Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iron catalyzed CO2 hydrogenation to formate enhanced by Lewis acid co-catalysts†
Yuanyuan
Zhang‡
a,
Alex D.
MacIntosh‡
a,
Janice L.
Wong
b,
Elizabeth A.
Bielinski
b,
Paul G.
Williard
a,
Brandon Q.
Mercado
b,
Nilay
Hazari
*b and
Wesley H.
Bernskoetter
*a
aThe Department of Chemistry, Brown University, Providence, RI 02912, USA. E-mail: wb36@brown.edu
bThe Department of Chemistry, Yale University, New Haven, CT 06520, USA. E-mail: nilay.hazari@yale.edu
First published on 28th May 2015
Abstract
A family of iron(II) carbonyl hydride complexes supported by either a bifunctional PNP ligand containing a secondary amine, or a PNP ligand with a tertiary amine that prevents metal–ligand cooperativity, were found to promote the catalytic hydrogenation of CO2 to formate in the presence of Brønsted base. In both cases a remarkable enhancement in catalytic activity was observed upon the addition of Lewis acid (LA) co-catalysts. For the secondary amine supported system, turnover numbers of approximately 9000 for formate production were achieved, while for catalysts supported by the tertiary amine ligand, nearly 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 turnovers were observed; the highest activity reported for an earth abundant catalyst to date. The LA co-catalysts raise the turnover number by more than an order of magnitude in each case. In the secondary amine system, mechanistic investigations implicated the LA in disrupting an intramolecular hydrogen bond between the PNP ligand N–H moiety and the carbonyl oxygen of a formate ligand in the catalytic resting state. This destabilization of the iron-bound formate accelerates product extrusion, the rate-limiting step in catalysis. In systems supported by ligands with the tertiary amine, it was demonstrated that the LA enhancement originates from cation assisted substitution of formate for dihydrogen during the slow step in catalysis.
000 turnovers were observed; the highest activity reported for an earth abundant catalyst to date. The LA co-catalysts raise the turnover number by more than an order of magnitude in each case. In the secondary amine system, mechanistic investigations implicated the LA in disrupting an intramolecular hydrogen bond between the PNP ligand N–H moiety and the carbonyl oxygen of a formate ligand in the catalytic resting state. This destabilization of the iron-bound formate accelerates product extrusion, the rate-limiting step in catalysis. In systems supported by ligands with the tertiary amine, it was demonstrated that the LA enhancement originates from cation assisted substitution of formate for dihydrogen during the slow step in catalysis.
Introduction
The increasing volatility in price and the negative environmental impact associated with fossil fuel utilization for energy and commodity chemical production continues to spur basic research into the exploitation of renewable carbon resources.1 CO2 is an attractive target for transitioning the chemical industry to sustainable feedstocks, due to its incredible abundance, cheap availability and low toxicity.2 Formic acid is an especially interesting CO2 reduction product given its use in numerous agrochemicals and preservatives,3 as well as its potential role as a material for chemical hydrogen storage (CHS) in renewable energy applications.4 The utilization of formic acid as a CHS material requires reversible hydrogenation/dehydrogenation between CO2 and formic acid, a reaction with a small thermodynamic preference (7 kcal mol−1) toward CO2 and H2 in the gas phase.5 Consequently, most catalysts for the hydrogenation of CO2 to formic acid rely on exogenous base to form formate, which drives the reaction.6 The most effective of these catalysts employ precious metals such as ruthenium,7 rhodium,8 and iridium,9 and turnover numbers (TONs) of approximately 3.5 × 106 and turnover frequencies (TOFs) near 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 have been achieved at elevated pressures and temperatures (49–59 atm; 120–220 °C).10 These findings demonstrate the remarkable potential for catalytic CO2 hydrogenation to formate, but also motivate the development of earth abundant catalytic systems, which are expected to enhance the sustainability and economic feasibility of this transformation.
000 h−1 have been achieved at elevated pressures and temperatures (49–59 atm; 120–220 °C).10 These findings demonstrate the remarkable potential for catalytic CO2 hydrogenation to formate, but also motivate the development of earth abundant catalytic systems, which are expected to enhance the sustainability and economic feasibility of this transformation.
Although homogeneous catalysts for CO2 hydrogenation to formate containing first-row transition metals were first described in 1976,11 TONs were low. As a result for many decades significantly more attention was devoted to the study of heterogeneous catalysts containing first-row transition metals.12 However, in recent years, the development of homogenous catalysts has been reinvigorated by the discovery of several more active cobalt and iron based systems.13 For example, Fujita et al. reported a Cp*Co (Cp* = η5-C5Me5) complex supported by a dihydroxy-bipyridine ligand that is capable of 59 turnovers to formate in aqueous bicarbonate,14 while Linehan and coworkers described an even more impressive TON of 9400 using (Me2PCH2CH2PMe2)2CoH, although the use of a costly and strong base (Verkade's base) is required for high conversion.15 During the same time period, Beller and coworkers described both cobalt and iron catalysts supported by tetraphosphine ligands, with the [P(o-C6H4PPh2)3Fe]2+ congener affording the highest TON for an iron catalyst to date.16 This in situ generated system afforded 1897 turnovers to formate in methanol/water with excess NEt3 under 60 atm of CO2/H2 at 100 °C.16b Another noteworthy iron system was reported by Milstein and coworkers who showed that a complex supported by pincer ligand with a pyridine backbone could give 708 turnovers under remarkably mild pressures (8–10 atm) in 2 M aqueous NaOH.17 Collectively these discoveries establish that earth abundant metals are capable of promoting CO2 hydrogenation, but their activities lag far beyond those of precious metal catalysts.
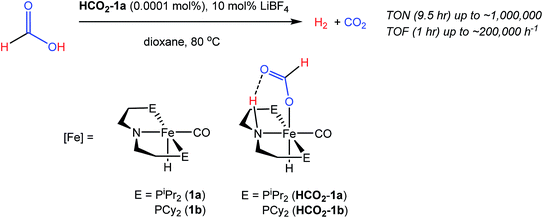
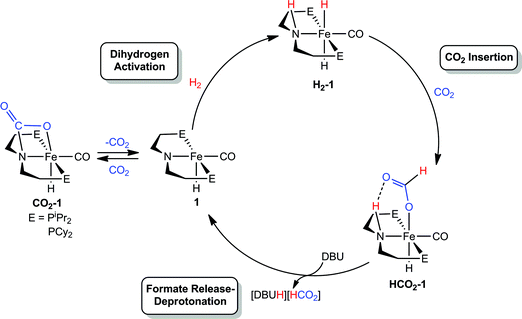
Our laboratories recently identified iron catalysts for formic acid dehydrogenation (FADH), the reverse of CO2 hydrogenation, which surpass even precious metal catalysts in activity.18 The iron(II) formate carbonyl hydride species, (RPNP)Fe(H)CO(HCO2) (RPNP = HN{CH2CH2(PR2)}2; R = iPr (HCO2-1a), R = Cy (HCO2-1b)), bearing a bifunctional amine ligand give 1 × 106 turnovers for FADH with a TOF near 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 (Fig. 1). Slightly diminished performance was also observed using the five-coordinate iron(II) species, ((RPNP)Fe(H)CO; R = iPr (1a), R = Cy (1b)), which readily form HCO2-1a and HCO2-1b upon exposure to formic acid. The impressive activity is dependent on the presence of a Lewis acid (LA) co-catalyst, such as LiBF4, which preliminary mechanistic studies indicate aids in the decarboxylation of an iron-formate intermediate. Herein we report the development of a collection of iron complexes based on this PNP ligand motif, which catalyze the hydrogenation of CO2 to formate with TONs approaching 60
000 h−1 (Fig. 1). Slightly diminished performance was also observed using the five-coordinate iron(II) species, ((RPNP)Fe(H)CO; R = iPr (1a), R = Cy (1b)), which readily form HCO2-1a and HCO2-1b upon exposure to formic acid. The impressive activity is dependent on the presence of a Lewis acid (LA) co-catalyst, such as LiBF4, which preliminary mechanistic studies indicate aids in the decarboxylation of an iron-formate intermediate. Herein we report the development of a collection of iron complexes based on this PNP ligand motif, which catalyze the hydrogenation of CO2 to formate with TONs approaching 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, far greater than any previously described earth abundant metal catalyst. These high TONs are only achieved in the presence of a LA co-catalyst and we describe detailed mechanistic studies which elucidate the crucial role of the LA.
000, far greater than any previously described earth abundant metal catalyst. These high TONs are only achieved in the presence of a LA co-catalyst and we describe detailed mechanistic studies which elucidate the crucial role of the LA.
Results and discussion
CO2 hydrogenation activity of (RPNP)Fe(H)CO
The mildly endergonic profile of CO2 hydrogenation to formic acid has led researchers to employ a wide variety of exogenous bases to drive this reaction. Given the limited stability of 1a and 1b in aqueous environments,19 our initial catalytic experiments focused on identifying suitable bases with moderate to good solubility in organic solvents. A brief screen of bases using 1b in THF and a combined 69 atm of CO2/H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C indicated that Cs2CO3 and 1,8-diazabicycloundec-7-ene (DBU) were among the most effective bases in promoting formate production (Table S1†). For subsequent investigations, DBU was selected as the base of choice owing to its higher solubility in THF and moderately better TON compared to Cs2CO3. The TON of 78 observed using DBU indicates that 1b is, at best, a modest catalyst for CO2 reduction by itself. However, addition of LiBF4 dramatically improved the conversion.20 Conducting catalytic reactions using 1a and 1b in the presence of a LA (2
1) at 80 °C indicated that Cs2CO3 and 1,8-diazabicycloundec-7-ene (DBU) were among the most effective bases in promoting formate production (Table S1†). For subsequent investigations, DBU was selected as the base of choice owing to its higher solubility in THF and moderately better TON compared to Cs2CO3. The TON of 78 observed using DBU indicates that 1b is, at best, a modest catalyst for CO2 reduction by itself. However, addition of LiBF4 dramatically improved the conversion.20 Conducting catalytic reactions using 1a and 1b in the presence of a LA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of DBU
1 ratio of DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiBF4) afforded a ca. 3–4 fold increase in TON, with the –PCy2 substituted 1b showing a slightly higher conversion (Table 1). A screen of LiBF4 loadings between DBU
LiBF4) afforded a ca. 3–4 fold increase in TON, with the –PCy2 substituted 1b showing a slightly higher conversion (Table 1). A screen of LiBF4 loadings between DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiBF4 ratios of 150
LiBF4 ratios of 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed an onset of saturation behavior below 6
1 showed an onset of saturation behavior below 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S2†), thus a DBU
1 (Table S2†), thus a DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA ratio of 7.5
LA ratio of 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was employed as the benchmark co-catalyst loading for most catalytic experiments reported here. This loading balances the higher conversions at increased LA loadings with possible complications arising from solubility limitations.
1 was employed as the benchmark co-catalyst loading for most catalytic experiments reported here. This loading balances the higher conversions at increased LA loadings with possible complications arising from solubility limitations.
| Entry | Catalyst | DBU/LiBF4 | TONb | Yieldc (%) |
|---|---|---|---|---|
a Reaction conditions: 69 atm of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (1 H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.78 μmol of 1a or 1b in 5 mL THF (ca. 0.015 M), 180 mg DBU at 80 °C.
b Formate production quantified by 1H NMR spectroscopy; reported values are the average of three trials.
c Reported yields are based on DBU 1), 0.78 μmol of 1a or 1b in 5 mL THF (ca. 0.015 M), 180 mg DBU at 80 °C.
b Formate production quantified by 1H NMR spectroscopy; reported values are the average of three trials.
c Reported yields are based on DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formate of 1 formate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||||
| 1 | 1a | No LiBF4 | 240 | 16 |
| 2 | 1b | No LiBF4 | 430 | 28 |
| 3 | 1a | 2/1 | 1010 | 67 |
| 4 | 1b | 2/1 | 1220 | 82 |
In our previous work on LA enhanced FADH we screened a large range of Lewis acidic salts and identified LiBF4 as the optimum co-catalyst.18 A more limited examination of LAs was conducted for the CO2-to-formate reaction with an emphasis on using readily available alkali metal salts (Table 2). Entries 2–4 show the superior performance of the trifluoromethanesulfonate anion (OTf−) compared to BF4− or Cl−. In the case of Cl−, an inhibition of the reaction compared to no added LA was observed, likely due to coordination of Cl− to the iron catalyst. Additional comparison of the three lightest alkali metal OTf salts (entries 4–6) indicated good TON for all species, with a slightly higher conversion for the Li cation.
| Entry | LA | DBU/LA | TONb | Yielde (%) |
|---|---|---|---|---|
a Reaction conditions: 69 atm of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (1 H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.78 μmol of 1b in 5 mL THF (ca. 0.015 M), 600 mg DBU (3.94 mmol) at 80 °C.
b Formate production quantified by 1H NMR spectroscopy.
c Reported value is the average of two trials.
d LiCl was not fully soluble at ambient temperature under these conditions.
e Reported yields are based on DBU 1), 0.78 μmol of 1b in 5 mL THF (ca. 0.015 M), 600 mg DBU (3.94 mmol) at 80 °C.
b Formate production quantified by 1H NMR spectroscopy.
c Reported value is the average of two trials.
d LiCl was not fully soluble at ambient temperature under these conditions.
e Reported yields are based on DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formate of 1 formate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
||||
| 1 | No LA | No LA | 880 | 17 |
| 2 | LiCld | 7.5/1 | 190 | 4 |
| 3 | LiBF4 | 7.5/1 | 2250 | 45 |
| 4 | LiOTf | 7.5/1 | 3070c | 61 |
| 5 | NaOTf | 7.5/1 | 2520 | 50 |
| 6 | KOTf | 7.5/1 | 2680 | 54 |
The incongruous reaction conditions employed across most iron and cobalt catalyzed CO2 hydrogenation reactions make a definitive comparison of catalyst activity challenging. The ca. 3000 TON observed for the LiOTf/1b catalyzed CO2-to-formate reaction (Table 2; entry 4) is higher than any other iron mediated system to date and it functions under comparable reaction conditions to the [P(o-C6H4PPh2)3Fe]2+ catalyst described by Beller.16b The LA/Fe co-catalyzed reaction is also far more active than cobalt catalysts when DBU is employed as the common base, although higher TONs are achieved with cobalt under different reaction conditions.15
Synthesis and characterization of [RPNMePFe] complexes
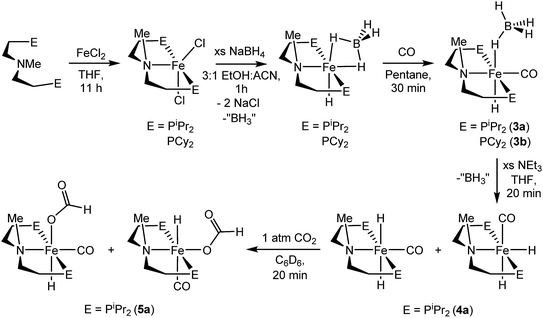
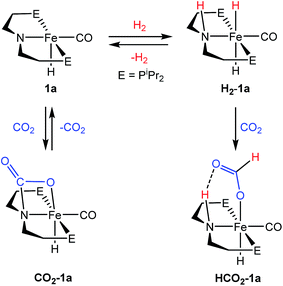
A potentially important feature of 1 is that it can undergo reactions in which there is metal–ligand cooperation21 due to the ability of the PNP ligand to be in either a protonated or deprotonated form.18,19,22 Beller and coworkers have recently reported the synthesis of MeN{CH2CH2(PiPr2)}2 and its coordination to iron as part of control experiments relating to catalytic nitrile and ester hydrogenation.23 To further explore the role of the bifunctional ligand we were interested in comparing the activity for CO2 hydrogenation of iron complexes supported by both RPNHP and RPNMeP ligands. In our hands both the isopropyl and cyclohexyl versions of RPNMeP were coordinated to FeCl2 by stirring in THF solution to give excellent yields of (RPNMeP)FeCl2 (Fig. 2). Each species displays a set of broad peaks between ca. −5 and 75 ppm in the 1H NMR spectrum, indicative of a paramagnetic substance. The molecular structure of (iPrPNMeP)FeCl2 was confirmed by X-ray diffraction (Fig. S16†) and exhibited a distorted square pyramidal geometry (τ = 0.37).24 Treatment of these iron(II) dichloride species with sodium borohydride in MeCN/EtOH afforded the six-coordinate (RPNMeP)Fe(H)BH4 species (Fig. 2). The structure of the PiPr2 congener was characterized by X-ray diffraction as depicted in Fig. S17.† The data were of sufficient quality that all hydrogen atoms, including those bound to iron and boron, were located in the difference map and clearly indicate a κ2-coordination of the BH4 ligand.The (RPNMeP)Fe(H)BH4 species each react readily with 1 atm of CO to yield (RPNMeP)Fe(H)CO(BH4) (R = iPr (3a), R = Cy (3b)) as yellow compounds (Fig. 2). The highest purity materials were obtained from syntheses conducted in pentane over short reaction times (30–45 minutes). 1H NMR spectra of 3a and 3b in benzene-d6 each display an iron-hydride resonance near −20 ppm and a very broad borohydride signal around −2.5 ppm. The broad resonance of the BH4 fragment is typical of κ1-coordinated species and suggests a rapid interchange of the bound B–H bond on the NMR timescale.25 The PiPr2 congener, 3a, again provided a solid state structure from X-ray diffraction experiments (Fig. S18†), which confirms the κ1-coordination of BH4 and the binding of CO ligand trans to the tertiary amine.
The thermal stability of 3a in benzene or THF was limited. Even upon standing under an N2 atmosphere for 1 hour, new Fe–H resonances began to appear in the 1H NMR spectrum. These resonances, along with signals in the 31P NMR spectrum, are consistent with those previously described by Beller and coworkers for the cis and trans dihydride isomers of (iPrPNMeP)Fe(H)2CO (4a).23,26 The conversion of 3a to 4a appears to be influenced by solvent and exposure to vacuum, with use of THF and lower pressures enhancing formation of the iron(II) dihydride species. Pure samples of 4a were obtained by treatment of 3a with a large excess of NEt3 and crystallization from pentane at low temperature. Crystal samples of 4a obtained at −30 °C consistently afforded a molecular structure of the cis dihydride isomer (Fig. S19†), including characterization of two polymorphs of the material.27 However, solutions prepared from the crystalline material consistently show a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio favoring the trans dihydride isomer. EXSY NMR experiments (mixing time 800 ms at 22 °C) do not display correlations indicative of rapid isomer interconversion on the NMR timescale, but the consistent ratio from multiple samples suggests isomerization likely occurs over longer time periods.
1 ratio favoring the trans dihydride isomer. EXSY NMR experiments (mixing time 800 ms at 22 °C) do not display correlations indicative of rapid isomer interconversion on the NMR timescale, but the consistent ratio from multiple samples suggests isomerization likely occurs over longer time periods.
The addition of 1 atm of CO2 to 4a generated the iron formate complex 5a as a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of two isomers (Fig. 2). The major isomer of 5a exhibits an Fe–H resonance at −23.89 ppm (3JP–H = 52 Hz) and a formate C–H peak at 9.22 ppm in the 1H NMR spectrum along with a signal at 84.81 ppm in the 31P {1H} NMR spectrum. The minor isomer displays similar resonances, which are illustrated in the ESI.† A structural assignment of the isomers of 5a was based on a combination of 2D NOESY, 13CO isotopic labeling and X-ray diffraction experiments. Cooling a diethyl ether solution of 5a to −35 °C yielded small yellow needles which weakly diffracted X-rays. While the data was marginal (requiring all hydrogens not bound to iron to be calculated) the refinement did afford a satisfactory solution with the molecular structure depicted in Fig. S20.† The crystallized isomer of 5a contains a meridional chelate ligand with the formate moiety positioned proximal to the N–Me substituent. The Fe–H bond is located trans to the formate ligand and cis to the iron-carbonyl. Additional structural evidence was obtained from 2D NOESY NMR spectra (23 °C, 300 ms mixing time) which indicated a through space correlation between the Fe–H and N–CH3 resonances for at least one of the isomers of 5a, though overlap between the isomers obviated assignment for this correlation to a specific isomer. Still, the NOESY NMR data indicated that the Fe–H and N–CH3 fragments are on the same face of the iron coordination environment for one isomer, presumably the one not identified by X-ray diffraction.28 Isotopic labeling of 5a with 13CO afforded 2JC–H coupling constants between the bound 13CO and Fe–H of 19.5 and 23.9 Hz for the major and minor isomers, respectively. The larger coupling constant for the minor isomer indicates a trans disposition of these fragments, which along with the NOESY NMR data and X-ray diffraction study is consistent with the isomers depicted in Fig. 2. This collection of data also suggests the structure determined by X-ray diffraction is the major isomer.
1 mixture of two isomers (Fig. 2). The major isomer of 5a exhibits an Fe–H resonance at −23.89 ppm (3JP–H = 52 Hz) and a formate C–H peak at 9.22 ppm in the 1H NMR spectrum along with a signal at 84.81 ppm in the 31P {1H} NMR spectrum. The minor isomer displays similar resonances, which are illustrated in the ESI.† A structural assignment of the isomers of 5a was based on a combination of 2D NOESY, 13CO isotopic labeling and X-ray diffraction experiments. Cooling a diethyl ether solution of 5a to −35 °C yielded small yellow needles which weakly diffracted X-rays. While the data was marginal (requiring all hydrogens not bound to iron to be calculated) the refinement did afford a satisfactory solution with the molecular structure depicted in Fig. S20.† The crystallized isomer of 5a contains a meridional chelate ligand with the formate moiety positioned proximal to the N–Me substituent. The Fe–H bond is located trans to the formate ligand and cis to the iron-carbonyl. Additional structural evidence was obtained from 2D NOESY NMR spectra (23 °C, 300 ms mixing time) which indicated a through space correlation between the Fe–H and N–CH3 resonances for at least one of the isomers of 5a, though overlap between the isomers obviated assignment for this correlation to a specific isomer. Still, the NOESY NMR data indicated that the Fe–H and N–CH3 fragments are on the same face of the iron coordination environment for one isomer, presumably the one not identified by X-ray diffraction.28 Isotopic labeling of 5a with 13CO afforded 2JC–H coupling constants between the bound 13CO and Fe–H of 19.5 and 23.9 Hz for the major and minor isomers, respectively. The larger coupling constant for the minor isomer indicates a trans disposition of these fragments, which along with the NOESY NMR data and X-ray diffraction study is consistent with the isomers depicted in Fig. 2. This collection of data also suggests the structure determined by X-ray diffraction is the major isomer.
CO2 hydrogenation activity of [RPNMePFe] complexes
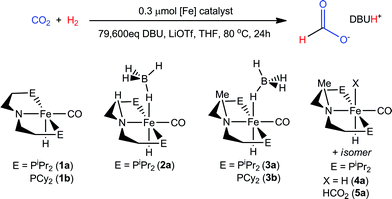
With several [RPNMePFe] complexes in hand, the metal-hydride containing species (iPrPNMeP)Fe(H)BH4, 3a and 4a were each screened for CO2 hydrogenation under the conditions described for 1b/LiOTf in Table 2. Although (iPrPNMeP)Fe(H)BH4 proved to be an ineffective catalyst with a TON = 52 (a conversion comparable to the reaction without iron catalyst), both 3a and 4a afforded very high conversions with TONs of 7660 and 6900, respectively (Table 3). The observed formate yields were in excess of the equivalents of DBU employed; however, stabilization of multiple formate ions by a single DBU via homoconjugation has been previously observed.15b,29 The dramatic improvement in catalyst performance using the N-methylated ligand necessitated trials at lower catalyst and higher DBU loadings to better elucidate their optimum performance. Given the comparable activity of 3a and 4a in preliminary experiments, the relative ease in obtaining 3a made it a more convenient choice for exploratory catalytic trials (Table 3). Only when the catalyst loading was dropped to 0.30 μmol and the DBU/Fe ratio raised to ca. 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 did the yield of formate decrease below the concentration of DBU employed. At ca. 80
000 did the yield of formate decrease below the concentration of DBU employed. At ca. 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 equivalents of base per iron an impressive 42
000 equivalents of base per iron an impressive 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347 turnovers to formate were observed. Further enhancement of the conversion to nearly 60
347 turnovers to formate were observed. Further enhancement of the conversion to nearly 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TON was achieved by raising the LiOTf co-catalyst loading to 5/1 with base, however, at this loading not all of the LiOTf appeared to dissolve and further increasing LiOTf amounts did not enhance the conversion. The central role of the LA co-catalyst was demonstrated in a control experiment where the absence of LiOTf drops the TON to a meager 2790. Overall, this remarkably active catalyst system affords TONs more than an order of magnitude greater than any previously reported iron catalysts.
000 TON was achieved by raising the LiOTf co-catalyst loading to 5/1 with base, however, at this loading not all of the LiOTf appeared to dissolve and further increasing LiOTf amounts did not enhance the conversion. The central role of the LA co-catalyst was demonstrated in a control experiment where the absence of LiOTf drops the TON to a meager 2790. Overall, this remarkably active catalyst system affords TONs more than an order of magnitude greater than any previously reported iron catalysts.
| Catalyst | [Fe] (μmol) | DBU/Fe | DBU/LiOTf | TONb | Yieldd (%) |
|---|---|---|---|---|---|
a Reaction conditions: 69 atm of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (1 H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.30 or 0.78 μmol of 3a or 4a in 5 mL or 10 mL THF at 80 °C for 24 hours.
b Formate production quantified by 1H NMR spectroscopy.
c Reported values are average of two trials.
d Reported yields are based on DBU 1), 0.30 or 0.78 μmol of 3a or 4a in 5 mL or 10 mL THF at 80 °C for 24 hours.
b Formate production quantified by 1H NMR spectroscopy.
c Reported values are average of two trials.
d Reported yields are based on DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formate of 1 formate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||||
| 3a | 0.78 | 5000 | 7.5/1 | 7660 | >99 |
| 4a | 0.78 | 5000 | 7.5/1 | 6900 | >99 |
| 3a | 0.30 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
7.5/1 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
85 |
| 3a | 0.30 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7.5/1 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350c 350c |
53 |
| 3a | 0.30 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5/1 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990c 990c |
74 |
| 3a | 0.30 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
No LiOTf | 2790 | 4 |
Table 4 provides a comparison of each of the iron CO2 hydrogenation catalysts described herein.30 Each trial was conducted under 69 atm of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (1
H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.30–0.78 μmol catalyst, and 79
1) with 0.30–0.78 μmol catalyst, and 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 equiv. of DBU with a 7.5
600 equiv. of DBU with a 7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 loading of base to LiOTf in THF at 80 °C. Under these conditions the secondary amine containing complexes 1a and 1b (entries 2 and 3) improve their TONs to 6030 and 8,910, respectively, over 24 hours. Trials conducted at longer reaction times did not improve the conversion indicating the catalysts had completely deactivated after 1 day.
1 loading of base to LiOTf in THF at 80 °C. Under these conditions the secondary amine containing complexes 1a and 1b (entries 2 and 3) improve their TONs to 6030 and 8,910, respectively, over 24 hours. Trials conducted at longer reaction times did not improve the conversion indicating the catalysts had completely deactivated after 1 day.
| Entry | Catalyst | DBU/LiOTf | TOFb (1 h) | TONb,c (24 h) | Yieldd (%) |
|---|---|---|---|---|---|
a Reaction conditions: 69 atm of CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 (1 H2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.3 μmol of catalyst in 10 mL THF (ca. 0.01 M), 3.600 g DBU at 80 °C.
b Formate production quantified by 1H NMR spectroscopy. TOF was measured after first hour including a temperature equilibration period (<10 min).
c Reported values are average of two trials.
d Reported yields are based on DBU 1), 0.3 μmol of catalyst in 10 mL THF (ca. 0.01 M), 3.600 g DBU at 80 °C.
b Formate production quantified by 1H NMR spectroscopy. TOF was measured after first hour including a temperature equilibration period (<10 min).
c Reported values are average of two trials.
d Reported yields are based on DBU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formate of 1 formate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||||
| 1 | 1a | 7.5/1 | 1290 | 6030 | 8 |
| 2 | 1b | 7.5/1 | 1830 | 8910 | 11 |
| 3 | 2a | 7.5/1 | 680 | 1500 | 2 |
| 4 | 3a | 7.5/1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
53 |
| 5 | 3b | 7.5/1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
58 |
| 6 | 4a | 7.5/1 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 970 |
49 |
| 7 | 5a | 7.5/1 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190 190 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
58 |
Use of the borohydride analog, (iPrPNP)Fe(H)CO(BH4) (2a), a feasible precatalyst for 1a, showed dramatically lower activity (entry 3).25c While the secondary amine [RPNPFe] complexes are highly active catalysts with respect to most previously reported iron and cobalt catalysts, they pale in comparison to the tertiary amine [RPNMePFe] systems. As noted above, 3a delivers a remarkable 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 conversions to formate over 24 hours (entry 4). Much like the analogous secondary amine supported catalyst, the cyclohexyl phosphine substituted 3b (entry 5) offers a small enhancement over the isopropyl phosphine counterpart. Use of the iron(II) dihydride carbonyl species 4a in place of the borohydride precursor (entries 4 and 6) afforded very similar TONs and TOFs (TOF were measured after the first hour of reaction). This is consistent with a rapid conversion of 3a to 4a under catalytic conditions, with both species then proceeding via a common mechanism for CO2 hydrogenation (vide infra).1 Use of 5a as a catalyst (entry 7) gave activity comparable to 3a and 4a, consistent with it being an intermediate in catalysis using 3a or 4a. It is notable that for all the [RPNMePFe] catalysts (entries 4–7) almost half the total conversion occurs during the first hour of the reaction. Again, no additional conversion was observed for experiments conducted for longer than 24 hours. This is indicative of highly active catalysts whose overall productivity declines significantly as the reaction proceeds.
350 conversions to formate over 24 hours (entry 4). Much like the analogous secondary amine supported catalyst, the cyclohexyl phosphine substituted 3b (entry 5) offers a small enhancement over the isopropyl phosphine counterpart. Use of the iron(II) dihydride carbonyl species 4a in place of the borohydride precursor (entries 4 and 6) afforded very similar TONs and TOFs (TOF were measured after the first hour of reaction). This is consistent with a rapid conversion of 3a to 4a under catalytic conditions, with both species then proceeding via a common mechanism for CO2 hydrogenation (vide infra).1 Use of 5a as a catalyst (entry 7) gave activity comparable to 3a and 4a, consistent with it being an intermediate in catalysis using 3a or 4a. It is notable that for all the [RPNMePFe] catalysts (entries 4–7) almost half the total conversion occurs during the first hour of the reaction. Again, no additional conversion was observed for experiments conducted for longer than 24 hours. This is indicative of highly active catalysts whose overall productivity declines significantly as the reaction proceeds.
Mechanistic considerations of (RPNP)Fe(H)CO/Li+ catalyzed CO2 hydrogenation
We were interested in understanding the role of the LA in systems supported by both RPNP and RPNMeP ligands and the increased activity of the tertiary amine supported species. The rate influencing role of the LA co-catalyst was first explored by studying the elementary reaction steps in isolation via NMR spectroscopy in systems with the bifunctional RPNP ligand. On the basis of our related studies,18 a plausible pathway for CO2 hydrogenation starting from 1 could proceed via (1) 1,2-addition of H2 across the Fe–N bond, followed by (2) insertion of CO2 into an Fe–H, and then (3) N–H deprotonation accompanied by formate extrusion to regenerate 1 (Scheme 1). Since the activities of 1a and 1b were comparable, –PiPr2 substituted 1a was selected for NMR experiments due to its simplified spectra.The H2 activation reaction can be observed directly by addition of H2 to 1a in THF or benzene solution, and results in near instantaneous bleaching of the dark red color and a corresponding appearance of NMR signals previously described for (iPrPNP)Fe(H)2CO (H2-1a).18 Though H2-1a was not isolable in the absence of an H2 atmosphere, CO2 insertion was immediately observed upon addition of 1 atm of CO2 to an in situ generated solution of H2-1a (Fig. 3). Careful examination of the NMR spectra over the first 15 minutes following CO2 addition revealed sufficient signals to account for the formation of two products. Two triplet Fe–H resonances were observed in the 1H NMR spectrum, at −25.43 and −25.83 ppm and 31P NMR spectra exhibited peaks at 95.40 and 93.99 ppm. The more upfield resonance in each of these pairs was assigned to HCO2-1a, which was previously prepared by addition of formic acid to 1a.18 Over the course of 1 hour the resonances originating from the second product diminished with concomitant growth in the signals for HCO2-1a. The reaction sequence was repeated using 13CO2 in order to gain insight into the transient product which afforded two enhanced resonances in the 13C NMR spectrum at 165.37 and 174.81 ppm. 1H-13C HSQC NMR spectra showed correlation between the resonance at 174.81 ppm and the formate C–H resonance of HCO2-1a at 9.51 ppm in the 1H-dimension; however, no one-bond correlations were observed for the signal at 165.37 ppm. This indicated that the transient species was not simply an isomer of HCO2-1a. Instead complex CO2-1a is the formal product of CO2 addition across the Fe–N bond, and separate experiments show that this species may also be obtained as the sole product from the reaction of CO2 to 1a. Definitive characterization of CO2-1a was established by single crystal X-ray diffraction as depicted in Fig. S21.† To the best of our knowledge, addition of CO2 across an Fe–NR2 bond has not previously been reported, but the transformation is closely related to the more commonly observed cycloaddition of CO2 to transition metal imides and CO2 insertion into transition metal amides.31 Though CO2-1a has limited stability to vacuum, small quantities of pure material were isolated by low temperature crystallization from pentane solution under N2. Notably, addition of 1 atm of H2 to CO2-1a affords HCO2-1a cleanly over 6–8 hours at ambient temperature with no observable intermediates. If these results are extrapolated to the catalytic conditions, this suggests that formation CO2-1a is likely of minimal consequence to CO2 hydrogenation, but may serve as a reversibly formed off-cycle catalytic intermediate.
Overall, the rapidity of H2-1a and HCO2-1a formation (even under temperature and pressure conditions far more mild than the catalytic reaction) suggests that extrusion of formate and/or N–H deprotonation from HCO2-1a are likely key to the rate of (RPNP)Fe(H)CO/Li+ catalyzed CO2 hydrogenation. This was supported by in situ NMR spectroscopy of a catalytic reaction under modified conditions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ratio of HCO2-1a:LiBF4
40 ratio of HCO2-1a:LiBF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
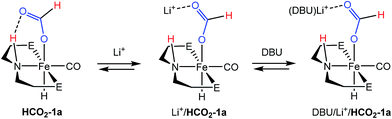
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBU in THF under 2 atm of CO2/H2 at ambient temperature) which showed 31P and 1H NMR resonances approximate to HCO2-1a as the primary organometallic species. To better assign these resonances and gain further insight into roles of Fe, Li+ and DBU in this portion of the reaction, a series of stoichiometric NMR scale reactions was performed (Fig. S1†). First, samples of HCO2-1a were independently treated with 1 equiv. of DBU and LiBF4 in THF-d8. The sample treated with base showed no reaction, but the NMR spectra of the sample containing LA exhibited several changes indicative of an interaction between HCO2-1a and Li+. LiBF4 addition resulted in a ∼1.5 ppm upfield shift of the 31P NMR resonance and a corresponding downfield shift of ∼0.2 ppm for the Fe–H peak in the 1H NMR spectrum (Fig. S1†). A more dramatic upfield movement of the N–H resonance from 8.52 to 5.74 ppm was also observed. This change in chemical shift is consistent with a disruption of the hydrogen bonding interaction between the secondary amine and the bound formate, an interaction which has been predicted by computational analysis.18,19 While an exact structure for the Li-bound HCO2-1a complex has not been established, 7Li NMR spectroscopy exhibited a resonance at −2.12 ppm which is consistent with a Li–O interaction.32 Subsequent addition of 1 equiv. of DBU to the Li+/HCO2-1a complex produced only a minimal change in the 31P NMR spectrum, and a very modest shift of the N–H proton resonance back downfield to 6.20 ppm. This indicates that DBU does not significantly alter the hydrogen bonding interaction. Notably, no conversion to 1a and free formate was detected by NMR spectroscopy.
DBU in THF under 2 atm of CO2/H2 at ambient temperature) which showed 31P and 1H NMR resonances approximate to HCO2-1a as the primary organometallic species. To better assign these resonances and gain further insight into roles of Fe, Li+ and DBU in this portion of the reaction, a series of stoichiometric NMR scale reactions was performed (Fig. S1†). First, samples of HCO2-1a were independently treated with 1 equiv. of DBU and LiBF4 in THF-d8. The sample treated with base showed no reaction, but the NMR spectra of the sample containing LA exhibited several changes indicative of an interaction between HCO2-1a and Li+. LiBF4 addition resulted in a ∼1.5 ppm upfield shift of the 31P NMR resonance and a corresponding downfield shift of ∼0.2 ppm for the Fe–H peak in the 1H NMR spectrum (Fig. S1†). A more dramatic upfield movement of the N–H resonance from 8.52 to 5.74 ppm was also observed. This change in chemical shift is consistent with a disruption of the hydrogen bonding interaction between the secondary amine and the bound formate, an interaction which has been predicted by computational analysis.18,19 While an exact structure for the Li-bound HCO2-1a complex has not been established, 7Li NMR spectroscopy exhibited a resonance at −2.12 ppm which is consistent with a Li–O interaction.32 Subsequent addition of 1 equiv. of DBU to the Li+/HCO2-1a complex produced only a minimal change in the 31P NMR spectrum, and a very modest shift of the N–H proton resonance back downfield to 6.20 ppm. This indicates that DBU does not significantly alter the hydrogen bonding interaction. Notably, no conversion to 1a and free formate was detected by NMR spectroscopy.
The stoichiometric experiments suggest a three component equilibrium exists between HCO2-1a, Li+/HCO2-1a, and DBU/Li+/HCO2-1a complexes (Fig. 4). The inability to observe separate NMR resonances for these species (even at −80 °C) suggests that equilibration is rapid. The relative upfield N–H 1H NMR chemical shift also indicates a thermodynamic preference for the Li+/HCO2-1a and DBU/Li+/HCO2-1a complexes. However, it is important to consider that the conditions of these stoichiometric NMR experiments are far removed from the prevailing Li+ and DBU concentrations under catalytic conditions.
In order to better model the catalytic reaction a J. Young NMR tube was charged with DBU/LiBF4/HCO2-1a in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Initial NMR spectra were nearly identical to those for the stoichiometric DBU/Li+/HCO2-1a mixture (Fig. S2†). Addition of 1.5 atm each of H2 and CO2 produced a gradual downfield shift in the N–H resonance along with the growth of a new peak near 9.30 ppm assigned to the free formate product. After 2 hours the conversion was complete and the N–H resonance remained at ∼7.80 ppm. This experiment is consistent with a reaction model where the initial resting state of the catalyst is dominated by the Li+ and DBU bound forms of HCO2-1a, but as DBU is consumed and the product ammonium formate reduces the available Li+ concentration via equilibration between the salts, the resting state equilibrium shifts toward the pure HCO2-1a complex.
1 ratio. Initial NMR spectra were nearly identical to those for the stoichiometric DBU/Li+/HCO2-1a mixture (Fig. S2†). Addition of 1.5 atm each of H2 and CO2 produced a gradual downfield shift in the N–H resonance along with the growth of a new peak near 9.30 ppm assigned to the free formate product. After 2 hours the conversion was complete and the N–H resonance remained at ∼7.80 ppm. This experiment is consistent with a reaction model where the initial resting state of the catalyst is dominated by the Li+ and DBU bound forms of HCO2-1a, but as DBU is consumed and the product ammonium formate reduces the available Li+ concentration via equilibration between the salts, the resting state equilibrium shifts toward the pure HCO2-1a complex.
Overall, the combination of data collected from the NMR experiments point toward the extrusion of formate from iron and/or the deprotonation of the N–H bond as the limiting steps of (RPNP)Fe(H)CO/Li+ co-catalyzed CO2 hydrogenation. The addition of LA appears to enhance catalysis primarily by assisting removal of the anionic formate and making the N–H fragment more available for DBU deprotonation through disruption of its hydrogen bond.
Mechanistic considerations for [RPNMePFe] catalysts
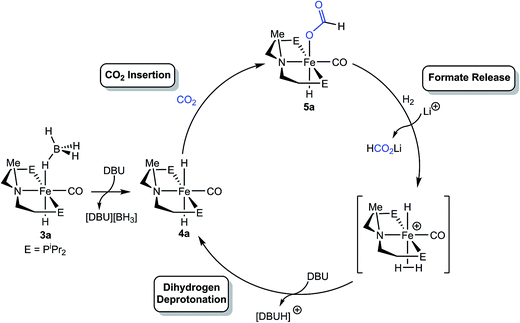
The mechanism of CO2 hydrogenation for the [RPNMePFe] catalysts was investigated through a series of NMR spectroscopy experiments. The similar catalytic performance of the iron borohydride and dihydride catalysts, 3a and 4a (Table 4), as well as the synthesis of 4a from 3a in the presence of base, suggests that both catalysts function via the same mechanism. It is likely that 3a simply serves as a precatalyst, which rapidly forms 4a upon exposure to the high concentrations of DBU present at the initiation of the reaction. This hypothesis was supported by in situ monitoring using NMR spectroscopy of a catalytic reaction using 3a or 4a with 3 and 40 equiv. of LiBF4 and DBU, respectively, under 1.5 atm each of H2 and CO2 (Fig. S3–S6†). In both cases, catalytically active (iPrPNMeP)Fe(H)CO(HCO2) (5a) (see Table 4), was observed as the resting state during formate production, although some residual 3a remained in the experiment using the iron borohydride catalyst.The iron formate catalyst resting state for the [RPNMePFe] systems parallels that observed for the secondary amine complexes, suggesting that formate extrusion still limits the rate of catalysis. In the case of 1a and 1b, our mechanistic experiments demonstrated that the Li+ co-catalyst assisted with this step, in part, by disrupting an intramolecular hydrogen bond between the formate and amide ligand. The role of Li+ was similarly probed for the [RPNMePFe] system through stoichiometric NMR experiments. A sample of 5a in THF-d8 was first treated with 5 equiv. of DBU under 1 atm H2 and monitored for 16 hours at ambient temperature, resulting in no observable formation of free formate or the iron dihydride complex 4a. However, addition of 3 equiv. of LiBF4 immediately afforded full conversion to 4a and extrusion of a formate ion (Fig. S12 and S13†). This observation is consistent with LA assistance of formate release from the iron coordination sphere, likely via stabilization of the anionic formate by the Li+ center. Further evidence for this interaction was obtained by the addition of 3 equiv. of LiBF4 to a THF-d8 solution of 5a, which immediately shifted the Fe–H 1H NMR resonances upfield by approximately 0.5 ppm and dramatically broadened both these signals and the peaks corresponding to the formate C–H protons. A broadening and upfield shift of signals was also observed in the 31P NMR spectrum, suggestive of a reversible coordination of Li+ to 5a (Fig. S14 and S15†).
The mechanistic information available suggests a pathway for [RPNMePFe] catalyzed CO2 hydrogenation which shares some common features with the secondary amine containing [RPNPFe] catalyst (Scheme 2), including the insertion of CO2 into an Fe–H bond followed by rate limiting formate extrusion. Yet the absence of a bifunctional amide moiety requires distinct mechanisms for the elementary reaction steps of H2 activation and deprotonation by DBU. It is proposed that for the [RPNMePFe] catalysts, Li+ facilitates the displacement of formate by dihydrogen to generate a transient iron(II) dihydrogen cationic complex, which is then deprotonated by DBU to regenerate the iron(II) dihydride species. No spectroscopic evidence for the iron(II) dihydrogen cationic complex has been observed during catalysis, but several closely related iron complexes have been observed by others and implicated as intermediates in CO2 hydrogenation.3d,13a,33 Given the prior precedent, a formate release/H2 deprotonation sequence was deemed more likely than an Fe–H deprotonation/H2 oxidative addition pathway, which would require the intermediacy of a zerovalent iron species.
Conclusions
The catalytic activity of a family of PNP supported iron hydrides, containing either secondary or tertiary amines was investigated. In both cases dramatic improvements in TON and TOF were observed when LA co-catalysts were present. Our best system, involving the tertiary amine supported complex (iPrPNMeP)Fe(H)CO(BH4), achieved approximately 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 turnovers, more than an order of magnitude greater than other iron catalysts and far superior to any earth abundant metal catalysts reported to date. In systems containing a secondary amine ligand, NMR spectroscopy identified the catalyst resting state as (RPNP)Fe(H)CO(HCO2) and suggested a key role for LA was disrupting a stabilizing hydrogen bond between N–H and Fe–O2CH moieties in this species. Mechanistic consideration of the [RPNMePFe] catalysts afforded a model whereby (RPNMeP)Fe(H)CO(BH4) was activated by base to produce a (RPNMeP)Fe(H)2CO species which rapidly inserts CO2. The resulting formate complex, (iPrPNMeP)Fe(H)CO(HCO2), was identified as the catalytic resting state. In this case, the primary role of LA was its assistance in a formate for dihydrogen substitution which yields a transient cationic iron(II) dihydrogen complex. Subsequent deprotonation of the dihydrogen fragment by DBU regenerates (RPNMeP)Fe(H)2CO. This pathway for CO2 hydrogenation resulted in remarkable activity, providing approximate TOFs of 20
000 turnovers, more than an order of magnitude greater than other iron catalysts and far superior to any earth abundant metal catalysts reported to date. In systems containing a secondary amine ligand, NMR spectroscopy identified the catalyst resting state as (RPNP)Fe(H)CO(HCO2) and suggested a key role for LA was disrupting a stabilizing hydrogen bond between N–H and Fe–O2CH moieties in this species. Mechanistic consideration of the [RPNMePFe] catalysts afforded a model whereby (RPNMeP)Fe(H)CO(BH4) was activated by base to produce a (RPNMeP)Fe(H)2CO species which rapidly inserts CO2. The resulting formate complex, (iPrPNMeP)Fe(H)CO(HCO2), was identified as the catalytic resting state. In this case, the primary role of LA was its assistance in a formate for dihydrogen substitution which yields a transient cationic iron(II) dihydrogen complex. Subsequent deprotonation of the dihydrogen fragment by DBU regenerates (RPNMeP)Fe(H)2CO. This pathway for CO2 hydrogenation resulted in remarkable activity, providing approximate TOFs of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. Given that most precious and earth abundant metal catalysts are postulated to operate via similar mechanisms for CO2 hydrogenation, it is possible that the use of LA co-catalysts could dramatically enhance performance across of a range of other CO2 reduction systems. Such improvements in CO2 hydrogenation at iron may enable these or related catalyst systems to produce even higher value products, such as methanol, under optimized conditions. Targeting these CO2 functionalization products, as well as further elucidating the structure-reactivity relationships in the [RPNMePFe] system are the foci of on-going efforts in our laboratories.
000 h−1. Given that most precious and earth abundant metal catalysts are postulated to operate via similar mechanisms for CO2 hydrogenation, it is possible that the use of LA co-catalysts could dramatically enhance performance across of a range of other CO2 reduction systems. Such improvements in CO2 hydrogenation at iron may enable these or related catalyst systems to produce even higher value products, such as methanol, under optimized conditions. Targeting these CO2 functionalization products, as well as further elucidating the structure-reactivity relationships in the [RPNMePFe] system are the foci of on-going efforts in our laboratories.
Acknowledgements
This material is based upon work supported by the National Science Foundation through the Centre for the Capture and Conversion of CO2 (C4) (CHE-1240020). N.H. and W.H.B are fellows of the Alfred P. Sloan Foundation.References
- (a) I. Dincer, Energy Policy, 1999, 27, 845 CrossRef; (b) N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed; (c) N. Dimitratos, J. Lopez-Sanchez and G. Hutchings, Top. Catal., 2009, 52, 258 CrossRef CAS.
- (a) Feedstocks for the Future, ed. J. Bozell and M. K. Patel, ACS Symposium Series 921, American Chemical Society, Washington, DC, 2006 Search PubMed; (b) Renewable Raw Materials: New Feedstocks for the Chemical Industry, ed. R. Ulber, D. Sell and T. Hirth, Wiley-VCH, Weinheim, 2011 Search PubMed; (c) Carbon Dioxide as Chemical Feedstock, ed. M. Aresta, Wiley-VCH, Weinheim, 2011 Search PubMed; (d) Transformation and Utilization of Carbon Dioxide, ed. B. M. Bhanage and M. Arai, Springer, 2014 Search PubMed.
- (a) P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1995, 95, 259 CrossRef CAS; (b) P. G. Jessop, F. Joo and C.-C. Tai, Coord. Chem. Rev., 2004, 248, 2425 CrossRef CAS; (c) C. Federsel, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 6254 CrossRef CAS PubMed; (d) C. Ziebart and M. Beller, in Transformation and Utilization of Carbon Dioxide, ed. B. M. Bhanage and M. Arai, Springer, Berlin, 2014 Search PubMed; (e) P. Kang, Z. Chen, M. Brookhart and T. J. Meyer, Top. Catal., 2015, 58, 30 CrossRef CAS; (f) I. Knopf and C. C. Cummins, Organometallics, 2015, 34, 1601 CrossRef CAS.
- (a) S. Enthaler, J. von Langermann and T. Schmidt, Energy Environ. Sci., 2010, 3, 1207 RSC; (b) M. Grasemann and G. Laurenczy, Energy Environ. Sci., 2012, 5, 8171 RSC.
- Y. Yasaka, C. Wakai, N. Matubayasi and M. Nakahara, J. Phys. Chem. A, 2010, 114, 3510 CrossRef CAS PubMed.
- An alternative strategy to drive the reaction, which is both more costly and conceptually different, is to use a sacrificial silane or borane. Hydrolysis of a siloxy or boroxy species subsequently generates formic acid. For examples using first row transition metals see: (a) R. Shintani and K. Nozaki, Organometallics, 2013, 32, 2459 CrossRef CAS; (b) K. Motokura, D. Kashiwame, A. Miyaji and T. Baba, Org. Lett., 2012, 14, 2642 CrossRef CAS PubMed.
- (a) G. Laurenczy, F. Joó and L. Nádasdi, Inorg. Chem., 2000, 39, 5083 CrossRef CAS PubMed; (b) J. Elek, L. Nádasdi, G. Papp, G. Laurenczy and F. Joó, Appl. Catal., A, 2003, 255, 59 CrossRef CAS; (c) C. Federsel, R. Jackstell, A. Boddien, G. Laurenczy and M. Beller, ChemSusChem, 2010, 3, 1048 CrossRef CAS PubMed; (d) A. Boddien, F. Gärtner, C. Federsel, P. Sponholz, D. Mellmann, R. Jackstell, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 6411 CrossRef CAS PubMed; (e) J. L. Drake, C. M. Manna and J. A. Byers, Organometallics, 2013, 32, 6891 CrossRef CAS; (f) C. A. Huff and M. S. Sanford, ACS Catal., 2013, 3, 2412 CrossRef CAS; (g) S. Moret, P. J. Dyson and G. Laurenczy, Nat. Commun., 2014, 5, 5017 CrossRef PubMed; (h) G. A. Filonenko, R. van Putten, E. N. Schulpen, E. J. M. Hensen and E. A. Pidko, ChemCatChem, 2014, 6, 1526 CrossRef CAS.
- (a) F. Gassner and W. Leitner, J. Chem. Soc., Chem. Commun., 1993, 1465 RSC; (b) W. Leitner, E. Dinjus and F. Gassner, in Aqueous-Phase Organometallic Catalysis, Concepts and Applications, ed. B. Cornils and W. A. Herrmann, Wiley-VCH, Weinheim, 1998, p. 486 Search PubMed.
- (a) F. Joo, F. Joo, L. Nadasdi, J. Elek, G. Laurenczy and L. Nadasdi, Chem. Commun., 1999, 971 RSC; (b) Y. Himeda, N. Onozawa-Komatsuzaki, H. Sugihara, H. Arakawa and K. Kasuga, Organometallics, 2004, 23, 1480 CrossRef CAS; (c) T. J. Schmeier, G. E. Dobereiner, R. H. Crabtree and N. Hazari, J. Am. Chem. Soc., 2011, 133, 9274 CrossRef CAS PubMed; (d) A. Azua, S. Sanz and E. Peris, Chem.–Eur. J., 2011, 17, 3963 CrossRef CAS PubMed; (e) F. J. Fernández-Alvarez, M. Iglesias, L. A. Oro and V. Polo, ChemCatChem, 2013, 5, 3481 CrossRef; (f) J. F. Hull, Y. Himeda, W.-H. Wang, B. Hashiguchi, R. Periana, D. J. Szalda, J. T. Muckerman and E. Fujita, Nat. Chem., 2012, 4, 383 CrossRef CAS PubMed; (g) Y. Maenaka, T. Suenobu and S. Fukuzumi, Energy Environ. Sci., 2012, 5, 7360 RSC.
- (a) R. Tanaka, M. Yamashita and K. Nozaki, J. Am. Chem. Soc., 2009, 131, 14168 CrossRef CAS PubMed; (b) R. Tanaka, M. Yamashita, L. W. Chung, K. Morokuma and K. Nozaki, Organometallics, 2011, 30, 6742 CrossRef CAS.
- Y. Inoue, H. Izumida, Y. Sasaki and H. Hashimoto, Chem. Lett., 1976, 863 CrossRef CAS.
- J. B. Hansen, Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knötzinger and J. Weitkamp, VCH, 1997, vol. 4 Search PubMed.
- (a) H. Fong and J. C. Peters, Inorg. Chem., 2015 DOI:10.1021/ic502508p; (b) O. Rivada-Wheelaghan, A. Dauth, G. Leitus, Y. Diskin-Posner and D. Milstein, Inorg. Chem., 2015, 54, 4526 CrossRef CAS PubMed.
- Y. M. Badiei, W.-H. Wang, J. F. Hull, D. J. Szalda, J. T. Muckerman, Y. Himeda and E. Fujita, Inorg. Chem., 2013, 52, 12576 CrossRef CAS PubMed.
- (a) M. S. Jeletic, M. T. Mock, A. M. Appel and J. C. Linehan, J. Am. Chem. Soc., 2013, 135, 11533 CrossRef CAS PubMed; (b) M. S. Jeletic, M. L. Helm, E. B. Hulley, M. T. Mock, A. M. Appel and J. C. Linehan, ACS Catal., 2014, 4, 3755 CrossRef CAS.
- (a) C. Federsel, A. Boddien, R. Jackstell, R. Jennerjahn, P. J. Dyson, R. Scopelliti, G. Laurenczy and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9777 CrossRef CAS PubMed; (b) C. Ziebart, C. Federsel, P. Anbarasan, R. Jackstell, W. Baumann, A. Spannenberg and M. Beller, J. Am. Chem. Soc., 2012, 134, 20701 CrossRef CAS PubMed; (c) C. Federsel, C. Ziebart, R. Jackstell, W. Baumann and M. Beller, Chem.–Eur. J., 2012, 18, 72 CrossRef CAS PubMed.
- R. Langer, Y. Diskin-Posner, G. Leitus, L. J. W. Shimon, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 9948 CrossRef CAS PubMed.
- E. A. Bielinski, P. O. Lagaditis, Y. Zhang, B. Q. Mercado, C. Würtele, W. H. Bernskoetter, N. Hazari and S. Schneider, J. Am. Chem. Soc., 2014, 136, 10234 CrossRef CAS PubMed.
- E. A. Bielinski, M. Förster, Y. Zhang, W. H. Bernskoetter, N. Hazari and M. C. Holthausen, ACS Catal., 2015, 5, 2404 CrossRef CAS.
- To the best of our knowledge the use of Lewis acids to improve CO2 hydrogenation to formate is conceptually novel. Previously Leitner and coworkers have used Brønsted acids to improve TONs in the hydrogenation of CO2 to methanol. See: S. Wesselbaum, T. von Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 7499 CrossRef CAS PubMed.
- (a) L. Fan, B. M. Foxman and O. V. Ozerov, Organometallics, 2004, 23, 326 CrossRef CAS; (b) H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 1814 CrossRef PubMed; (c) D. G. H. Hetterscheid, J. I. van der Vlugt, B. de Bruin and J. N. H. Reek, Angew. Chem., Int. Ed., 2009, 48, 8178 CrossRef CAS PubMed; (d) M. L. Scheuermann, U. Fekl, W. Kaminsky and K. I. Goldberg, Organometallics, 2010, 29, 4749 CrossRef CAS; (e) C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS PubMed; (f) S. Schneider, J. Meiners and B. Askevold, Eur. J. Inorg. Chem., 2012, 2012, 412 CrossRef CAS.
- S. Chakraborty, W. W. Brennessel and W. D. Jones, J. Am. Chem. Soc., 2014, 136, 8564 CrossRef CAS PubMed.
- (a) C. Bornschein, S. Werkmeister, B. Wendt, H. Jiao, E. Alberico, W. Baumann, H. Junge, K. Junge and M. Beller, Nat. Commun., 2014, 5, 4111 CAS; (b) S. Werkmeister, K. Junge, B. Wendt, E. Alberico, H. Jiao, W. Baumann, H. Junge, F. Gallou and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 8722 CrossRef CAS PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) T. J. Marks and J. R. Kolb, Chem. Rev., 1977, 77, 263 CrossRef CAS; (b) R. Langer, M. A. Iron, L. Konstantinovski, Y. Diskin-Posner, G. Leitus, Y. Ben-David and D. Milstein, Chem.–Eur. J., 2012, 18, 7196 CrossRef CAS PubMed; (c) I. Koehne, T. J. Schmeier, E. A. Bielinski, C. J. Pan, P. O. Lagaditis, W. H. Bernskoetter, M. K. Takase, C. Würtele, N. Hazari and S. Schneider, Inorg. Chem., 2014, 53, 2133 CrossRef CAS PubMed.
- Similar spectra have been observed for the cyclohexyl congener, 3b, but no attempt has been made to isolate (CyPNMeP)Fe(H)2CO.
- The metrical parameters of each polymorph of 4a are quite similar. The cifs for both polymorphs are provided in the ESI.†.
- No exchange correlations between isomers of 5a were observed under these conditions.
- (a) P. Munshi, A. D. Main, J. C. Linehan, C.-C. Tai and P. G. Jessop, J. Am. Chem. Soc., 2002, 124, 7963 CrossRef CAS PubMed; (b) A. D. Getty, C.-C. Tai, J. C. Linehan, P. G. Jessop, M. M. Olmstead and A. L. Rheingold, Organometallics, 2009, 28, 5466 CrossRef CAS.
- In the absence of iron catalyst, only 0.1% conversion to formate was observed under these conditions.
- S. Chakraborty, O. Blacque and H. Berke, Dalton Trans., 2015, 6560 RSC.
- (a) H. J. Reich, J. P. Borst, R. R. Dykstra and P. D. Green, J. Am. Chem. Soc., 1993, 115, 8728 CrossRef CAS; (b) M. C. Masiker, C. L. Mayne, B. J. Boone, A. M. Orendt and E. M. Eyring, Magn. Reson. Chem., 2010, 48, 94 CAS.
- (a) J. D. Gilbertson, N. K. Szymczak and D. R. Tyler, Inorg. Chem., 2004, 43, 3341 CrossRef CAS PubMed; (b) J. D. Gilbertson, N. K. Szymczak, J. L. Crossland, W. K. Miller, D. K. Lyon, B. M. Foxman, J. Davis and D. R. Tyler, Inorg. Chem., 2007, 46, 1205 CrossRef CAS PubMed; (c) Y. Lee, R. A. Kinney, B. M. Hoffman and J. C. Peters, J. Am. Chem. Soc., 2011, 133, 16366 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and characterizing data, X-ray CIFs, and selected NMR spectra. CCDC 1061151–1061157. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01467k |
| ‡ These authors made equal contribution. |
| This journal is © The Royal Society of Chemistry 2015 |