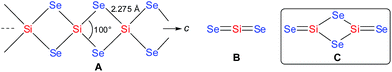Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A soluble molecular variant of the semiconducting silicondiselenide†
Kartik
Chandra Mondal
a,
Sudipta
Roy
a,
Birger
Dittrich
*b,
Bholanath
Maity
c,
Sayan
Dutta
c,
Debasis
Koley
*c,
Suresh Kumar
Vasa
d,
Rasmus
Linser
d,
Sebastian
Dechert
a and
Herbert W.
Roesky
*a
aInstitut für Anorganische Chemie, Georg-August-Universität, 37077 Göttingen, Germany. E-mail: hroesky@gwdg.de
bInstitut für Anorganische und Angewandte Chemie, Universität Hamburg, 20146 Hamburg, Germany, Raum AC 15c (Erdgeschoss)
cDept. of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur – 741246, India
dMax-Planck-Institut für Biophysikalische Chemie, Abtl. NMR-basierte Strukturbiologie, 37077 Göttingen, Germany
First published on 18th June 2015
Abstract
Silicondiselenide is a semiconductor and exists as an insoluble polymer (SiSe2)n which is prepared by reacting elemental silicon with selenium powder in the temperature range of 400–850 °C. Herein, we report on the synthesis, isolation, and characterization of carbene stabilized molecular silicondiselenide in the form of (cAAC)2Si2Se4 (3) [cAAC = cyclic alkyl(amino)carbene]. 3 is synthesized via reaction of diatomic silicon(0) compound (cAAC)2Si2 (2) with black selenium powder at −78 °C to room temperature. The intensely orange colored compound 3 is soluble in polar organic solvents and stable at room temperature for a month under an inert atmosphere. 3 decomposes above 245 °C. The molecular structure of 3 has been confirmed by X-ray single crystal diffraction. It is also characterized by UV-vis, IR, Raman spectroscopy and mass spectrometry. The stability, bonding, and electron density distributions of 3 have been studied by theoretical calculations.
Introduction
The synthesis of silicon diselenide (SiSe2; A in Scheme 1) was first reported by P. Sabatier in 1891.1 Six decades later crystalline SiSe2 was prepared by Weiss et al.2 who reported the Bravais lattice of the crystal structure of SiSe2 as body-centered orthorhombic. Structural analysis showed that it crystallizes in space group Ibam.3a–b SiSe2 has a unique crystalline structure which consists of infinite nonintersecting chains of edge-sharing tetrahedra (A) as shown in Scheme 1.3a–b The structural scenario of amorphous SiSe2 is more complicated,3c where the Si atoms are linked via intra- and inter-tetrahedral bonds connected by Se atoms. The predominant structural motif of amorphous SiSe2 follows the sequence involving both corner- and edge-sharing connections,3c which are importantly more frequent than those constructed exclusively by edge-sharing Si atoms in crystalline SiSe2.3a–bSeveral modified synthesis routes have been developed in quest of the physical properties of SiSe2 in a single phase. Large red rods of SiSe2 have been grown for 4–7 days inside a quartz tube at 1000 °C under high vacuum placed in a furnace.4–6 They are highly moisture sensitive and undergo hydrolysis on the surface to produce silicon- and selenium dioxide.4 The glass transition temperature and crystallization temperature of SiSe2 are 460 and 610 °C, respectively.5 Further studies showed that SiSe2 can exist as three different polymorphs depending upon the temperature (400–850 °C) of preparation.6 Crystalline SiSe2 is considered to be a semiconductor4,7a–b and has a huge potential to be used in solar cells.7c
After the first synthesis report of N-heterocyclic carbenes (NHCs) in 1991, they have been utilized as strong σ-donating ligands in different areas of chemistry.8 The mono-atomic and diatomic variants of silicon are stabilized by NHCs and cAACs in form of L: → Si(0) = Si(0) ← :L, and L: → Si(0) ← :L [L: = NHC or cAAC (cyclic alkyl(amino)carbene)].9,10 Several variants of phosphorus are stabilized by NHCs and cAACs, respectively.10,11 G. Bertrand et al. have stated10 that carbene stabilized variants of these elements are synthesized not only for academic curiosity but also for their solubility in organic solvents. Since organic ligand-anchored variants are more soluble in organic solvents, their chemical transformations are much easier and faster. They are suggested to be utilized for the synthesis of the smaller units of the larger framework/polymer such as silicon dioxide (Si2O4).10 Very recently, the syntheses and characterizations of (NHC)2Si2O3 and (NHC)2Si2O4 were reported by Robinson et al.12a Reaction of (NHC)2Si2 with excess of oxygen donor (N2O or O2) led to the isolation of free NHC and uncharacterized decomposed product of silicon oxide. The light yellow colored (NHC)2Si2O3 was isolated only when three equivalents of N2O are allowed to react with (NHC)2Si2. Note, that (NHC)2Si2O3 does not convert to higher oxide analogue (NHC)2Si2O4 when the former compound is further reacted with N2O. The synthesis of colorless compound (NHC)2Si2O4 is successful only when (NHC)2Si2 is reacted with two equivalents of molecular oxygen (O2).12a Y. Apeloig has summarized the compounds containing Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds giving high emphases on NHC stabilized Si2O3 and Si2O4 oxides.12b However, monomeric or dimeric variants of SiSe2 (B, C; Scheme 1) in the molecular form are even not reported by low temperature matrix-isolation. Herein, we report on the synthesis and characterization of carbene-stabilized (SiSe2)2 in the molecular form of (cAAC)2Si2Se4 (3). The reaction employed cAAC-supported diatomic silicon(0) (cAAC)2Si2 (2) which is reacted with black selenium powder in the temperature range of −78 °C to rt (Scheme 2). Moreover, the stability and bonding of 3 are studied by theoretical calculations.
O double bonds giving high emphases on NHC stabilized Si2O3 and Si2O4 oxides.12b However, monomeric or dimeric variants of SiSe2 (B, C; Scheme 1) in the molecular form are even not reported by low temperature matrix-isolation. Herein, we report on the synthesis and characterization of carbene-stabilized (SiSe2)2 in the molecular form of (cAAC)2Si2Se4 (3). The reaction employed cAAC-supported diatomic silicon(0) (cAAC)2Si2 (2) which is reacted with black selenium powder in the temperature range of −78 °C to rt (Scheme 2). Moreover, the stability and bonding of 3 are studied by theoretical calculations.
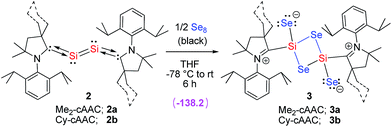 | ||
| Scheme 2 Synthesis of compound 3 from 2. The energy term in parenthesis is ΔG (kcal mol−1) at M06-2X/TZVP/SMD//M06-2X/SVP level of theory for 2a to 3a. | ||
Result and discussion
Both cAAC and NHC form stable adducts with SiCl4.9a,c The colorless adducts (cAAC) → SiCl4 (1a, Me2-cAAC; 1b, Cy-cAAC) are obtained when cAACs react with SiCl4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in THF.15a,9c The diatomic silicon(0) (cAAC)2Si2 (2a–b) has been synthesized by complete dechlorination of 1 with four equiv of KC8 in THF (see ESI†). 2a–b are isolated as dark black-purple needles from n-hexane solution (see ESI for details, Schemes S1–S2†). The yield of 2a is 52% which is higher than that of red colored (NHC)2Si2 (21%)9a but comparable to that of 2b.9c Theoretical calculation showed that cAAC is more firmly bound to the Si2 unit and thus 2 is even characterized by EI-mass spectrometry,9c while its NHC analogue (NHC)2Si2 is not. The latter species dissociates under such conditions and only free NHC is found in the mass spectrum. This is due to the weaker π-accepting property13 which makes NHC-containing compounds more labile.14
1 molar ratio in THF.15a,9c The diatomic silicon(0) (cAAC)2Si2 (2a–b) has been synthesized by complete dechlorination of 1 with four equiv of KC8 in THF (see ESI†). 2a–b are isolated as dark black-purple needles from n-hexane solution (see ESI for details, Schemes S1–S2†). The yield of 2a is 52% which is higher than that of red colored (NHC)2Si2 (21%)9a but comparable to that of 2b.9c Theoretical calculation showed that cAAC is more firmly bound to the Si2 unit and thus 2 is even characterized by EI-mass spectrometry,9c while its NHC analogue (NHC)2Si2 is not. The latter species dissociates under such conditions and only free NHC is found in the mass spectrum. This is due to the weaker π-accepting property13 which makes NHC-containing compounds more labile.14
(NHC)2Si2,9a and (cAAC)2Si2 (2a, Me2-cAAC; 2b, Cy-cAAC) are studied by solid state 29Si NMR at room temperature (rt) to compare the electronic environments of the silicon atoms. (NHC)2Si2 shows its 29Si resonance at 202.4–204.3 ppm which is close to the corresponding value of 224.5 ppm (singlet) in solution.9a Analogous measurement on 2a exhibits a singlet at 254.6 ppm, while two resonances (190.1 and 318.3 ppm) are observed for 2b. Note, that the calculated [(190.1 + 318.3)/2 = 254.2] mean value is ∼254 ppm. There is no resonance around 254 ppm in the solid state 29Si NMR of 2b suggesting that the two silicon atoms are not equivalent. The changes in the chemical shift values of NHC and cAAC derivatives are attributed to the higher π-accepting property of cAAC over NHC.16 However, the reason behind the two well separated (128.2 ppm) 29Si resonances in 2b is not apparently understood. The temperature dependent X-ray single crystal studies revealed that both 2a and 2b crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The center of inversion in between the two silicon atoms in 2b is absent at 100 K (see ESI†), but it is present at 23 K.9c In comparison, the center of inversion in between the two silicon atoms in 2a is present even at 100 K (see ESI†). Structural comparison between 2a and 2b clearly shows that the carbene moieties are evenly disordered in 2a but not in 2b (Fig. S5–S8†). The combined solid state NMR and temperature dependent X-ray structural correlation unambiguously clarify the acute differences of electronic environments of silicon atoms in 2a–b. In the solution phase both 2a and 2b have a similar coordination environment and hence their 29Si resonances are close to each other (252.3 ppm for 2a and 249.1 ppm for 2b) (Schemes S5–S6, Fig. S12–S13, see ESI† for details).
space group. The center of inversion in between the two silicon atoms in 2b is absent at 100 K (see ESI†), but it is present at 23 K.9c In comparison, the center of inversion in between the two silicon atoms in 2a is present even at 100 K (see ESI†). Structural comparison between 2a and 2b clearly shows that the carbene moieties are evenly disordered in 2a but not in 2b (Fig. S5–S8†). The combined solid state NMR and temperature dependent X-ray structural correlation unambiguously clarify the acute differences of electronic environments of silicon atoms in 2a–b. In the solution phase both 2a and 2b have a similar coordination environment and hence their 29Si resonances are close to each other (252.3 ppm for 2a and 249.1 ppm for 2b) (Schemes S5–S6, Fig. S12–S13, see ESI† for details).
Compound 2 is dissolved in THF to obtain a dark purple solution which is cooled to −78 °C and passed into another flask containing black selenium powder (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se = 1
Se = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio). A light green color is obtained after stirring the solution for ten minutes. The green color of the solution is becoming more intense when stirring is continued for twenty minutes. Then the mixture is stirred for 3 h to obtain a brown solution with unreacted selenium powder. Stirring is continued for another 2.5 h to produce a clear orange solution. The volume of the THF solution is reduced to 2 mL under vacuum. Finally, 3 mL of toluene are added and the orange solution is stored at −32 °C in a freezer to form small orange blocks/needles of 3 in 30–32% yield. Compound 3b is comparatively more soluble in THF than 3a. Very recently the synthesis and isolation of (NHC)2Si2O3 and (NHC)2Si2O4 were reported by reacting (NHC)2Si with N2O and O2, respectively.12 The formation of (NHC)2Si2O3 and (NHC)2Si2O4 depends on the source of oxygen. Importantly, when (NHC)2Si2O3 was further reacted with N2O led to the isolation of decomposed by-products and thus (NHC)2Si2O4 was not obtained. It is well known that NHC mostly favors the formation of coordinate σ-bonds while cAAC forms both coordinate and electron sharing covalent σ-bonds depending on the electronic situation of the involved silicon atom.15
4 molar ratio). A light green color is obtained after stirring the solution for ten minutes. The green color of the solution is becoming more intense when stirring is continued for twenty minutes. Then the mixture is stirred for 3 h to obtain a brown solution with unreacted selenium powder. Stirring is continued for another 2.5 h to produce a clear orange solution. The volume of the THF solution is reduced to 2 mL under vacuum. Finally, 3 mL of toluene are added and the orange solution is stored at −32 °C in a freezer to form small orange blocks/needles of 3 in 30–32% yield. Compound 3b is comparatively more soluble in THF than 3a. Very recently the synthesis and isolation of (NHC)2Si2O3 and (NHC)2Si2O4 were reported by reacting (NHC)2Si with N2O and O2, respectively.12 The formation of (NHC)2Si2O3 and (NHC)2Si2O4 depends on the source of oxygen. Importantly, when (NHC)2Si2O3 was further reacted with N2O led to the isolation of decomposed by-products and thus (NHC)2Si2O4 was not obtained. It is well known that NHC mostly favors the formation of coordinate σ-bonds while cAAC forms both coordinate and electron sharing covalent σ-bonds depending on the electronic situation of the involved silicon atom.15
We have carried out the reaction of 2a and Se-powder in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratios. The reaction in a 1
4 molar ratios. The reaction in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio does not produce the green intermediate color. A dark brown solution was obtained. The solvent was removed and the dry residue was extracted with n-hexane to obtain a brown filtrate and crystalline 3a in 10–12% yield. The concentrated n-hexane solution was stored at −32 °C in a freezer. No crystals were formed. The removal of solvent (n-hexane) produced an oily material. The reaction in a 1
2 molar ratio does not produce the green intermediate color. A dark brown solution was obtained. The solvent was removed and the dry residue was extracted with n-hexane to obtain a brown filtrate and crystalline 3a in 10–12% yield. The concentrated n-hexane solution was stored at −32 °C in a freezer. No crystals were formed. The removal of solvent (n-hexane) produced an oily material. The reaction in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio proceeds first to a lighter green intermediate color. In the following step a lighter orange solution was obtained from which the crystalline powder of 3a was isolated in 17–20% yield.
3 molar ratio proceeds first to a lighter green intermediate color. In the following step a lighter orange solution was obtained from which the crystalline powder of 3a was isolated in 17–20% yield.
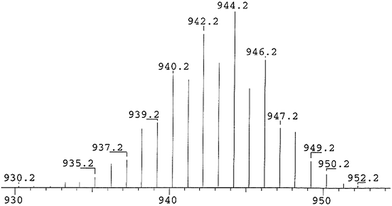
The crystals of 3a–b are stable in air for several days and retain their dark orange color for a week while THF solutions of 3a–b slowly loose their color when exposed to air. Orange powders of 3a–b decompose above 285 °C (3a), 245 °C (3b) to give light yellow solids of cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se.17 This is concluded from mass spectrometry (see ESI†). Compound 3a was further characterized by EI-MS mass spectrometry (m/z (100%); 944.2) (see ESI†). The UV-vis spectra of compounds 3a–b recorded in THF solution show absorption bands at 422 nm (3a) and 402 nm (3b), respectively (see ESI†) which are close to the values obtained from theoretical TD-DFT calculation (400–440 nm; Tables S10 and S11†). Relative transitions are explained from KS-MO of 3a shown in Fig. S15.† The infrared (IR) spectrum of 3a (measured in the range of 400–4000 cm−1) showed a sharp absorption band at 547 cm−1. It is close to the theoretically calculated values of 533.3 cm−1 (νSi
Se.17 This is concluded from mass spectrometry (see ESI†). Compound 3a was further characterized by EI-MS mass spectrometry (m/z (100%); 944.2) (see ESI†). The UV-vis spectra of compounds 3a–b recorded in THF solution show absorption bands at 422 nm (3a) and 402 nm (3b), respectively (see ESI†) which are close to the values obtained from theoretical TD-DFT calculation (400–440 nm; Tables S10 and S11†). Relative transitions are explained from KS-MO of 3a shown in Fig. S15.† The infrared (IR) spectrum of 3a (measured in the range of 400–4000 cm−1) showed a sharp absorption band at 547 cm−1. It is close to the theoretically calculated values of 533.3 cm−1 (νSi![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se) and 355.1 cm−1 (νSi–Se) of 3a. Additionally, 3a is investigated by Raman spectroscopy (see ESI†). Raman spectra are recorded on solid sample of 3a which exhibit Raman bands at 1490.9 cm−1 and 1475.9 cm−1 with a shoulder. A strong Raman band (νSi
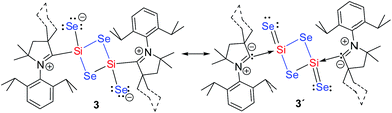
Se) and 355.1 cm−1 (νSi–Se) of 3a. Additionally, 3a is investigated by Raman spectroscopy (see ESI†). Raman spectra are recorded on solid sample of 3a which exhibit Raman bands at 1490.9 cm−1 and 1475.9 cm−1 with a shoulder. A strong Raman band (νSi![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si)9c was observed at 478 cm−1 for (cAAC)2Si2 which is not present in 3a. Both the compounds 3a–b are studied by solution and solid state NMR measurements. 1H and 13C NMR resonances are very broad and hence not much informative (Fig. S11†). 29Si and 77Se NMR resonances are not observed. The zwitterionic nature (Schemes 2 and 3) of compound 3 might be the reason for the broadening of the NMR resonances. However, the corresponding chemical shift values of carbene carbon, silicon, and selenium atoms are theoretically calculated and given in the ESI.†
Si)9c was observed at 478 cm−1 for (cAAC)2Si2 which is not present in 3a. Both the compounds 3a–b are studied by solution and solid state NMR measurements. 1H and 13C NMR resonances are very broad and hence not much informative (Fig. S11†). 29Si and 77Se NMR resonances are not observed. The zwitterionic nature (Schemes 2 and 3) of compound 3 might be the reason for the broadening of the NMR resonances. However, the corresponding chemical shift values of carbene carbon, silicon, and selenium atoms are theoretically calculated and given in the ESI.†
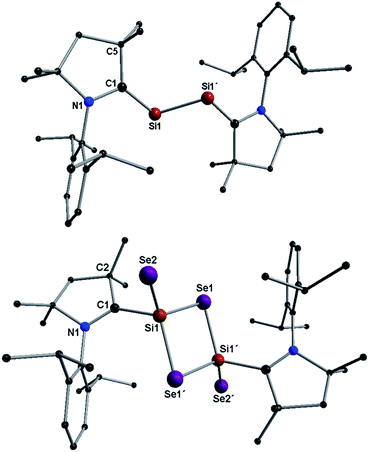
Structural descriptions of 2a–b are given in ESI.† Compound 3a crystallizes in the space group P21/n and possesses a center of inversion within the molecule. The asymmetric unit of 3a contains the (Me2-cAAC)SiSe2 fragment. The complete molecular structure of 3a generated from applying inversion symmetry is shown in Fig. 1.
The Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(μ-Se)2Si
Si(μ-Se)2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se unit (alike C shown in Scheme 1) is coordinated by two Me2-cAAC ligands. Both silicon atoms adopt a four coordinate distorted tetrahedral geometry. The CcAAC–Si bond length of 3a is 1.931 (4) Å which is close to that in 1a (1.944 (2) Å)15a but larger than that in 2a (1.887(4) Å) (Table S1†). This suggests that the bond between carbene carbon and silicon is a donor bond (CcAAC → Si) (Scheme 3), rather than a donor–acceptor partial double bond9c in 2a as illustrated in Scheme 2. The Si1–Se1 and Si1–Se2 bond distances of 3a are 2.2874(10)/2.3046(10), and 2.1510(10) Å, respectively, suggesting single bond (Si1–Se1, Si1–Se1′) and double bond (Si1
Se unit (alike C shown in Scheme 1) is coordinated by two Me2-cAAC ligands. Both silicon atoms adopt a four coordinate distorted tetrahedral geometry. The CcAAC–Si bond length of 3a is 1.931 (4) Å which is close to that in 1a (1.944 (2) Å)15a but larger than that in 2a (1.887(4) Å) (Table S1†). This suggests that the bond between carbene carbon and silicon is a donor bond (CcAAC → Si) (Scheme 3), rather than a donor–acceptor partial double bond9c in 2a as illustrated in Scheme 2. The Si1–Se1 and Si1–Se2 bond distances of 3a are 2.2874(10)/2.3046(10), and 2.1510(10) Å, respectively, suggesting single bond (Si1–Se1, Si1–Se1′) and double bond (Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se2) character (Scheme 3).14 The Si1–Se1/Se1′ bond distances are close to the values reported for (SiSe2)n (A) (2.275 Å). The Se–Si–Se bond angle in Si2Se2 four membered ring of 3a is 96.55(4)° which is sharper (by ∼3.5°) than that of A (100.0(1)°).3b This might be due to the coordination of Me2-cAAC to each silicon atom. The Si2Se4 core of compound 3a is structurally similar to that of (NHC)2Si2O4 compound reported by Robinson et al.12a The silicon–silicon bond distance is 3.056 Å in 3a while that of (NHC)2Si2O4 is 2.3980(11) Å which is due to the larger size of the selenium atoms.
Se2) character (Scheme 3).14 The Si1–Se1/Se1′ bond distances are close to the values reported for (SiSe2)n (A) (2.275 Å). The Se–Si–Se bond angle in Si2Se2 four membered ring of 3a is 96.55(4)° which is sharper (by ∼3.5°) than that of A (100.0(1)°).3b This might be due to the coordination of Me2-cAAC to each silicon atom. The Si2Se4 core of compound 3a is structurally similar to that of (NHC)2Si2O4 compound reported by Robinson et al.12a The silicon–silicon bond distance is 3.056 Å in 3a while that of (NHC)2Si2O4 is 2.3980(11) Å which is due to the larger size of the selenium atoms.
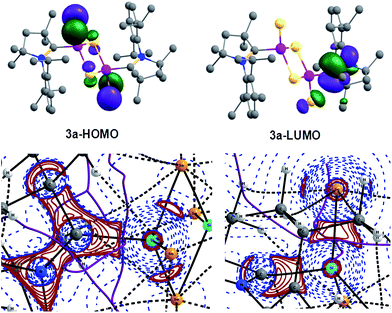
We have performed DFT calculations to illustrate the electronic structure and bonding scenario of 3a (refer Computational Details in ESI†). The optimized geometry of 3a at the M06-2X/SVP level shows a strong resemblance with the X-ray crystal structure of 3a (Fig. 1 and S14†). The electronic structure and bonding features of 3a are illustrated using NBO analysis as implemented in Gaussian09. The calculations reveal that C1 is connected to Si1 by a single bond with electron occupancy of 1.94701 e which is primarily located on the C1 (77%) center. The N1–C1 bond in 3a is significantly shorter (1.309 Å) than in 2a (1.343 Å) due to the strong π-bonding interaction to disrupt C1←Si1 back donation. This finding also reveals that the C1 is bound to the Si1 as a singlet carbene donor (C1→Si). On the other hand Si1 also binds to Se2 by a single bond and Se contains three lone pairs. But the Si1–Se2 bond length is 2.15 Å which is significantly shorter than the single bond length (2.28 Å and 2.07 Å in H3Si–SeH and H2Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se, respectively). It is surprising to see that the lone pair occupancies on the Se2 are 1.960, 1.713, and 1.717 e, respectively. The lowering in occupancy of the last two lone pairs can be envisaged as some sort of donor–acceptor type interaction with the Si1 atom, in turn making the bond shorter (Scheme 3).
Se, respectively). It is surprising to see that the lone pair occupancies on the Se2 are 1.960, 1.713, and 1.717 e, respectively. The lowering in occupancy of the last two lone pairs can be envisaged as some sort of donor–acceptor type interaction with the Si1 atom, in turn making the bond shorter (Scheme 3).
Topological and topographical analyses are also performed for further illustration of the bonding features in 3a using QTAIM (Quantum Theory of Atoms in Molecules) calculations (see computational details in ESI†).
The electron density, ρ(r), at the (3,−1) bond critical points (BCPs) of C1–Si1 (0.095) and Si1–Se2 (0.104) bonds along with the respective Laplacian [∇2ρ(r); +0.240 and +0.051] indicate closed-shell interaction i.e., donor–acceptor bond (Table S9†). This is further supported by 2D Laplacian plot of (3,−3) critical points (Fig. 2, bottom). The Delocalization Index (DI) value of C1–Si1 (0.43) is lower than for an ordinary C–Si bond in H3C–SiH3 (0.55), indicating the presence of weak C1–Si1 donor type bonding. In case of the Si1–Se2 bond the DI value donor (0.6; Si1→Se2) is close to that of the Si–Se single bond (0.58) in H3Si–SeH in accordance with the NBO results discussed above. The real bonding in 3 is a combination of two resonating structures as shown in Scheme 3. In contrast the lower but positive value of Laplacian (+0.051) indicates closed-shell binding nature to lesser extent. We presume that the more electronegative Se is reluctant to share its lone pairs to the adjacent Si center in turn contributing towards equal sharing between the partners.
Conclusions
We have shown that carbene coordinated diatomic silicon(0) species (cAAC)2Si2 (2a–b) can react with black selenium powder in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio in THF at −78 °C to rt to produce molecular compound (cAAC)2Si2Se4 (3a–b). Orange powders of 3a–b are stable under an inert atmosphere for nearly a month and decompose above 245 °C. Dark orange crystals of 3a–b retain their colors in air for a week. They are soluble in polar organic solvents, such as THF, while partially soluble in toluene and n-hexane. The molecular structure of 3a was confirmed by X-ray single crystal diffraction and EI-mass spectrometry (Fig. 3). A comparison between the bond parameters of 2a and 3a unambiguously led to the conclusion that the Si2(0) unit in 2a is stabilized by a donor acceptor type bond between 2cAAC and (0)Si
4 molar ratio in THF at −78 °C to rt to produce molecular compound (cAAC)2Si2Se4 (3a–b). Orange powders of 3a–b are stable under an inert atmosphere for nearly a month and decompose above 245 °C. Dark orange crystals of 3a–b retain their colors in air for a week. They are soluble in polar organic solvents, such as THF, while partially soluble in toluene and n-hexane. The molecular structure of 3a was confirmed by X-ray single crystal diffraction and EI-mass spectrometry (Fig. 3). A comparison between the bond parameters of 2a and 3a unambiguously led to the conclusion that the Si2(0) unit in 2a is stabilized by a donor acceptor type bond between 2cAAC and (0)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(0), while its derivative Si2Se4 of 3a has been prevented from undergoing polymerization by strong σ-donation of the cAAC molecules (Schemes 2 and 3). The bonding and stability of 3a have been further studied by theoretical calculations. To the best of our knowledge, this is the first report on a neutral ligand-stabilized molecular Si2Se4 species.18,19
Si(0), while its derivative Si2Se4 of 3a has been prevented from undergoing polymerization by strong σ-donation of the cAAC molecules (Schemes 2 and 3). The bonding and stability of 3a have been further studied by theoretical calculations. To the best of our knowledge, this is the first report on a neutral ligand-stabilized molecular Si2Se4 species.18,19
Acknowledgements
Dedicated to Professor Robert R. Holmes for his outstanding contributions in chemistry. H. W. R. thanks the Deutsche Forschungsgemeinschaft (DFG Funder ID http://dx.doi.org/10.13039/501100001659, RO 224/64-1) for financial support. We thank Dr. S. Demeshko for solid state UV-vis measurements. B. M. and S. D. thank CSIR, India for SRF and JRF fellowships, respectively. D. K. thanks to CSIR, India for project fund (http://dx.doi.org/10.13039/501100001332, 01(2770)/13/EMR-II). We thank M. Tretiakov for preparing Me2-cAAC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se and (NHC)2Si2.
Se and (NHC)2Si2.
Notes and references
- P. C. R. Sabatier, Comptes Rendus Hebdomadaires des Séances de l'Académie des Sciences, 1891, 113, 132 Search PubMed.
- A. Weiss and A. Weiss, Naturforschung, 1952, 7, 483 Search PubMed.
- (a) E. Parthé, Crystal Chemistry of Tetrahedral Structures, Gordan and Breach, New York, 1964 Search PubMed; (b) J. Peters and B. Krebs, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 1270 CrossRef; (c) M. Celino and C. Massobrio, Phys. Rev. Lett., 2003, 90, 125502 CrossRef PubMed.
- E. A. Hauschild and C. R. Kannewurf, J. Phys. Chem. Solids, 1969, 30, 353 CrossRef CAS.
- M. Tenhover, M. A. Hazle and R. K. Grasselli, Phys. Rev. Lett., 1983, 51, 404 CrossRef CAS.
- A. Pradel, V. Michel-Lledos, M. Ribes and H. Eckert, Chem. Mater., 1993, 5, 377 CrossRef CAS.
- (a) E. Mooser and W. B. Pearson, J. Electron., 1956, 1, 629 CAS; (b) D. N. Tafen and D. A. Drabold, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 165208 CrossRef; (c) Recently came in the news of phys.org, http://phys.org/news/2014-04-material-scientist-exploring-ways-efficiency.html.
- M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS PubMed.
- (a) Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. V. R. Schleyer and G. H. Robinson, Science, 2008, 321, 1069 CrossRef CAS PubMed; (b) Y. Xiong, S. Yao, S. Inoue, J. D. Epping and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 7147 ( Angew. Chem. , 2013 , 125 , 7287 ) CrossRef CAS PubMed; (c) K. C. Mondal, P. P. Samuel, H. W. Roesky, R. R. Aysin, L. A. Leites, S. Neudeck, J. Lübben, B. Dittrich, M. Hermann and G. Frenking, J. Am. Chem. Soc., 2014, 136, 8919 CrossRef CAS PubMed; (d) K. C. Mondal, H. W. Roesky, M. C. Schwarzer, G. Frenking, B. Niepötter, H. Wolf, R. Herbst-Irmer and D. Stalke, Angew. Chem., Int. Ed., 2013, 52, 2963 ( Angew. Chem. , 2013 , 125 , 3036 ) CrossRef CAS PubMed.
- C. A. Dyker and G. Bertrand, Science, 2008, 321, 1050 CrossRef CAS PubMed.
- (a) J. D. Masuda, W. W. Schoeller, B. Donnadieu and G. Bertrand, Angew. Chem., Int. Ed., 2007, 46, 7052 ( Angew. Chem. , 2007 , 119 , 7182 ) CrossRef CAS PubMed; (b) O. Back, G. Kuchenbeiser, B. Donnadieu and G. Bertrand, Angew., Chem. Int. Ed., 2009, 48, 5530 ( Angew. Chem. , 2009 , 121 , 5638 ) CrossRef CAS PubMed; (c) Y. Wang, Y. Xie, P. Wie, R. B. King, H. F. Schaefer III, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2008, 130, 14970 CrossRef CAS PubMed.
- (a) Y. Wang, M. Chen, Y. Xie, P. Wei, H. F. Schaefer III, P. V. R. Schleyer and G. H. Robinson, Nat. Chem., 2015, 7, 509–513 CrossRef CAS PubMed; (b) Y. Apeloig, Nat. Chem., 2015, 7, 468–470 CrossRef CAS PubMed.
- R. Tonner, G. Heydenrych and G. Frenking, Chem.–Asian J., 2007, 2, 1555 CrossRef CAS PubMed.
- M. Y. Abraham, Y. Wang, Y. Xie, P. Wei, H. F. Schaefer, P. V. R. Schleyer and G. H. Robinson, J. Am. Chem. Soc., 2011, 133, 8874 CrossRef CAS PubMed.
- (a) K. C. Mondal, H. W. Roesky, A. C. Stückl, F. Ihret, W. Kaim, B. Dittrich, B. Maity and D. Koley, Angew. Chem., Int. Ed., 2013, 52, 11804 ( Angew.Chem. , 2013 , 125 , 12020 ) CrossRef CAS PubMed; (b) K. C. Mondal, H. W. Roesky, M. C. Schwarzer, G. Frenking, I. Tkach, H. Wolf, D. Kratzert, R. Herbst-Irmer, B. Niepötter and D. Stalke, Angew. Chem., Int. Ed., 2013, 52, 1801 CrossRef CAS PubMed; (c) K. C. Mondal, B. Dittrich, B. Maity, D. Koley and H. W. Roesky, J. Am. Chem. Soc., 2014, 136, 9568 CrossRef CAS PubMed.
- (a) D. Martin, M. Melaimi, M. Soleilhavoup and G. Bertrand, Organometallics, 2011, 30, 5304 CrossRef CAS PubMed; (b) D. Martin, Y. Canac, V. Lavallo and G. Bertrand, J. Am. Chem. Soc., 2014, 136, 5023 CrossRef CAS PubMed.
- M. Tretiakov, Y. G. Shermolovich, A. P. Singh, P. P. Samuel, H. W. Roesky, B. Niepötter, A. Visscher and D. Stalke, Dalton Trans., 2013, 12940 RSC.
- Lighter congener such as SiS2 and SiS were studied at low temperature in a methane matrix but not SiSe2. See M. Friesen and H. Schnöckel, Z. Anorg. Allg. Chem., 1999, 625, 1097 CrossRef CAS.
- S.-H. Zhang, H.-X. Yeong and C.-W. So, Chem.–Eur. J., 2011, 17, 3490 CrossRef CAS PubMed and all references on Si-Se compounds therein.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses, NMR, UV-vis, Raman spectra, crystallographic table, and theoretical details. CCDC 926618, 927696, 948799, 983863, 1060365. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01516b |
| This journal is © The Royal Society of Chemistry 2015 |