Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
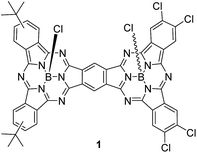
A push–pull unsymmetrical subphthalocyanine dimer†
Germán
Zango
a,
Johannes
Zirzlmeier
b,
Christian G.
Claessens
a,
Timothy
Clark
 *c,
M. Victoria
Martínez-Díaz
*a,
Dirk M.
Guldi
*b and
Tomás
Torres
*ad
*c,
M. Victoria
Martínez-Díaz
*a,
Dirk M.
Guldi
*b and
Tomás
Torres
*ad
aDepartamento de Química Orgánica, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain. E-mail: tomas.torres@uam.es; victoria.martinez@uam.es
bDepartment of Chemistry and Pharmacy, Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universitaet Erlangen-Nuernberg, Egerlandstraße 3, 91058 Erlangen, Germany. E-mail: dirk.guldi@fau.de
cComputer Chemie Centrum, Department of Chemistry and Pharmacy, Friedrich-Alexander-Universitaet Erlangen-Nuernberg, Naegelsbachstraße 25, 91052 Erlangen, Germany. E-mail: tim.clark@fau.de
dIMDEA-Nanociencia, c/Faraday 9, Cantoblanco, 28049 Madrid, Spain.
First published on 17th June 2015
Abstract
Unsymmetrical subphthalocyanine fused dimers have been prepared from appropriate ortho-dinitrile SubPc precursors. In particular, either electron-donating or electron-accepting substituents have been introduced on each SubPc constituent unit, resulting in unprecedented push–pull π-extended curved aromatic macrocycles. From fluorescence experiments in solvents of different polarity we conclude a dual fluorescence, namely a delocalized singlet excited state (1.73 eV) and a polarized charge transfer state (<1.7 eV). Pump probe experiments corroborate the dual nature of the fluorescence. On one hand, the delocalized singlet excited state gives rise to a several nanosecond lasting intersystem crossing yielding the corresponding triplet excited state. On the other hand, the polarized charge transfer state deactivates within a few picosesonds. Visualization of the charge transfer state was accomplished by means of molecular modeling with a slight polarization of the HOMO towards the electron donor and of the LUMO towards the electron acceptor.
Introduction
The interesting electronic properties of π-extended materials such as graphene1 are the result of their flat two-dimensional π-conjugated systems. Curved π-conjugated systems2 are much rarer, despite major advantages such as enhanced solubility, decreased aggregation by π–π stacking, etc. Curved polyaromatics, like corannulenes, circulenes, sumanenes and their derivatives, have been prepared and studied intensively.3 Introducing heteroatoms into the aromatic π-extended frameworks results in molecular building blocks that exhibit unusual properties, like, for instance, metal coordination provided by the presence of nitrogen atoms. In this respect, planar porphyrins and/or phthalocyanines (Pc)4 are among the most relevant examples of macroheterocyclic aromatic compounds.In contrast, the lower homologues of phthalocyanines, subphthalocyanines (SubPc)5 are curved aromatic macrocycles, whose geometry is imposed by the coordination of the three constituent diimino-isoindoles to a central tetrahedral boron. Dimers of subphthalocyanines (SubPc)2, that is, π-extended systems comprising two SubPcs fused through a common benzene ring, have been synthesized and fully characterized.6 The geometry of the constituent SubPcs endowed the corresponding dimers with an unusual bowl-shape topology, in which two topoisomers, the syn and the anti, are formed in equal amounts. The syn and anti SubPc dimers exhibit similar spectroscopic features and a remarkable bathochromic shift of ca. 120 nm of their Q-band absorptions to about 700 nm relative to their parent monomers.
SubPcs have been used successfully in evaporated planar heterojunction photovoltaic devices.7 Very recently, an outstanding 8.4% efficiency has been reported.8 Moreover, highly efficient all SubPc-based photovoltaic devices have been fabricated using an electron accepting (F8SubPc)2 dimer and a complementary absorbing SubPc monomer as electron donor.9
The preparation of unsymmetrical SubPc dimers has been pursued intensively in our group with the goal of modulating the optoelectronic properties of this family of molecular building blocks further. We now describe a push–pull SubPc–SubPc′ dimer 1 – Chart 1 – that exhibits an unsymmetrical electronic distribution along the curved aromatic surface.
In particular, electron-donating tert-butyl and electron-withdrawing chloro groups have been introduced into each SubPc-moiety. The stepwise route described goes through an unsymmetrically substituted ortho-dicyano SubPc 2, which is used as a π-extended o-dinitrile precursor. Recently, Kobayashi and Shibata have described a phthalocyanine-subphthalocyanine (Pc-SubPc) heterodinuclear fused dimer starting from an ortho-dicyano Pc.10 This new π-extended conjugate is a hybrid of the two original constituents, half flat–half concave, and exhibits intermediate spectroscopic properties, but no push–pull character was evident.
Results and discussion
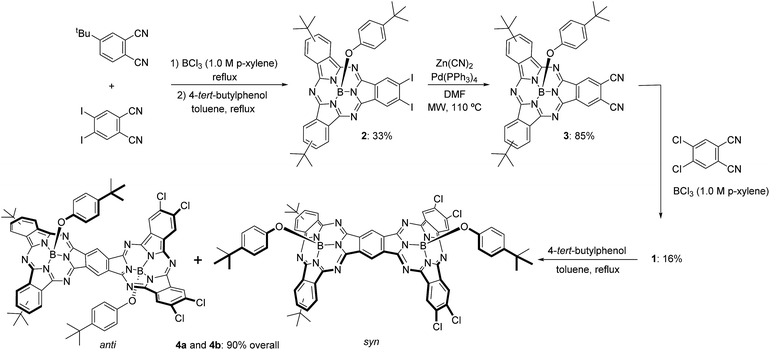
The synthetic pathway for SubPc–SubPc′ dimer 1 is shown in Scheme 1. DiiodoSubPc 2 was prepared in 33% yield by standard cross condensation of 4-tert-butylphthalonitrile and 4,5-diiodophthalonitrile11 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in the presence of BCl3 (1 eq.) in p-xylene, followed by axial chlorine atom substitution with 4-tert-butylphenol in excess. 2 consists of an equimolar mixture of three regioisomers with different symmetries (see ESI†), which can be separated by subsequent column chromatography. Despite SubPcs being sensitive to cyanide-anion,12 a mild palladium-mediated coupling reaction of SubPc 2 with zinc cyanide (2.4 equivalents) under microwave irradiation (20 W) in DMF at 110 °C during 18 min furnishes the desired ortho-dicyano SubPc 3 in 85% yield. Without microwave irradiation, a mixture of starting material 2, ortho-monocyano-monoiodo-SubPc, and SubPc 3 was obtained after 24 h of heating in DMF at 110 °C. Higher reaction temperatures and/or longer reaction times led to an increase in the degree of SubPc decomposition.
1 molar ratio) in the presence of BCl3 (1 eq.) in p-xylene, followed by axial chlorine atom substitution with 4-tert-butylphenol in excess. 2 consists of an equimolar mixture of three regioisomers with different symmetries (see ESI†), which can be separated by subsequent column chromatography. Despite SubPcs being sensitive to cyanide-anion,12 a mild palladium-mediated coupling reaction of SubPc 2 with zinc cyanide (2.4 equivalents) under microwave irradiation (20 W) in DMF at 110 °C during 18 min furnishes the desired ortho-dicyano SubPc 3 in 85% yield. Without microwave irradiation, a mixture of starting material 2, ortho-monocyano-monoiodo-SubPc, and SubPc 3 was obtained after 24 h of heating in DMF at 110 °C. Higher reaction temperatures and/or longer reaction times led to an increase in the degree of SubPc decomposition.
Finally, SubPc–SubPc′ dimer 1 was synthesized in 16% yield as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of syn- and anti-topoisomers, by reaction of SubPc 3 – either a mixture of regioisomers or a single regioisomer – with 4 equivalents of 4,5-dichlorophthalonitrile in the presence of 6 equivalents of BCl3 in p-xylene under reflux. Full substitution of the axial ligand in the starting ortho-dicyano SubPc 3 by a chlorine atom took place during the reaction.13 Separation of syn- and anti-topoisomers of dimer 1 was not possible by column chromatography on silica gel. However, replacement of the two chlorine atoms in 1 by 4-tert-butylphenol allowed for the separation of both topoisomers 4a and 4b in 90% overall yield. The structures of all compounds were confirmed by 1H-NMR, IR, UV-vis, and HR-MALDI-MS.
1 mixture of syn- and anti-topoisomers, by reaction of SubPc 3 – either a mixture of regioisomers or a single regioisomer – with 4 equivalents of 4,5-dichlorophthalonitrile in the presence of 6 equivalents of BCl3 in p-xylene under reflux. Full substitution of the axial ligand in the starting ortho-dicyano SubPc 3 by a chlorine atom took place during the reaction.13 Separation of syn- and anti-topoisomers of dimer 1 was not possible by column chromatography on silica gel. However, replacement of the two chlorine atoms in 1 by 4-tert-butylphenol allowed for the separation of both topoisomers 4a and 4b in 90% overall yield. The structures of all compounds were confirmed by 1H-NMR, IR, UV-vis, and HR-MALDI-MS.
The 1H-NMR (500 MHz) spectrum of 1 in CDCl3 exhibits several diagnostic singlets between 10.40 and 10.35 ppm, corresponding to the two highly deshielded protons of the central benzene ring. The absorption spectra of 1–3 show a B/Soret band at ca. 320 nm and a Q band in the visible region. The maximum of the Q band absorption is subject to a bathochromic shift on going from diiodoSubPc 2 (580 nm) to dicyanoSubPc 3 (598 nm). This trend indicates the strong influence of the ortho-dinitriles on the π-conjugation of the SubPc aromatic structure. In contrast, the absorption spectrum of SubPc–SubPc′ dimer 1 reveals a Q band maximum at 712 nm, which is 114 nm red-shifted relative to that of SubPc 3. It is notable that the extinction coefficients also increase as the extent of the π-system increases.
The electrochemical behaviour of 1–4 was studied by cyclic voltammetry in THF – see Table 1 and ESI, Fig. S25 and S26.† DiiodoSubPc 2 exhibits a first reversible reduction at −1481 mV followed by a second one at −2008 mV relative to Fc/Fc+. An irreversible oxidative process is observed at 668 mV. Incorporating two nitriles in the periphery of SubPc 3 renders the reduction much easier; SubPc 3 shows a first reversible reduction at −1221 mV, 260 mV positively shifted relative to that of SubPc 2. A second reversible and a third irreversible reduction are seen at −1744 and −2289 mV, respectively. Compared to SubPc 2, a more difficult irreversible oxidation is observed for SubPc 3 at 806 mV.
| E 11/2,red (mV) | E 1ox , (mV) | E LUMO (eV) | E 0–0 (eV) | E HOMO (eV) | |
|---|---|---|---|---|---|
| a [10−3 M] in THF vs. Fc/Fc+, Pt working electrode, Pt counter electrode, 20 °C, 0.1 TBAPF6, scan rate = 100 mV s−1. b Peak oxidation potential by square wave voltammetry. c Calculated with respect to ferrocene, EHOMO: −4.8 eV. d Estimated from the intersection between the absorption and emission spectra. | |||||
| 1 | −1088 | 640 | −3.71 | 1.73 | −5.44 |
| 2 | −1481 | 668 | −3.32 | 2.13 | −5.45 |
| 3 | −1221 | 806 | −3.58 | 2.07 | −5.65 |
| 4a | −1215 | 589 | −3.59 | 1.72 | −5.31 |
| 4b | −1217 | 569 | −3.58 | 1.73 | −5.31 |
SubPc–SubPc′ dimer 1 exhibits reduction processes at −1088, −1392, and −1801 mV. Hereby, the first reduction process is shifted anodically by 133 mV relative to SubPc 3. An irreversible oxidation process is observed at 640 mV, shifted cathodically by 164 mV relative to 3. As expected, dimers 4a and 4b present slight differences in their reduction and oxidation processes compared to 1 due to axial substitution with donor 4-tert-butylphenoxy groups, and both topoisomers show exactly the same electrochemical behaviour.
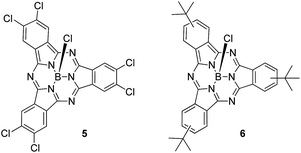
First insights into potential push–pull interactions in the SubPc–SubPc′ dimer 1 came from absorption and fluorescence measurements. In the two references, hexachloro-substituted SubPc 5 and tri-tert-butyl-substituted SubPc 6 – Chart 2 – the long wavelength absorptions are seen at 574 and 569 nm in toluene – see ESI.† Importantly, changing the solvent polarity from toluene to benzonitrile exerts only a marginal impact on the vibrational progression of the absorption features, in general, and the long wavelength absorption maxima, in particular. In direct contrast to the absorption, chloro-substituted SubPc 5 (2.15 eV) and tert-butyl-substituted SubPc 6 (2.16 eV) exhibit fluorescence with distinct vibrational progression and with Stokes shifts of 211 and 229 cm−1. Here, the fluorescence maxima, fluorescence quantum yields, and fluorescence lifetimes in toluene are 581 nm, 28.6 ± 2.5%, and 3.65 ns, respectively, for the electron accepting chloro-substituted SubPc 5, and 577 nm, 26.5 ± 2.5%, and 3.29 ns for the electron donating tert-butyl-substituted SubPc 6. It is notable that none of the fluorescence features change when the solvent polarity is varied systematically.
At first glance, the absorption and fluorescence of SubPc–SubPc′ dimer 1 – Fig. 1 – are similar to those seen for the references described above despite a substantial red shift. For example, in toluene the vibrationally fine structured absorption and fluorescence include a long wavelength maximum at 715 nm and a short wavelength maximum at 721 nm, respectively. In THF and benzonitrile, the corresponding features peak at 708/720 and at 716/727 nm, respectively. In contrast to the invariance of the maxima, the fluorescence quantum yields drop as the solvent polarity increases from cyclohexane (19.2%) and toluene (16.1%) to THF (8.3%) and benzonitrile (7.3%). A closer look reveals, however, that the fluorescence features an additional, rather broad component, whose maximum is subject to some red shifts as the solvent polarity is increased, but determination of the exact location turned out to be difficult. We postulate that a delocalized singlet excited state (1.73 eV) is the origin of the former, while the latter is due to a polarized charge transfer state (<1.7 eV).
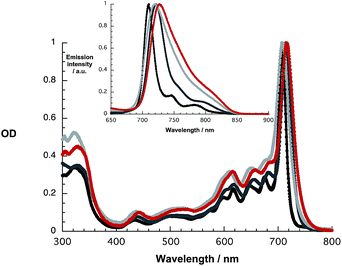 | ||
| Fig. 1 Absorption and fluorescence spectra (inset) upon 610 nm photoexcitation of 1 in cyclohexane (black), toluene (dark grey), THF (light grey), and benzonitrile (red). | ||
This hypothesis was confirmed in pump-probe experiments, in which chloro-substituted SubPc 5, tert-butyl-substituted SubPc 6, and SubPc–SubPc′ dimer 1 were photoexcited at 530 and 656 nm. For 5 and 6, we find differential absorption spectra immediately upon photoexcitation – see ESI.† These include the features of the singlet excited states. For example, for chloro-substituted SubPc 5 in toluene, transient bleaching from 510 to 608 nm with a minimum at 578 nm reflects the ground-state absorption. Maxima at 428 and 648 nm, a shoulder at 620 nm, and a broad NIR band complete the singlet excited state features of chloro-substituted SubPc 5. The differential absorption spectrum of tert-butyl-substituted SubPc 6 reveals a minimum at 574 and maxima at 446 and 620 nm, in addition to a shoulder at 645 nm. From multi-wavelength analyses, we estimate solvent-independent singlet excited state lifetimes of 2.9 ± 0.1 ns for chloro-substituted SubPc 5 and 3.0 ± 0.15 ns for tert-butyl-substituted SubPc 6. These decay lifetimes imply an efficient intersystem crossing to the corresponding triplet excited states. For the triplet excited state, the most prominent features in toluene are a 574 nm minimum with a 553 nm shoulder and 444, 617 and 672 nm maxima for chloro-substituted SubPc 5 and a 571 nm minimum with a shoulder at 550 nm and 458, 610 and 670 nm maxima for the tert-butyl-substituted SubPc 6. They decay with lifetimes on the order of 7.7 ± 0.2 μs and 1.2 ± 0.1 μs and give place to the singlet ground state.
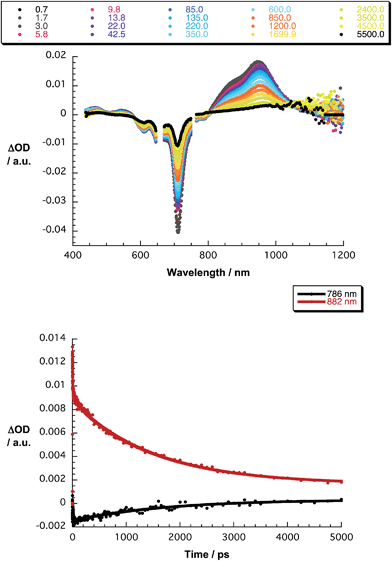
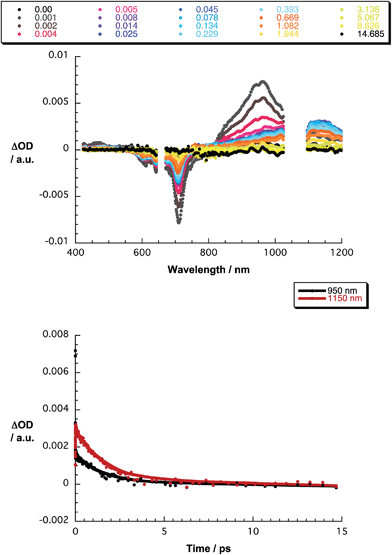
The final set of experiments is concerned with the SubPc–SubPc′ dimer 1 in pump-probe assays – Fig. 2 and 3. In line with the absorption spectra, the ground-state bleaching in the singlet excited state (1.73 eV) is red shifted relative to those described above for chloro-substituted SubPc 5 (2.15 eV) and tert-butyl-substituted SubPc 6 (2.16 eV). In particular, minima develop immediately upon photoexcitation at 656 nm in toluene at 618, 684, and 716 nm, in THF at 611 and 710 nm, and in benzonitrile at 616 and 717 nm. Additionally, maxima are found at 468, 544, and 952 nm in toluene, 470, 541, and 948 nm in THF, and in benzonitrile at 470, 547, and 955 nm. The time evolution of these differential absorption spectra allows us to derive solvent-dependent intersystem crossing lifetimes for the delocalized singlet excited state with values that range from 2.28 ± 0.01 ns in cyclohexane to 1.69 ± 0.03 ns in benzonitrile. These values are in close agreement with those determined in fluorescence lifetime measurements; 2.4 ns in cyclohexane and 1.7 ns in benzonitrile. Evidence for the shorter-lived polarized charge transfer state came in the form of ground-state bleaching at 710 nm and a 870 nm shoulder, but only in the polar solvents THF and benzonitrile. The lifetimes are 3.2 ± 0.3 ps in THF and 5.3 ± 0.5 ps in benzonitrile.
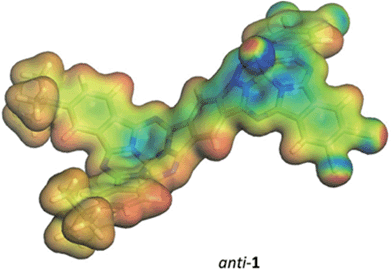
We turned to modeling to rationalize the experimental observations. Geometries were initially optimized for the syn- and anti-isomers of 1 and for a model phthalocyanine dimer 7 – the structure is shown in the ESI† – using density-functional theory (DFT) with the B3LYP hybrid functional,14,15 and the 6-31G(d) basis set.16–18 All DFT calculations used the Gaussian 09 program.19 The optimized geometries were confirmed to be local minima by calculating their normal vibrations within the harmonic approximation. Electronic spectra were calculated for these geometries in the gas phase using the AM1 Hamiltonian20,21 and a configuration-interaction with only single excitations (CIS). CIS calculations used 100 occupied and 100 virtual orbitals in the active space. All semiempirical calculations were performed with our development version of VAMP.22 The optimized structures of these three compounds are given in the ESI.†Syn- and anti-1 are strongly non-planar, as expected, whereas 7 optimizes to a planar structure. 7 was included in the study in order to quantify the effects of non-planarity on the subphthalocyanine dimers by comparison with a planar model compound.
The calculated spectra of syn- and anti-1 are very similar, so that only that for the anti-isomer will be discussed in the following. The data for the syn-isomer are given in the ESI.† A simulation of the calculated UV/vis spectrum for anti-1 is shown in Fig. S38 of the ESI.† The calculated spectrum shows respectable agreement with the experiment, giving us confidence that AM1/CIS is able to reproduce the major features of the excited states of 1 and 7.
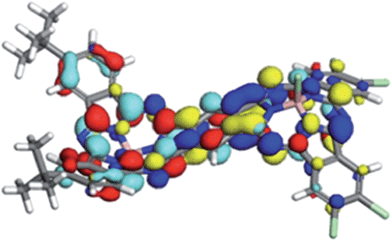
A detailed analysis of the states involved in the excitations reveals that the S1 to S4 states have dipole moments that differ by less than 1.5 Debye from the ground state for both syn- and anti-1. A charge-separated state is found in both isomers for S5, which occurs close to 410 nm in both cases and gives an excited state with a dipole moment of 19.4 or 18.2 Debyes for syn and anti, respectively. The planar phthalocyanine dimer 7 shows similar behavior. S1 to S4 are π–π local excitations and S5 (475 nm) is a charge-separated state with a dipole moment of 36.5 Debyes. As the charge-separated states are embedded energetically in a plethora of locally excited ones, strong solvent shifts of the charge-separated state will not necessarily be evident in the experimental spectra. Fig. 4 shows the calculated molecular electrostatic potentials on the 0.001 a.u. isodensity surface of the charge-separated (S5) states of anti-1. It is evident that charge separation is far more pronounced in the planar phthalocyanine dimer 7. The oscillator strength calculated for the S0 → S5 transition in 7 (0.27) is also slightly lower than those found for syn- (0.31) and anti-1 (0.36), which is consistent with lower charge-separation in the non-planar species 1.
The ground states of both isomers of 1 show a small amount of charge separation between the two unsymmetrical halves of the dimer. Fig. 5 shows the calculated (DFT) HOMO and LUMO of anti-1.
The slight polarization of the HOMO towards the donor and of the LUMO towards the acceptor demonstrate nicely the effect of the donor and acceptor substituents. Self-consistent reaction field (SCRF) AM1-CIS calculations23 in water suggest that the locally excite S1 and a polar charge-shifted state close in energy are the first two excited states in solution.
Conclusions
Unsymmetrically substituted SubPc fused dimers have been prepared for the first time by a stepwise methodology, which implies the preparation of ortho-dicyano SubPcs, used as π-extended phthalonitrile precursor. The successful key transformation of o-diiodo SubPcs into o-dicyano derivatives by a palladium-mediated reaction required two equivalents of zinc cyanide and the use of microwave irradiation. Subsequent cross-condensation between ortho-dicyano SubPcs and a differently substituted phthalonitrile allowed the preparation of unsymmetrical SubPc dimers as equimolar mixture of syn and anti isomers, which exhibit similar spectroscopic features. Separation of syn and anti isomers was achieved by further axial functionalization.Physico-chemical studies allowed us to probe the push–pull character of SubPc–SubPc′ dimer 1, bearing tert-butyl and chlorine substituents on each SubPc half, and have provided evidence for a charge-polarized curved π-extended system. For example, from fluorescence experiments in solvents of different polarity we conclude a dual fluorescence, namely a delocalized singlet excited state (1.73 eV) and a polarized charge transfer state (<1.7 eV). Further corroboration for the dual nature of the fluorescence came from pump-probe experiments. The delocalized singlet excited state gives rise to intersystem crossing that lasts several nanoseconds to yield the corresponding triplet excited state, while the polarized charge-transfer state deactivates within a few picosesonds. The nature of the charge-transfer state was visualized using the results of DFT calculations. They show a slight polarization of the HOMO towards the electron donor and of the LUMO towards the electron acceptor. Semiempirical CIS calculations confirm the existence of charge-transfer states above low-lying locally excited singlets in vacuum. These charge-transfer states are stabilized relative to the non-polar singlets in SCRF calculations.
Acknowledgements
This work was supported by the Spanish MINECO (CTQ2014-52869-P), by the Comunidad de Madrid (S2013/MIT-2841, FOTOCARBON), by the Deutsche Forschungsgemeinschaft as part of the Excellence Cluster Engineering of Advanced Materials and SFB953 Synthetic Carbon Allotropes, and by the Bavarian State Government as part of the Solar Technologies go Hybrid initiative.Notes and references
- (a) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 306, 666 CrossRef CAS PubMed; (b) A. Ambrosi, C. K. Chua, A. Bonanni and M. Pumera, Chem. Rev., 2014, 114, 7150 CrossRef CAS PubMed.
- (a) X. Lu and Z. Chen, Chem. Rev., 2005, 105, 3643 CrossRef CAS PubMed; (b) T. Yao, H. Yu, R. J. Vermeij and G. J. Bodwell, Pure Appl. Chem., 2008, 80, 533 CrossRef CAS; (c) B. Zhang, G. P. Manning, M. A. Dobrowolski, M. K. Cyranski and G. J. Bodwell, Org. Lett., 2008, 10, 273 CrossRef CAS PubMed.
- (a) Y.-T. Wu and J. S. Siegel, Chem. Rev., 2006, 106, 4843 CrossRef CAS PubMed; (b) Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions, and Coordination Chemistry, ed. M. A. Petrukhina and L. T. Scott, John Wiley & Sons, Inc., New Jersey, 2012 Search PubMed; (c) C.-N. Feng, M.-Y. Kuo and Y.-T. Wu, Angew. Chem., Int. Ed., 2013, 52, 7791 CrossRef CAS PubMed.
- (a) J. Mack and N. Kobayashi, Chem. Rev., 2011, 111, 281 CrossRef CAS PubMed; (b) G. de la Torre, C. G. Claessens and T. Torres, Chem. Commun., 2007, 2000 RSC; (c) G. Bottari, G. de la Torre, D. M. Guldi and T. Torres, Chem. Rev., 2010, 110, 6768 CrossRef CAS PubMed; (d) C. G. Claessens, U. Hahn and T. Torres, Chem. Rec., 2008, 8, 75 CrossRef CAS PubMed; (e) G. de la Torre, G. Bottari, U. Hahn and T. Torres, Struct. Bonding, 2010, 135, 1 CrossRef CAS; (f) N. Kobayashi, Synthesis and Spectroscopic Properties of Phthalocyanine Analogs, in The Porphyrin Handbook, ed. K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, San Diego, 2003, vol. 15, ch. 100, pp. 161–262 Search PubMed.
- (a) C. G. Claessens, D. González-Rodríguez, M. S. Rodríguez-Morgade, A. Medina and T. Torres, Chem. Rev., 2014, 114, 2192 CrossRef CAS PubMed; (b) C. G. Claessens, D. González-Rodríguez and T. Torres, Chem. Rev., 2002, 102, 835 CrossRef CAS PubMed; (c) S. Shimizu and N. Kobayashi, Chem. Commun., 2014, 50, 6949 RSC.
- (a) C. G. Claessens and T. Torres, Angew. Chem., Int. Ed., 2002, 41, 2561 CrossRef CAS; (b) T. Fukuda, J. R. Stork, R. J. Potucek, M. M. Olmstead, B. C. Noll, N. Kobayashi and W. S. Durfee, Angew. Chem., Int. Ed., 2002, 41, 2565 CrossRef CAS; (c) R. S. Iglesias, C. G. Claessens, T. Torres, M. A. Herranz, V. R. Ferro and J. M. García de la Vega, J. Org. Chem., 2007, 72, 2967 CrossRef CAS PubMed.
- (a) G. E. Morse and T. P. Bender, ACS Appl. Mater. Interfaces, 2012, 4, 5055 CrossRef CAS PubMed; (b) H. Gommans, T. Aernouts, B. Verreet, P. Heremans, A. Medina, C. G. Claessens and T. Torres, Adv. Funct. Mater., 2009, 19, 3435 CrossRef CAS PubMed; (c) N. Beaumont, S. W. Cho, P. Sullivan, D. Newby, K. E. Smith and T. S. Jones, Adv. Funct. Mater., 2012, 22, 561 CrossRef CAS PubMed; (d) N. Beaumont, J. S. Castrucci, P. Sullivan, G. E. Morse, A. S. Paton, Z.-H. Lu, T. P. Bender and T. S. Jones, J. Phys. Chem. C, 2014, 118, 14813 CrossRef CAS; (e) B. Verreet, K. Cnops, D. Cheyns, P. Heremans, A. Stesmans, G. Zango, C. G. Claessens, T. Torres and B. P. Rand, Adv. Funct. Mater., 2014, 4, 1301413 Search PubMed.
- K. Cnops, B. P. Rand, D. Cheyns, B. Verreet, M. A. Empl and P. Heremans, Nat. Commun., 2014, 5, 4406 Search PubMed.
- B. Verreet, B. P. Rand, D. Cheyns, A. Hadipour, T. Aernouts, P. Heremans, A. Medina, C. G. Claessens and T. Torres, Adv. Energy Mater., 2011, 1, 565 CrossRef CAS PubMed.
- N. Shibata, S. Mori, M. Hayashi, M. Umeda, E. Tokunaga, M. Shiro, H. Sato, T. Hoshi and N. Kobayashi, Chem. Commun., 2014, 50, 3040 RSC.
- D. S. Terekhov, K. J. M. Nolan, C. R. McArthur and C. C. Leznoff, J. Org. Chem., 1996, 61, 3034 CrossRef CAS PubMed.
- (a) E. Palomares, M. V. Martínez-Díaz, T. Torres and E. Coronado, Adv. Funct. Mater., 2006, 16, 1166 CrossRef CAS PubMed; (b) J. V. Ros-Lis, R. Martínez-Mañez and J. Soto, Chem. Commun., 2005, 42, 5260 RSC; (c) S. Xu, K. Chen and H. Tian, J. Mater. Chem., 2005, 15, 2676 RSC.
- A related reaction, the substitution of phenoxy ligands by fluorine has been described: M. S. Rodriguez-Morgade, C. G. Claessens, A. Medina, D. González-Rodríguez, E. Gutierrez-Puebla, A. Monge, I. Alkorta, J. Elguero and T. Torres, Chem.–Eur. J., 2008, 14, 1342 CrossRef CAS PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822 CrossRef.
- J. S. Binkley, J. A. Pople and W. J. Hehre, J. Am. Chem. Soc., 1980, 102, 939 CrossRef CAS.
- M. S. Gordon, Chem. Phys. Lett., 1980, 76, 163 CrossRef CAS.
- M. S. Gordon, J. S. Binkley, J. A. Pople, W. J. Pietro and W. J. Hehre, J. Am. Chem. Soc., 1982, 104, 2797 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- M. J. S. Dewar, E. G. Zoebisch, E. F. Healy and J. J. P. Stewart, J. Am. Chem. Soc., 1985, 107, 3902 CrossRef CAS.
- A. J. Holder, in Encyclopedia of Computational Chemistry, ed. P. v. R. Schleyer, N. L. Allinger, T. Clark, J. Gasteiger, P. A. Kollman, H. F. Schaefer III and P. R. Schreiner, John Wiley & Sons, Chichester, UK, 1998, vol. 1, pp. 8–11 Search PubMed.
- T. Clark, A. Alex, B. Beck, F. Burkhardt, J. Chandrasekhar, P. Gedeck, A. H. C. Horn, M. Hutter, B. Martin, G. Rauhut, W. Sauer, T. Schindler and T. Steinke, VAMP 11, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, 2014 Search PubMed.
- G. Rauhut, T. Clark and T. Steinke, J. Am. Chem. Soc., 1993, 115, 9174 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc01709b |
| This journal is © The Royal Society of Chemistry 2015 |