular ordering, as well as the OTFT performance of these polymers.
Results and discussion
PDQT polymers P1, P2a, and P3 were synthesized by the Stille coupling polymerization of the dibromo monomer M1a with equivalent 5,5′-bis(trimethylstannyl)-2,2′-bithiophene using three different Pd catalyst systems, Pd(PPh3)2Cl2, Pd2(dba)3/P(o-tol)3 (molar ratio: 1/4), and Pd(PPh3)4, as outlined in Scheme 1. Polymer P4a was prepared by the Yamamoto coupling polymerization38 of the dichloro monomer M2a using Ni(COD)2. Each repeat unit in P4a has one DBT and two thiophenes (T–DBT–T), which is equivalent to the repeat unit (DBT–BT) of P1, P2a, and P3 (Scheme 1). However, P4a can be considered a homopolymer and only one type of repeat unit would exist in the resulting polymer. A branched long chain hydrocarbon, 2-octyldodecyl (C20), was used on the DPP unit to improve the solubility of the polymers. P1, P2a, and P3 made via the Stille coupling showed good solubility in chloroform, while P4a made by the Yamamoto coupling is insoluble in the same solvent. A soluble fraction of P4a (34%) was obtained by Soxhlet extraction with 1,1,2,2-tetrachloroethane (TCE), which has a much higher boiling point (146.5 °C) than chloroform (61.2 °C). The extremely poor solubility of P4a is thought to be due to the highly regular structure of the polymer yielding an extremely rigid backbone. To improve the solubility, a longer side chain, 2-decyltetradecyl (C24), was used and the resulting polymer P4b exhibited much better solubility (62.1% and 24.8% yields were obtained from the fractions extracted with chloroform and TCE, respectively; only the fraction extracted with chloroform was used for characterization of this polymer in this study). In order to make a direct comparison with P4b, P2b with the same C24 side chain was prepared using the Pd2(dba)3/P(o-tol)3 catalyst.The molecular weight of these polymers was measured using a high temperature gel-permeation chromatography (HT-GPC) system at 140 °C with 1,2,4-trichlorobenzene as an eluent and polystyrene as standards. The results are summarized in Table 1. All the polymers have the number average molecular weight (Mn) greater than 20 kg mol−1. Due to the poor solubility of P4a, its molecular weight could not be measured with this method.
| Polymer | Catalyst | HT-GPC | MALDI-ToF | λ max (nm) | E g opt (eV) | E HOMO/ELUMO (eV) | ||
|---|---|---|---|---|---|---|---|---|
| M n (kg mol−1) | PDI | DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratiob BT ratiob |
Sol. | Film | ||||
| a HT-GPC, UV-Vis, and cyclic voltammetry data for P2a and P2b were reported previously.34,35 b DBT: 3,6-di([2,2′-bithiophen]-5-yl)-2,5-bis(2-octyldodecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione or 3,6-di([2,2′-bithiophen]-5-yl)-2,5-bis(2-tetradecyldecyl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione, BT: 2,2′-bithiophen-diyl. c n: the number of DBT units. d The fraction extracted with TCE. e The fraction extracted with chloroform. | ||||||||
| P1 | Pd(PPh3)2Cl2 | 21.4 | 2.03 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67–1.33 (n = 9)c 0.67–1.33 (n = 9)c |
775 | 783 | 1.24 | −5.3/−4.1 |
| P2a | Pd2(dba)3/P(o-tol)3 | 47.0 | 2.90 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43–1.14 (n = 7) 0.43–1.14 (n = 7) |
787 | 786 | 1.34 | −5.3/−4.0 |
| P2b | Pd2(dba)3/P(o-tol)3 | 54.9 | 3.12 | NA | 781 | 781 | 1.32 | −5.3/−4.0 |
| P3 | Pd(PPh3)4 | 38.3 | 2.16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.80–1.3 (n = 10) 0.80–1.3 (n = 10) |
798 | 787 | 1.38 | −5.3/−4.0 |
| P4a | Ni(COD)2/2,2′-bipyridyl | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
773 | 783 | 1.46 | −5.3/−3.9 |
| P4b | Ni(COD)2/2,2′-bipyridyl | 23.8 | 2.51 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
785 | 790 | 1.43 | −5.3/−3.9 |
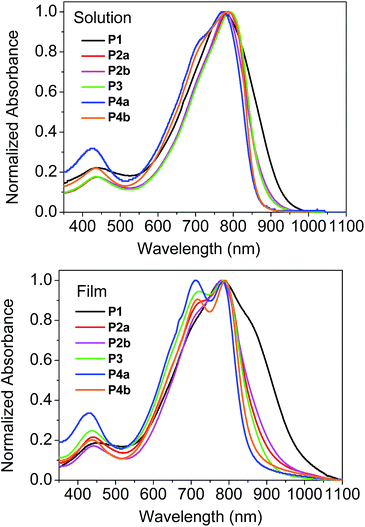
The UV-Vis spectra of the polymers are shown in Fig. 1. In solution, the polymers showed the wavelengths of maximum absorption (λmax) ranging from 773 nm to 798 nm. The relatively small λmax (773 nm) observed for P4a is thought to be due to the lower molecular weight of its soluble fraction in TCE. Most of the polymers showed a slight redshift (<10 nm) in λmax for the thin film when compared with the solution spectra due to intermolecular interaction in the solid state. It was also observed that these polymers showed dramatic differences in the long wavelength absorption side of the spectra (particularly in thin films) due to the presence of homocoupled defects in the polymer backbone.37 (This phenomenon will be further discussed later.) P1 displayed the most pronounced extension into the long wavelength region, followed by P2 then P3, with P4 showing the least. Therefore the optical band gaps for these polymers follow a trend of P4 > P3 > P2 > P1. The molecular weight of the polymers, which correlates to the main chain conjugation length, would influence the band gap of the polymers if the maximum effective conjugation length is not reached. However, the differences in the molecular weight do not seem to correlate with the different absorption profiles observed for these polymers synthesized using different catalyst systems. For instance, it can be seen that P1, with the lowest molecular weight among these polymers (except for P4a), showed the largest redshift. Therefore, the main reason for the redshifts observed for P1–P3 as compared to P4 might be due to their potentially different backbone structures. The highest occupied molecular orbital (HOMO) energy levels of these polymers are very similar (∼−5.3 eV) as determined by cyclic voltammetry with ferrocene as a reference (Table 1). The lowest unoccupied molecular orbital (LUMO) energy levels were estimated using the EHOMO and the optical band gaps to be around −4 eV with the highest value of −3.9 eV for P4a and P4b and the lowest value of −4.1 eV for P1.
1H NMR was utilized to investigate the molecular structures of these polymers. However 1H NMR spectra of all the polymers measured at room temperature in CDCl3 or deuterated 1,1,2,2-tetrachloroethane (TCE-d2) showed very broad peaks (ESI†), indicating that the polymer chains aggregated in solution, a phenomenon that has been observed for some other D–A polymers.33,39 The resolution of the 1H NMR spectra was improved by measuring the polymers in TCE-d2 at an elevated temperature of 125 °C to dissociate the polymer chain aggregates. This resulted in well-resolved peaks for the spectra of P4a and P4b (Fig. 2). As expected, these two polymers showed quite simple resonance peak patterns due to the presence of the exact repeat units enabled by the Yamamoto coupling polymerization. Some minor peaks are also observed and can be assigned to the end groups. Distinct peaks in the aromatic region for P1–P3, which correspond to those in P4, are observed, but several intense peaks at other positions are also present. The observation of more complex NMR patterns for P1–P3 compared to P4 is most likely due to their different backbone structures. This strongly suggests that side reactions occurred to form structural defects during the Stille coupling polymerization with the catalyst systems utilized in this study.
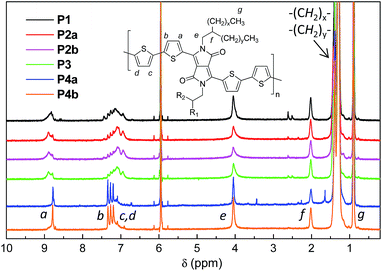 | ||
| Fig. 2 500 MHz 1H NMR spectra of P1–P4 acquired in deuterated 1,1,2,2-tetrachloroethane (TCE-d2) at 125 °C. | ||
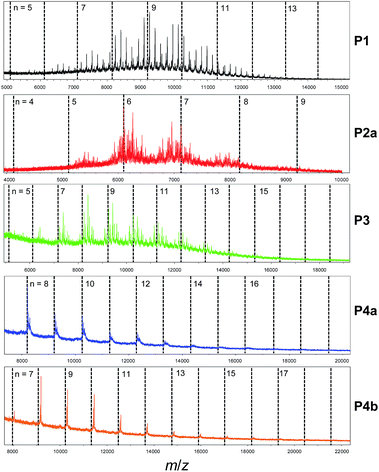
To shed more light on the polymer structures, MALDI-ToF mass spectroscopy was utilized to obtain the molecular masses for individual polymer chains after GPC separation. Fig. 3 shows that for P4a and P4b there is an excellent agreement of the measured molecular masses of the polymers with the calculated/theoretical values (represented by the vertical dashed lines), indicating their perfect polymer structures with exact repeat units. The offset from the theoretical values arises from the masses of the end groups. On the other hand, the molecular masses of P1 are mostly not observed at the theoretical molecular masses, but appear to be distributed more like a random copolymer yielding a distribution of distributions, i.e., there exist many chains containing less or more BT units than expected. There are dominant end group structures, so the spectrum is not further convoluted by overlapping end group distributions. In the spectrum of P2a, the distributions are more severely affected due to the extremely complex distribution of end group structures (with similar intensities) superimposed onto the broad comonomer distribution. The mass spectrum of P3 shows a better overall match to the theoretical values as compared to P1 and P2a. The broad comonomer distribution for each of the P1, P2a and P3 samples as compared to P4a and P4b indicates that there are significant amounts of structural defects in these polymers. For example, the ratio of the two comonomer units (DBT and BT) for P1 were calculated for polymer chains containing 9 DBT units (n = 9). It was found that these peaks correspond to molecules having DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios of 9
BT ratios of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–12 (1
6–12 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.67–1.33) (Table 1). The existence of polymer chains with DBT
0.67–1.33) (Table 1). The existence of polymer chains with DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios greater than the expected largest possible ratio of 1
BT ratios greater than the expected largest possible ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89 for polymers with alternating DBT and BT units (DBT
0.89 for polymers with alternating DBT and BT units (DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT = 9
BT = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 9
8, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, and 9
9, and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (or 1
10 (or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.89, 1
0.89, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1.11)) suggests that some DBT units are adjoined or homocoupled. For P2a, polymer chains around n = 7 have DBT
1, and 1.11)) suggests that some DBT units are adjoined or homocoupled. For P2a, polymer chains around n = 7 have DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios between 1
BT ratios between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43 and 1
0.43 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.14, indicating that there exist chains having larger than the DBT
1.14, indicating that there exist chains having larger than the DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios expected for molecules with alternating DBT
BT ratios expected for molecules with alternating DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT units (7
BT units (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 7
6, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and 7
7, and 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 (or 1
8 (or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.86, 1
0.86, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.14)). Again, this indicates that this polymer has many DBT–DBT homocoupling units. The DBT
1.14)). Again, this indicates that this polymer has many DBT–DBT homocoupling units. The DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios of P3 molecules around n = 10 are in the range of 10
BT ratios of P3 molecules around n = 10 are in the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–13 (1
8–13 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8–1.3), which are close to the expected ratios of 10
0.8–1.3), which are close to the expected ratios of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 10
9, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 10
10, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 (1
11 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9, 1
0.9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1), suggesting that this polymer has less homocoupling DBT–DBT units. Polymers 4a and 4b showed an exact DBT
1.1), suggesting that this polymer has less homocoupling DBT–DBT units. Polymers 4a and 4b showed an exact DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratio of 1
BT ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for all the peaks since their monomers have an intrinsic DBT
1 for all the peaks since their monomers have an intrinsic DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratio of 1
BT ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
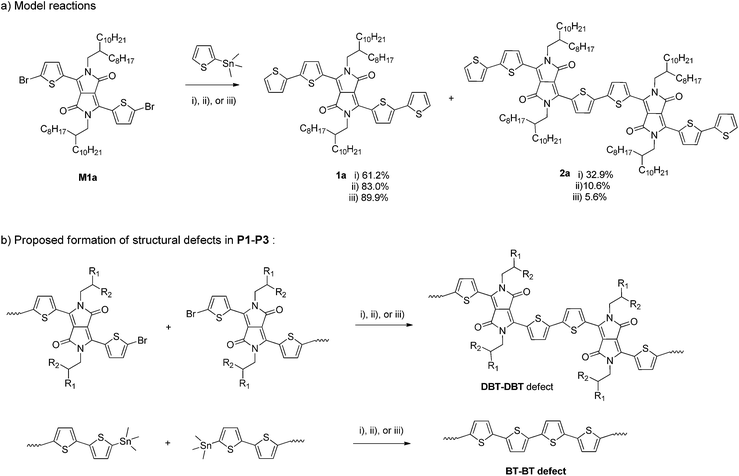
To further investigate the possible side reactions during the Stille coupling, model reactions between M1a and a monostannyl compound, 2-trimethylstannylthiophene, were conducted using the three catalyst systems under the same reaction conditions for synthesizing P1, P2a, and P3, respectively (Scheme 2a). The expected product 1a was isolated from the final reaction mixtures by column chromatography on silica gel with yields of 62.1%, 83.0%, and 89.9% with respect to the individual reaction conditions (i), (ii), and (iii) used for P1, P2a, and P3, respectively. A by-product 2a was isolated for all three model reactions. A surprisingly large amount of 2a (32.9% isolated yield) was obtained when Pd(PPh3)Cl2 was used as the catalyst, while 10.6% (ii) and 5.6% (iii) of compound 2a were isolated for reactions using Pd2(dba)3/P(o-tol)3 and Pd(PPh3)4, respectively. Compound 2a was most likely formed from the homocoupling of two C–Br bonds of two M1a molecules and/or the intermediate with one side capped with a thiophene, T–DBT–Br. Based on the yields of the homocoupling product 2a for the three catalyst systems, it is reasonable to assume that the same type of side reactions involving C–Br containing species occurred during the Stille coupling polymerization (Scheme 2b). The content of the DBT–DBT homocoupling defects in the polymers would follow the order of P1 > P2 > P3, which is in agreement with the MALDI-ToF results discussed previously. Similar side reactions were reported very recently for the Suzuki coupling reaction and was also suggested to occur during the Stille coupling polymerization in a different catalyst system Pd2(dba)3/PPh3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).37 A homopolymer of M1a (PDBT-20), which contains only DBT units, was observed to have a much longer λmax (917 nm in solution and 928 nm in thin films)40 than that of P4, suggesting that the higher the amount of the DBT–DBT homocoupling defects, the longer the absorption wavelength of the PDQT polymer. The absorption redshift trend of P1 > P2 > P3 with respect to P4 agrees well with the DBT–DBT defect contents of these polymers. The presence of the largest amount of DBT–DBT defects in P1 also leads to its deepest LUMO energy level among the polymers studied because of the strong electron-accepting effect of the DPP units. It should be noted that other side reactions such as the homocoupling of organostannanes, which is a common side reaction observed for the Stille coupling,41 might also occur during the polymerization reactions in this study. This type of side reaction would form the BT–BT homocoupling defects in P1–P3 as illustrated in Scheme 2b. This could explain the presence of polymer chains that have DBT
1).37 A homopolymer of M1a (PDBT-20), which contains only DBT units, was observed to have a much longer λmax (917 nm in solution and 928 nm in thin films)40 than that of P4, suggesting that the higher the amount of the DBT–DBT homocoupling defects, the longer the absorption wavelength of the PDQT polymer. The absorption redshift trend of P1 > P2 > P3 with respect to P4 agrees well with the DBT–DBT defect contents of these polymers. The presence of the largest amount of DBT–DBT defects in P1 also leads to its deepest LUMO energy level among the polymers studied because of the strong electron-accepting effect of the DPP units. It should be noted that other side reactions such as the homocoupling of organostannanes, which is a common side reaction observed for the Stille coupling,41 might also occur during the polymerization reactions in this study. This type of side reaction would form the BT–BT homocoupling defects in P1–P3 as illustrated in Scheme 2b. This could explain the presence of polymer chains that have DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios smaller than expected (with more BT units than expected) for P1 and P3 revealed by their MALDI-ToF spectra (Table 1 and Fig. 3). P2a showed the smallest DBT
BT ratios smaller than expected (with more BT units than expected) for P1 and P3 revealed by their MALDI-ToF spectra (Table 1 and Fig. 3). P2a showed the smallest DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratio 1
BT ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.14 (7
1.14 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) at n = 7, indicating that less BT
8) at n = 7, indicating that less BT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT defects present in this polymer, although the existence of the BT
BT defects present in this polymer, although the existence of the BT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT defects in this polymer cannot be completely ruled out solely based on its DBT
BT defects in this polymer cannot be completely ruled out solely based on its DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BT ratios. Attempts to isolate the possible bithiophene by-product from the model Stille coupling reaction mixtures with column chromatography or detect this by-product by mass spectroscopy were unsuccessful due to the extremely low anticipated mass yields of this by-product and the interference of other impurities. Nonetheless, the almost quantitative isolated yields of P1–P3 (97–99%) support that most of the BT units in the 5,5′-bis(trimethylstannyl)-2,2′-bithiophene monomer were incorporated into these polymers, which suggests that similarly significant amounts of homocouling BT–BT defects with respect to that of the DBT–DBT defects might be formed.
BT ratios. Attempts to isolate the possible bithiophene by-product from the model Stille coupling reaction mixtures with column chromatography or detect this by-product by mass spectroscopy were unsuccessful due to the extremely low anticipated mass yields of this by-product and the interference of other impurities. Nonetheless, the almost quantitative isolated yields of P1–P3 (97–99%) support that most of the BT units in the 5,5′-bis(trimethylstannyl)-2,2′-bithiophene monomer were incorporated into these polymers, which suggests that similarly significant amounts of homocouling BT–BT defects with respect to that of the DBT–DBT defects might be formed.
The above results revealed that P4a and P4b have a regular backbone structure with exact repeat units, while all the polymers (P1–P3) synthesized via the Stille coupling polymerization contain significant amounts of structural defects. Among the Pd catalyst systems used, Pd(PPh3)4 afforded the most regular backbone architecture observed in P3, while Pd(PPh3)2Cl2 produced the most irregular polymer backbone in P1. One would intuitively expect that the molecular ordering of these polymers in the solid state would follow the order of P4 > P3 > P2 > P1 based on their structural regularity. We investigated the chain packing of these polymers in spin-coated thin films using X-ray diffractometry (XRD). As shown in Fig. 4, P1–P3 films are highly crystalline with prominent primary (100) and secondary (200) diffraction peaks that represent the typical layer-by-layer lamellar polymer chain packing.5 The absence of the (010) peak that represents the π–π stacking distance (at 2θ = ∼20–25°) indicates that the polymer chains adopted an edge-on orientation.5 As anticipated, the crystallinity of the P1–P3 films follows the same order of P3 > P2a > P1 (having the same C20 side chains) as that of their structural regularity, judging from the intensities of their diffraction peaks. Much to our surprise, the structurally regular polymer P4a showed extremely low diffraction intensities, suggesting the very low crystallinity of this polymer. P4b, which has C24 side chains, also showed much poorer molecular ordering compared to P2b that has the same C24 side chains. The reason for the unexpected poor crystallinity of P4a and P4b is not fully understood at this time and needs to be further investigated. We suspect that the regular backbone structure of these two polymers would result in a high degree of rigidity for the polymer main chain, hampering the packing of the polymer molecules. On the other hand, the structural defects present in P1–P3 would provide a desirable flexibility to the polymer backbone, allowing for a slower crystallization process and a fine tuning of the chain packing. Transmission XRD measurements were conducted on polymer flakes to determine the π–π stacking distance between polymer backbones (Fig. 5). All polymers showed distinct (010) reflection peaks around 2θ = 10.6–10.8°, which correspond to π–π stacking distances of 0.38–0.39 nm (Table 2). These results suggest that the existence of the structural defects in P1–P3 did not alter the π–π stacking distance noticeably and would probably not markedly influence the hopping of charge carriers along the π–π stacks.
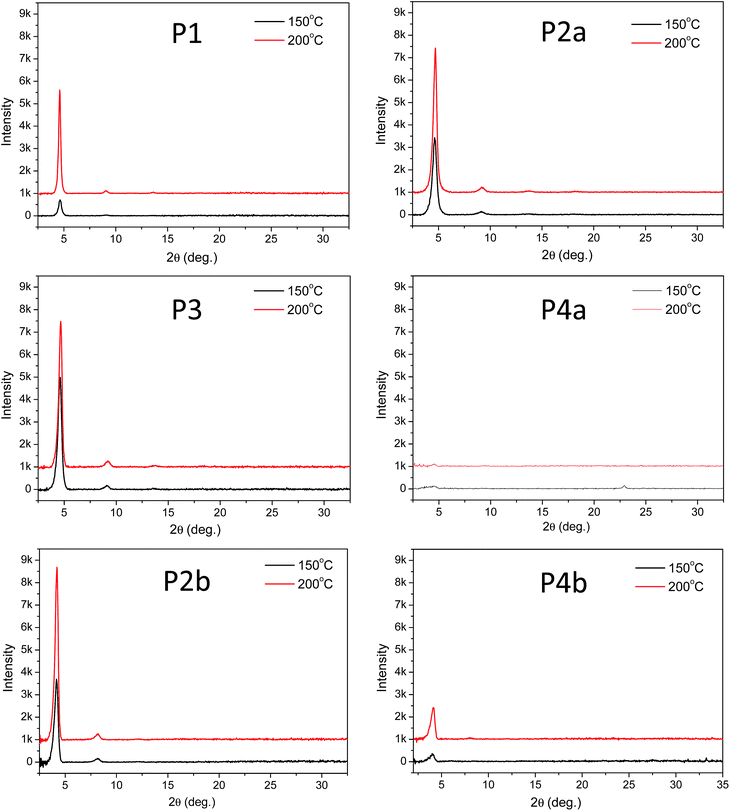 | ||
| Fig. 4 Reflective XRD diagrams of P1–P4 thin films (with similar thicknesses of ∼50 nm) spin coated on dodecyltrichlorosilane-modified SiO2/Si wafer and annealed at 150 °C or 200 °C using Cu Kα1 radiation (λ = 0.15406 nm). Note that the intensity scale for P4a is one-tenth of that in other diagrams due to the low diffraction intensities of this polymer. Data for P2a and P2b were reported previously.35 | ||
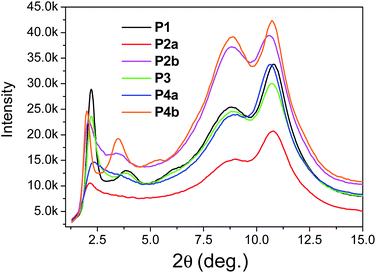 | ||
| Fig. 5 Transmission XRD diagrams of P1–P4 flakes (thicknesses were not intentionally controlled) annealed at 200 °C using Mo Kα radiation (λ = 0.071073 nm). Data for P2a and P2b were reported previously.35 | ||
| Polymer | Anneal. temp. (°C) | XRD | OTFT performance | |||
|---|---|---|---|---|---|---|
| d (100) (nm) | d (010) (nm) | Ave. (max) µh (cm2 V−1 s−1) | I on/Ioff | V th (V) | ||
| a Data for P2a and P2b were reported previously.34,35 | ||||||
| P1 | 150 | 1.91 | 1.60 (2.10) | ∼106 | −6 | |
| 200 | 1.92 | 0.38 | 2.84 (2.94) | ∼104 | −3 | |
| P2a | 150 | 1.91 | 1.58 (2.10) | ∼104 | −10 | |
| 200 | 1.88 | 0.38 | 3.57 (5.50) | ∼106 | −7 | |
| P2b | 150 | 2.13 | 2.65 (3.37) | ∼104 | 18 | |
| 200 | 2.11 | 0.39 | 1.46 (1.51) | ∼104 | 18 | |
| P3 | 150 | 1.91 | 1.62 (1.74) | ∼104 | 17 | |
| 200 | 1.89 | 0.38 | 1.35 (1.41) | ∼104 | 19 | |
| P4a | 150 | 1.98 | 0.065 (0.084) | ∼108 | −22 | |
| 200 | 1.98 | 0.38 | 0.029 (0.036) | ∼105 | −35 | |
| P4b | 150 | 2.15 | 0.90 (0.94) | ∼106 | −8 | |
| 200 | 2.13 | 0.39 | 1.10 (1.13) | ∼106 | −24 | |
The crystallinity of these polymers was also studied by differential scanning calorimetry (DSC) (data are presented in the ESI†). The DSC results showed that for all the polymers having the C20 side chains (P1, P2a, P3, and P4a) only P1 showed a melting point at 287 °C in the testing temperature range from −20 °C to 325 °C. P2a and P3 may have melting points higher than their decomposition temperatures, while P4a probably does not have an obvious melting point based on its poor crystallinity determined by the thin film XRD. For P2b and P4b, which have C24 side chains, P2b showed a distinct melting point at 293 °C, while there was no thermal transition observed for P4b. The melting point of P4b may also be above its thermal decomposition temperature. Obviously, the existence of structural defects could lower the melting points of the polymers as observed in P1 and P2b. The structural defects might have helped the creep of the polymer chains in achieving more ordered chain packing.
Interconnections of grains in polymer thin films are crucial for charge transport in polymer semiconductors. The atomic force microscopy (AFM) images of polymers P1, P2, and P3 exhibited similar surface morphologies composed of well-connected small grains (Fig. 6). The P2a and P2b films are very smooth followed by an increase in roughness for P1. P3 has the roughest surface among the four polymers synthesized by the Stille coupling polymerization. P4a and P4b showed dramatically different morphologies compared with P1–P3. P4a films showed a very rough morphology comprising of large flakes ∼100 nm in size with the films of P4b being quite smooth composed of small donut-shaped structures.
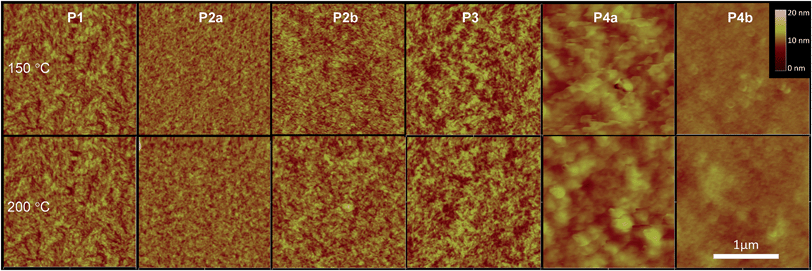 | ||
| Fig. 6 AFM height images (2 µm × 2 µm each) of P1–P4 thin films (∼50 nm) spin-coated on dodecyltrichlorosilane-modified Si/SiO2 wafers and annealed at 150 °C or 200 °C. Images of P2b were reported previously.35 | ||
The charge transport performance of these polymers was evaluated in bottom-gate bottom-contact OTFT devices fabricated on heavily n-doped Si/SiO2 wafers, where the Si layer was used as the gate electrode, the SiO2 layer as the dielectric, and gold pairs as source and drain electrodes. Output and transfer curves of typical OTFT devices are provided in Fig. 7. It can be seen in Table 2 that the 150 °C-annealed thin films of P1, P2a, and P3 with the same C20 side chains showed very similar hole mobilities with average values of ∼1.6 cm2 V−1 s−1. When the polymer films were annealed at 200 °C, the average mobility for P1 and P2a increased to 2.84 cm2 V−1 s−1 and 3.57 cm2 V−1 s−1, respectively. On the other hand, the average mobility for the 200 °C-annealed P3 dropped slightly to 1.35 cm2 V−1 s−1. P4a having the same C20 side chains showed strikingly lower mobilities of 0.065 and 0.029 cm2 V−1 s−1 for the 150 °C- and 200 °C-annealed thin films, respectively, which is most likely due to the very poor crystallinity (Fig. 4) and the poor intergranular connections (Fig. 6) of the polymer films. It was also noted that the mobilities of P4b (150 °C-annealed: 0.90 cm2 V−1 s−1; 200 °C-annealed: 1.10 cm2 V−1 s−1) are much lower than those of P2b having the same C24 side chains (150 °C-annealed: 2.65 cm2 V−1 s−1; 200 °C-annealed: 1.46 cm2 V−1 s−1), which can also be attributed to the poorer crystallinity of P4b. Some devices showed curved IDS1/2vs. VGS lines (Fig. 4) and thus gate-dependent mobilities (ESI†). This bending phenomenon was also observed previously for PDQT25,34,35 and other high mobility polymers,27,29,42,43 which was explained by the carrier supersaturation in the conduction channel at high source/drain current regimes or low-density shallow charge trapping in the semiconductor and/or interface.27
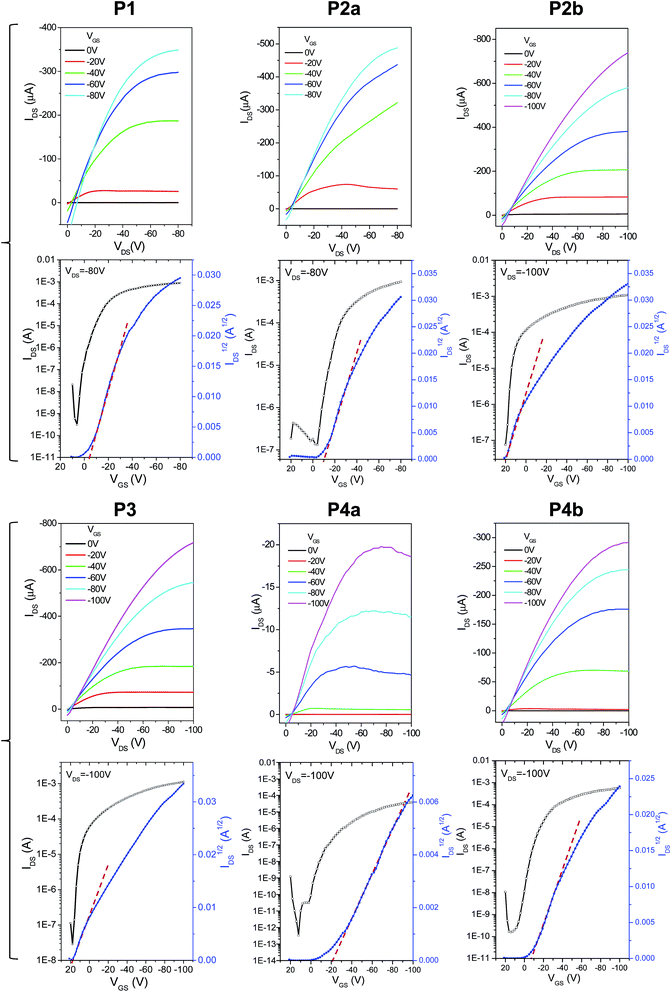 | ||
| Fig. 7 Output (top) and transfer (bottom) characteristics of typical bottom-gate bottom-contact OTFT devices using polymers P1–P4 as channel semiconductors. The polymer thin films were annealed at 150 °C for 15 min. Device dimensions: L = 30 µm; W = 1 mm. N-doped Si/SiO2 wafers were used as substrates. The thickness of the dielectric SiO2 layer is 200 nm for P1 and P2a and 300 nm for the other polymers. Curves for P2a and P2b were reported previously.35 | ||
Based on the above results, it appears that the presence of an appropriate amount of structural defects in the polymers synthesized by the Stille coupling polymerization could provide good solubility, increase molecular ordering, maintain short π–π stacking distance, and improve film morphology. These characteristics favorably result in the enhanced charge transport performance of these polymers in OTFTs. The structurally regular polymers P4a and P4b showed very poor solution processability (poor solubility), low crystallinity, and poor morphology, leading to much lower charge carrier mobility in comparison to the “defected” polymers P1–P3.
Conclusions
We analyzed the structures of PDQT polymers synthesized using different polymerization methods and observed a correlation between the structure and the optoelectronic properties of these polymers. Our results confirmed the formation of the DBT–DBT and BT–BT structural defects due to the homocoupling side reactions of the C–Br bonds and the organostannane species, respectively, during the Stille coupling polymerization in the presence of three different Pd catalysts. We found that Pd(PPh3)4 produced a PDQT polymer with fewest structural defects, while Pd(PPh3)2Cl2 gave the most irregular polymer backbone architecture. NMR measurements of the polymers at an elevated temperature of 125 °C revealed peaks representing the structural defects with an aid of the NMR spectra of the structurally well-defined polymers prepared by Yamamoto coupling with Ni(COD)2. MALDI-ToF mass spectroscopy further substantiated the presence of the DBT–DBT and BT–BT structural defects formed during the Stille coupling polymerization by detecting chains containing comonomer ratios not equal to the expected theoretical values. Isolation of a by-product from model Stille coupling reactions using these Pd catalyst systems validated that homocoupling involving the C–Br bonds occurred to a significant extent during the Stille coupling reactions, which is responsible for the formation of the DBT–DBT structural defects in the polymers. The existence of these structural defects was thought to result in the redshifts of the absorption profiles of the PDQT polymers. Surprisingly, the “perfect” structurally well-defined PDQT polymers prepared using Yamamoto coupling showed very poor molecular ordering in thin films presumably due to their rigid backbone that prevents the polymer chains from fine adjustment to form highly ordered molecular packing. As a result, these “perfect” polymers showed poor charge transport performance in OTFTs. On the contrary, polymers having significant amounts of structural defects prepared by the Stille coupling polymerization exhibited much improved molecular ordering and OTFT performance. Our results demonstrated that a “perfect” regular polymer may not lead to the best OTFT performance, while the existence of an appropriate/acceptable amount of structural defects in polymer chains can enhance the molecular ordering and thus the charge transport.Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for financial support (Discovery Grants No. 402566-2011) of this work. S.C. thanks the Overseas Study Program of Guangzhou Elite Project provided by Guangzhou City, China. The authors would also like to thank Robin Sheppard of Xerox Corp. for performing the high temperature GPC analyses.Notes and references
- H. Klauk, Chem. Soc. Rev., 2010, 39, 2643–2666 RSC.
- A. C. Arias, J. D. MacKenzie, I. McCulloch, J. Rivnay and A. Salleo, Chem. Rev., 2010, 110, 3–24 CrossRef CAS PubMed.
- A. Facchetti, Chem. Mater., 2010, 23, 733–758 CrossRef.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2011, 112, 2208–2267 CrossRef PubMed.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Nature, 1999, 401, 685–688 CrossRef CAS PubMed.
- H. Sirringhaus, Adv. Mater., 2005, 17, 2411–2425 CrossRef CAS.
- B. S. Ong, Y. Wu, P. Liu and S. Gardner, J. Am. Chem. Soc., 2004, 126, 3378–3379 CrossRef CAS PubMed.
- I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. MacDonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M. L. Chabinyc, R. J. Kline, M. D. McGehee and M. F. Toney, Nat. Mater., 2006, 5, 328–333 CrossRef CAS PubMed.
- B. S. Ong, Y. Wu, Y. Li, P. Liu and H. Pan, Chem.–Eur. J., 2008, 14, 4766–4778 CrossRef CAS PubMed.
- M. Zhang, H. N. Tsao, W. Pisula, C. Yang, A. K. Mishra and K. Müllen, J. Am. Chem. Soc., 2007, 129, 3472–3473 CrossRef CAS PubMed.
- J. Liu, R. Zhang, G. Sauvé, T. Kowalewski and R. D. McCullough, J. Am. Chem. Soc., 2008, 130, 13167–13176 CrossRef CAS PubMed.
- H. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dotz, M. Kastler and A. Facchetti, Nature, 2009, 457, 679–686 CrossRef CAS PubMed.
- Y. Li, P. Sonar, S. P. Singh, W. Zeng and M. S. Soh, J. Mater. Chem., 2011, 21, 10829–10835 RSC.
- X. Zhang, H. Bronstein, A. J. Kronemeijer, J. Smith, Y. Kim, R. J. Kline, L. J. Richter, T. D. Anthopoulos, H. Sirringhaus, K. Song, M. Heeney, W. Zhang, I. McCulloch and D. M. DeLongchamp, Nat. Commun., 2013, 4, 2238 Search PubMed.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1038–1044 CrossRef CAS PubMed.
- D. Venkateshvaran, M. Nikolka, A. Sadhanala, V. Lemaur, M. Zelazny, M. Kepa, M. Hurhangee, A. J. Kronemeijer, V. Pecunia, I. Nasrallah, I. Romanov, K. Broch, I. McCulloch, D. Emin, Y. Olivier, J. Cornil, D. Beljonne and H. Sirringhaus, Nature, 2014, 515, 384–388 CrossRef CAS PubMed.
- A. Tsumura, H. Koezuka and T. Ando, Appl. Phys. Lett., 1986, 49, 1210–1212 CrossRef CAS PubMed.
- Z. Bao, A. Dodabalapur and A. J. Lovinger, Appl. Phys. Lett., 1996, 69, 4108–4110 CrossRef CAS PubMed.
- J. Li, K. H. Ong, P. Sonar, S. L. Lim, G. M. Ng, H. K. Wong, H. S. Tan and Z. K. Chen, Polym. Chem., 2013, 4, 804–811 RSC.
- H. Li, F. Liu, X. Wang, C. Gu, P. Wang and H. Fu, Macromolecules, 2013, 46, 9211–9219 CrossRef CAS.
- I. Osaka, G. Sauvé, R. Zhang, T. Kowalewski and R. D. McCullough, Adv. Mater., 2007, 19, 4160–4165 CrossRef CAS.
- H. N. Tsao, D. Cho, J. W. Andreasen, A. Rouhanipour, D. W. Breiby, W. Pisula and K. Müllen, Adv. Mater., 2009, 21, 209–212 CrossRef CAS.
- Y. Li, S. P. Singh and P. Sonar, Adv. Mater., 2010, 22, 4862–4866 CrossRef CAS PubMed.
- C. Guo, W. Hong, H. Aziz and Y. Li, Reviews in Advanced Sciences and Engineering, 2012, 1, 200–224 CrossRef PubMed.
- Y. Li, P. Sonar, L. Murphy and W. Hong, Energy Environ. Sci., 2013, 6, 1684–1710 CAS.
- Y. He, W. Hong and Y. Li, J. Mater. Chem. C, 2014, 2, 8651–8661 RSC.
- J. Li, Y. Zhao, H. S. Tan, Y. Guo, C.-A. Di, G. Yu, Y. Liu, M. Lin, S. H. Lim, Y. Zhou, H. Su and B. S. Ong, Sci. Rep., 2012, 2, 754 Search PubMed.
- I. Kang, H. J. Yun, D. S. Chung, S. K. Kwon and Y. H. Kim, J. Am. Chem. Soc., 2013, 135, 14896–14899 CrossRef CAS PubMed.
- C. Luo, A. K. K. Kyaw, L. A. Perez, S. Patel, M. Wang, B. Grimm, G. C. Bazan, E. J. Kramer and A. J. Heeger, Nano Lett., 2014, 14, 2764–2771 CrossRef CAS PubMed.
- G. Kim, S. J. Kang, G. K. Dutta, Y. K. Han, T. J. Shin, Y. Y. Noh and C. Yang, J. Am. Chem. Soc., 2014, 136, 9477–9483 CrossRef CAS PubMed.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524 CrossRef.
- B. Carsten, F. He, H. J. Son, T. Xu and L. Yu, Chem. Rev., 2011, 111, 1493–1528 CrossRef CAS PubMed.
- Y. Li, P. Sonar, S. P. Singh, M. S. Soh, M. van Meurs and J. Tan, J. Am. Chem. Soc., 2011, 133, 2198–2204 CrossRef CAS PubMed.
- B. Sun, W. Hong, H. Aziz, N. M. Abukhdeir and Y. Li, J. Mater. Chem. C, 2013, 1, 4423 RSC.
- S. Chen, B. Sun, W. Hong, H. Aziz, Y. Meng and Y. Li, J. Mater. Chem. C, 2014, 2, 2183–2190 RSC.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- K. H. Hendriks, W. Li, G. H. L. Heintges, G. W. P. van Pruissen, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2014, 136, 11128–11133 CrossRef CAS PubMed.
- T. Yamamoto, A. Morita, Y. Miyazaki, T. Maruyama, H. Wakayama, Z. H. Zhou, Y. Nakamura, T. Kanbara, S. Sasaki and K. Kubota, Macromolecules, 1992, 25, 1214–1223 CrossRef CAS.
- J. R. Matthews, W. Niu, A. Tandia, A. L. Wallace, J. Hu, W.-Y. Lee, G. Giri, S. C. B. Mannsfeld, Y. Xie, S. Cai, H. H. Fong, Z. Bao and M. He, Chem. Mater., 2013, 25, 782–789 CrossRef CAS.
- Y. Li, B. Sun, P. Sonar and S. P. Singh, Org. Electron., 2012, 13, 1606–1613 CrossRef CAS PubMed.
- V. Farina, V. Krishnamurthy, W. J. Scott and W. J. Scott, The Stille Reaction, Wiley, New York, 1998 Search PubMed.
- H. J. Chen, Y. L. Guo, G. Yu, Y. Zhao, J. Zhang, D. Gao, H. T. Liu and Y. Q. Liu, Adv. Mater., 2012, 24, 4618–4622 CrossRef CAS PubMed.
- J. Mei, D. H. Kim, A. L. Ayzner, M. F. Toney and Z. Bao, J. Am. Chem. Soc., 2011, 133, 20130–20133 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc00843c |
| ‡ Wei Hong and Shaoyun Chen contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |