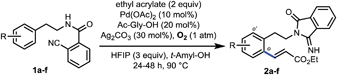Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd(II)-catalyzed remote regiodivergent ortho- and meta-C–H functionalizations of phenylethylamines†
Shangda
Li‡
a,
Huafang
Ji‡
a,
Lei
Cai
a and
Gang
Li
 *ab
*ab
aKey Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 West Yang-Qiao Road, Fuzhou, Fujian 350002, P. R. China. E-mail: gangli@fjirsm.ac.cn
bState Key Laboratory of Structural Chemistry, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China
First published on 25th June 2015
Abstract
Site selectivity control is of vital importance in the direct functionalization of inert C–H bonds. Reported here is a novel example of remote regiodivergent ortho- and meta-C–H bond functionalizations of phenylethylamine derivatives by using a novel 2-cyanobenzoyl group as the original directing functionality, where the regioselectivity was adjusted by a methylation. The potential of the method was exemplified by sequential functionalizations of both ortho- and meta-C–H bonds of a phenylethylamine derivative in a streamlined manner.
Introduction
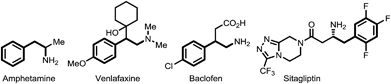
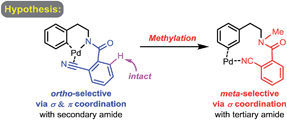
Controlling site selectivity is an outstanding challenge in the direct functionalization of inert C–H bonds that are ubiquitous in organic molecules.1 The increasing applications of these type of transformations in organic synthesis also demand accessibility to diverse site selectivities.2 While numerous directing groups have been introduced to assist the cleavage of proximal ortho-C–H bonds in most cases with transition metals,1,3–10 directing group assisted meta-selective C–H functionalization of arenes has proved especially challenging and is still very rare.5,6,8,9 In 2009, a remarkable breakthrough was reported by Gaunt et al., who developed a carbonyl group directed unprecedented meta-selective C–H arylation of anilides by using a Cu(II) catalyst and diaryliodonium salts.5a This method was later extended to α-aryl carbonyl compounds by the same group.5b Another impressive breakthrough came from the Frost group, who introduced an ingenious method of meta-selective C–H sulfonation of 2-phenylpyridines via cyclometalated ruthenium intermediates.6a,b A similar strategy was then employed by Ackermann to realize a meta-selective C–H alkylation with secondary alkyl halides.6c Recently, a small number of ground-breaking examples of Pd(II) catalyzed directed meta-selective C–H functionalizations of arenes that were attached with elegantly devised nitrile-based templates were disclosed, pioneered by Yu and then further studied by Tan and Maiti.8 By using the above directing group assisted meta-selective C–H functionalization of arenes, elegant regiodivergent functionalizations of ortho- and meta-C–H bonds have been reported by Gaunt,4b,5b Frost6b and Yu,8b and examples of reactions reported by Gaunt4b,5b and Frost6b could even be performed sequentially.8i,11,12 However, the use of analogous directing groups to achieve remote-selective regiodivergent activation of ortho- and meta-C–H bonds has not been examined and remains a significant challenge.13,14 We envision that such methodology is highly desirable for drug discovery and material sciences, since it only requires a single operation to achieve a different remote regioselectivity.2f Herein, we report a novel strategy for regiodivergent ortho- and meta-C–H functionalizations of phenylethylamine derivatives.To test our hypothesis of a regiodivergent C–H functionalization strategy by using analogous directing groups, we selected phenylethylamines as the testing compounds, since they are a class of aromatic compounds that are important core structures of numerous drug molecules (Fig. 1). Moreover, although ortho-C–H functionalizations have been reported for phenylethylamine derivatives, their meta-selective C–H functionalization remains elusive.15 Inspired by recent studies on directed meta-selective C–H functionalizations of arenes,8 we proposed that a 2-cyanobenzoyl group could act as the key directing functionality for both ortho- and meta-C–H functionalizations of phenylethylamines with a Pd(II) catalyst by taking advantage of the σ and π coordination ability of the nitrile group (Scheme 1).16 However, during our study we found that our proposed mode of ortho-selective C–H bond cleavage was not feasible and a novel remote-selective ortho-C–H bond cleavage was observed instead (vide infra).13,14
Results and discussion
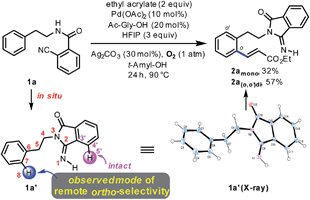
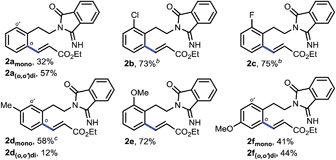
To examine our original hypothesis (Scheme 2), we chose olefination as the model reaction.1o,17 After extensive condition screening with Pd(OAc)2 as the catalyst (see ESI†), we were able to produce a high combined yield of ortho-olefinated products by treating 1a with ethyl acrylate under oxygen with hexafluoroisopropanol (HFIP) as an additive and N-acetyl-glycine (Ac-Gly-OH) ligand.8a,18 Interestingly, the 2-cyanobenzoyl motif cyclized to an imidamide derivative in the products. To ascertain the mechanism of this olefination, 1a was subjected to the above reaction conditions without adding ethyl acrylate, affording 1a′ that was believed to be the reactive substrate for the olefination. Indeed, after 1a′ was treated with the same olefination conditions, the desired products were generated in similar yields (see ESI†). Although this reaction pathway is not desired from our original hypothesis, the site selectivity of this reaction is surprisingly uncommon since the imino group of 1a′, the most likely directing group on 1a′, directed the cleavage of a remote ortho-C–H bond rather than a proximal ortho-C–H bond on the arene attached to the imidamide, which is in marked contrast to the ortho-C–H functionalizations of arylimine derivatives.19 The exact origin of the selectivity is unclear at present, and the study of the mechanism is under way.14Several representative substrates were then surveyed briefly (Table 1). It was found that electron-withdrawing groups like chloride and fluoride were tolerated (2b–c), giving good yields of desired products. Good to excellent yields of products were also generated with substrates containing electron-donating groups such as methyl at the meta-position (2d) and methoxy at the ortho- (2e) and para-position (2f).
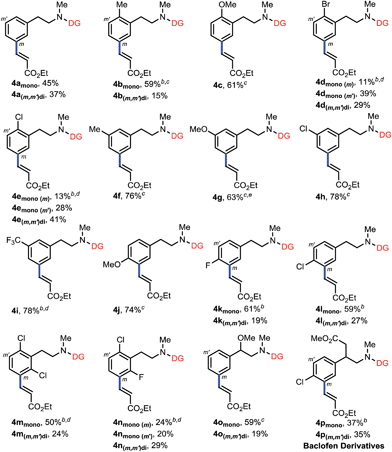
Having established the remote-selective ortho-C–H olefination of the secondary phenylethylamide, we were eager to test whether the selectivity could be switched to a remote-selective meta-C–H olefination after the secondary amide is methylated into a tertiary one (see the ESI† for methylation with MeI). Starting with the above ortho-olefination reaction conditions, we were very delighted to find that N-methyl amide 3a could lead to a 10% yield of the desired product with a trace of other regioisomers (Table 2, entry 1). Inspired by the previous discovery that HFIP was a compatible solvent with nitrile-based templates,8 we switched the solvent to HFIP and found that the combined yield of desired products was increased dramatically to 58% with silver acetate as the sole oxidant (entry 2). Since when using weakly acidic HFIP as the sole solvent some substrate might decompose, DCE was added as the co-solvent, resulting in an increased yield of 73% (entry 3). To optimize the solvent system, we decreased the volume of HFIP to 15% and found that the combined yield was only slightly improved (entry 4). However, a further decreased volume of HFIP led to a much diminished yield (entry 5). Other solvents were also screened, but the combination of DCE and HFIP proved to be the best. The addition of a weak base, such as KHCO3, to tune the acidity of the reaction system was not effective either (entry 6). Since a higher catalytic turnover of the Pd catalyst was observed with 50% volume of HFIP, we repeated the reaction with this solvent system at 80 °C and found that the combined yield was improved to 90% in 32 hours under nitrogen (entry 7), representing the highest catalytic turnover of the Pd catalyst. Finally, by adding 5 equivalents of DMF we were able to get more mono-olefinated product in 28 hours while maintaining the overall efficiency (entry 8, see the ESI† for more condition screenings). However, further screening of reaction conditions could not result in better mono- vs. di-olefination selectivity at present, and a study on this issue is actively being carried out in our laboratory. The meta-selectivity was unambiguously verified by X-ray crystallographic analysis of a derivative obtained by hydrolyzing the ester group of 4amono (see the ESI†).
| Entry | Solvents [v/v] | T (°C) | Yield (%) [4amono, 4a(m,m′)di] | 3a (%) |
|---|---|---|---|---|
| a Reaction conditions: 3a (0.2 mmol), ethyl acrylate (0.4 mmol), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (0.6 mmol), solvent (2 mL), 24 h, 80–90 °C. Yield was determined by 1H NMR analysis using CH2Br2 as the internal standard. b Using the same conditions as in Scheme 2. c KHCO3 (2 equiv.) was added. d Under N2. e 32 h. Isolated yields were 45% of 4amono and 37% of 4a(m,m′)di. f 28 h, DMF (5 equiv.) was added. | ||||
| 1b | t-Amyl-OH | 90 | 10 [10, 0] | 90 |
| 2 | HFIP | 90 | 58 [13, 45] | Trace |
| 3 | DCE/HFIP [50/50] | 90 | 71 [32, 39] | Trace |
| 4 | DCE/HFIP [85/15] | 90 | 73 [48, 25] | 10 |
| 5 | DCE/HFIP [95/5] | 90 | 39 [32, 7] | 44 |
| 6c | DCE/HFIP [85/15] | 90 | 26 [26, 0] | 55 |
| 7 , | DCE/HFIP [50/50] | 80 | 90 [46, 44] | Trace |
| 8d,f | DCE/HFIP [50/50] | 80 | 90 [58, 32] | Trace |
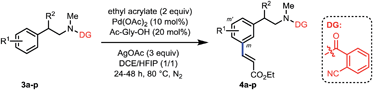
With the optimized conditions at hand, we examined the scope of this remote meta-selective olefination protocol (Table 3). Ortho-substituted substrates with both electron-donating methyl and methoxy and electron-withdrawing bromo and chloro groups proved to be suitable substrates, producing good combined yields of meta-olefinated products (4b–4e). It is worth noting that arenes bearing bromo or chloro substituents (4d and 4e) were compatible substrates, enabling further elaboration at the halogenated positions. Moreover, although we could not circumvent di-olefination (4b, 4d–e), the fact that both meta-positions of 2-substituted substrates could be functionalized provides a great opportunity for synthesis of diversely substituted arenes, which is particularly beneficial for the drug discovery industry. The remaining meta-position of meta-substituted substrates was also selectively olefinated in high yields (4f–4i). Para-substituted compounds carrying methoxy or halide groups were also viable substrates for obtaining high yields of the desired products (4j–4l). Notably, despite the steric hindrance, the olefin partner could also be installed selectively at the meta-position with poly-substituted substrates (4m–4n), displaying an uncommon procedure for constructing new penta-substituted phenylethylamines. It is interesting to note that the reaction was not sensitive to the difference of steric hindrance and both meta-positions of 3n could be olefinated. Finally, substituents at the benzylic position were also tolerated (4o and 4p), presenting the potential utility of this protocol with a drug molecule (4p). The meta-selectivity of various substrates was generally excellent with only minor amounts of other isomers whose amounts were hard to determine due to the presence of rotamers in the 1H NMR spectra of the crude olefinated products. The exceptional substrate is 3g, which also generated around 10% of other isomeric products owing to the electron-donating methoxy substituent. However, it is notable that the intrinsic electronic biases of the molecules were overall successfully overridden (4d–4f, 4k–4n). Moreover, removal of the directing group was smoothly realized by hydrolysis with HCl to afford high yields of new meta-substituted phenylethylamines (see the ESI†).
| a Reaction conditions: 3 (0.2 mmol), ethyl acrylate (0.4 mmol), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (0.6 mmol), DCE (1 mL), HFIP (1 mL), 24–48 h, 80 °C, N2. Isolated yields are reported, see the ESI† for details. b 90 °C. c DMF (1 mmol) was added. d DCE (0 mL)/HFIP (2 mL). e Around 10% of other isomers detected by 1H NMR. |
|---|
|
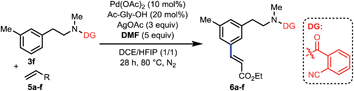
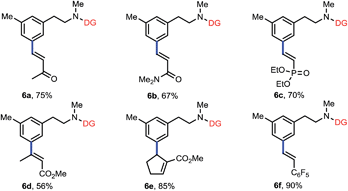
To further expand the scope of this reaction, we examined various olefin coupling partners and found olefination of 3f with α,β-unsaturated ketone, amide and phosphonate afforded desired products in good yields (Table 4, 6a–6c). We were also pleased to find trans-2-butenoate reacted stereoselectively with 3f to give 6d in moderate yield. It is noteworthy that this reaction was also compatible with cyclic tri-substituted olefin to give high yield of allylated product (6e). Finally, electron deficient styrene such as pentafluorostyrene 5f was also effective with this method to produce excellent yield of product (6f), albeit electron-rich styrenes were not applicable coupling partners.
| a Reaction conditions: 3f (0.1 mmol), 5 (0.2 mmol), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (0.3 mmol), DMF (0.5 mmol), DCE (0.5 mL), HFIP (0.5 mL), 28 h, 80 °C, N2. Isolated yields are reported. |
|---|
|
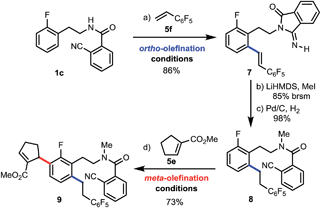
Finally, to demonstrate the potential of our method for streamlined synthesis of highly substituted arenes, we first subjected 1c to our standard ortho-olefination conditions with 5f and obtained ortho-olefinated 7 in 86% yield (Scheme 3). Then, much to our delight, we were able to convert 7 to the desired amide 8 with the required nitrile group which was reconstructed simultaneously with methylation by using LiHMDS, followed by hydrogenation of the double bond.20 Lastly, the meta-selective allylation proceeded efficiently with tri-substituted olefin 5e to afford tetrasubstituted arene 9 in good yield, enabling the building of complexity in a concise manner.
Conclusions
In summary, a novel example of remote regiodivergent ortho- and meta-C–H functionalizations has been developed with phenylethylamine derivatives by introducing a novel 2-cyanobenzoyl group as the original directing functionality. A single methylation was sufficient to switch the remote regioselectivity. This method also enabled the novel sequential functionalizations of ortho- and meta-C–H bonds of a phenylethylamine derivative. Further development of this strategy will improve C–H functionalization to become a more versatile synthetic tool.Acknowledgements
This work was supported by the Fujian Institute of Research on the Structure of Matter, NSFC (21402198) and “The 1000 Youth Talents Program”. We gratefully appreciate Prof. Jin-Quan Yu at The Scripps Research Institute, Prof. Weiping Su at FJIRSM and Prof. Richard P. Hsung at the University of Wisconsin-Madison for helpful comments. We also thank Prof. Daqiang Yuan at FJIRSM for crystallographic analysis.Notes and references
- Selected recent reviews: (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (b) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC; (c) F. Kakiuchi and T. Kochi, Synthesis, 2008, 2008, 3013 CrossRef; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (e) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (g) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed; (h) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Rev., 2010, 111, 1293 CrossRef PubMed; (i) T. Satoh and M. Miura, Synthesis, 2010, 2010, 3395 CrossRef; (j) O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902 RSC; (k) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992 RSC; (l) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (m) M. S. Sigman and E. W. Werner, Acc. Chem. Res., 2012, 45, 874 CrossRef CAS PubMed; (n) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (o) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (p) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936 CrossRef CAS PubMed; (q) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236 CrossRef CAS PubMed; (r) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (s) M. C. White, Science, 2012, 335, 807 CrossRef CAS PubMed; (t) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (u) L. Ackermann, Acc. Chem. Res., 2014, 47, 281 CrossRef CAS PubMed; (v) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843 RSC; (w) J. J. Topczewski and M. S. Sanford, Chem. Sci., 2015, 6, 70 RSC.
- (a) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417 CrossRef CAS PubMed; (b) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885 RSC; (c) W. R. Gutekunst and P. S. Baran, Chem. Soc. Rev., 2011, 40, 1976 RSC; (d) D. Y. K. Chen and S. W. Youn, Chem.–Eur. J., 2012, 18, 9452 CrossRef CAS PubMed; (e) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (f) W.-D. Joanna and G. Frank, Nat. Chem., 2013, 5, 369 CrossRef PubMed.
- Selected examples of Pd-catalyzed regioselective C–H functionalizations: (a) N. R. Deprez, D. Kalyani, A. Krause and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 4972 CrossRef CAS PubMed; (b) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS PubMed; (c) C. S. Yeung, X. Zhao, N. Borduas and V. M. Dong, Chem. Sci., 2010, 1, 331 RSC; (d) K. Ueda, S. Yanagisawa, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2010, 49, 8946 CrossRef CAS PubMed; (e) A. J. Hickman and M. S. Sanford, ACS Catal., 2011, 1, 170 CrossRef CAS; (f) J. Karthikeyan and C.-H. Cheng, Angew. Chem., Int. Ed., 2011, 50, 9880 CrossRef CAS PubMed; (g) A. N. Campbell, E. B. Meyer and S. S. Stahl, Chem. Commun., 2011, 47, 10257 RSC; (h) X. Wang, D. Leow and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 13864 CrossRef CAS PubMed; (i) K. Sun, Y. Li, T. Xiong, J. Zhang and Q. Zhang, J. Am. Chem. Soc., 2011, 133, 1694 CrossRef CAS PubMed; (j) L. Jiao and T. Bach, J. Am. Chem. Soc., 2011, 133, 12990 CrossRef CAS PubMed; (k) Z. Wu, F. Luo, S. Chen, Z. Li, H. Xiang and X. Zhou, Chem. Commun., 2013, 49, 7653 RSC; (l) R. Shrestha, P. Mukherjee, Y. Tan, Z. C. Litman and J. F. Hartwig, J. Am. Chem. Soc., 2013, 135, 8480 CrossRef CAS PubMed; (m) D.-T. D. Tang, K. D. Collins, J. B. Ernst and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 1809 CrossRef CAS PubMed.
- Selected examples of non-Pd-catalyzed regioselective C–H functionalizations: (a) H. Chen, S. Schlecht, T. C. Semple and J. F. Hartwig, Science, 2000, 287, 1995 CrossRef CAS; (b) C.-L. Ciana, R. J. Phipps, J. R. Brandt, F.-M. Meyer and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 458 CrossRef CAS PubMed; (c) S. Mochida, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2011, 76, 3024 CrossRef CAS PubMed; (d) J. Kwak, M. Kim and S. Chang, J. Am. Chem. Soc., 2011, 133, 3780 CrossRef CAS PubMed; (e) B. Li, Z.-H. Wu, Y.-F. Gu, C.-L. Sun, B.-Q. Wang and Z.-J. Shi, Angew. Chem., Int. Ed., 2011, 50, 1109 CrossRef CAS PubMed; (f) J. Wencel-Delord, C. Nimphius, F. W. Patureau and F. Glorius, Chem.–Asian J., 2012, 7, 1208 CrossRef CAS PubMed; (g) T. Andou, Y. Saga, H. Komai, S. Matsunaga and M. Kanai, Angew. Chem., Int. Ed., 2013, 52, 3213 CrossRef CAS PubMed; (h) K. Ueda, K. Amaike, R. M. Maceiczyk, K. Itami and J. Yamaguchi, J. Am. Chem. Soc., 2014, 136, 13226 CrossRef CAS PubMed.
- (a) R. J. Phipps and M. J. Gaunt, Science, 2009, 323, 1593 CrossRef CAS PubMed; (b) H. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps and M. J. Gaunt, Angew. Chem., Int. Ed., 2011, 50, 463 CrossRef CAS PubMed.
- (a) O. Saidi, J. Marafie, A. E. W. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey and C. G. Frost, J. Am. Chem. Soc., 2011, 133, 19298 CrossRef CAS PubMed; (b) C. Frost, W. Reynolds, P. Liu and G. Kociok-Köhn, Synlett, 2013, 24, 2687 CrossRef; (c) N. Hofmann and L. Ackermann, J. Am. Chem. Soc., 2013, 135, 5877 CrossRef CAS PubMed.
- For other examples of non-Pd-catalyzed meta-C–H functionalizations: (a) J.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka and M. R. Smith, Science, 2002, 295, 305 CrossRef CAS PubMed; (b) T. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi and J. F. Hartwig, J. Am. Chem. Soc., 2002, 124, 390 CrossRef CAS PubMed; (c) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS PubMed; (d) B.-J. Li and Z.-J. Shi, Chem. Sci., 2011, 2, 488 RSC; (e) C. Cheng and J. F. Hartwig, Science, 2014, 343, 853 CrossRef CAS PubMed.
- For Pd-catalyzed meta-C–H functionalizations using a chelating directing template: (a) D. Leow, G. Li, T.-S. Mei and J.-Q. Yu, Nature, 2012, 486, 518 CrossRef CAS PubMed; (b) H.-X. Dai, G. Li, X.-G. Zhang, A. F. Stepan and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 7567 CrossRef CAS PubMed; (c) L. Wan, N. Dastbaravardeh, G. Li and J.-Q. Yu, J. Am. Chem. Soc., 2013, 135, 18056 CrossRef CAS PubMed; (d) R.-Y. Tang, G. Li and J.-Q. Yu, Nature, 2014, 507, 215 CrossRef CAS PubMed; (e) G. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R.-Y. Tang, M. Movassaghi and J.-Q. Yu, J. Am. Chem. Soc., 2014, 136, 10807 CrossRef CAS PubMed; (f) Y. Deng and J.-Q. Yu, Angew. Chem., Int. Ed., 2015, 54, 888 CrossRef CAS PubMed; (g) S. Lee, H. Lee and K. L. Tan, J. Am. Chem. Soc., 2013, 135, 18778 CrossRef CAS PubMed; (h) M. Bera, A. Modak, T. Patra, A. Maji and D. Maiti, Org. Lett., 2014, 16, 5760 CrossRef CAS PubMed; (i) M. Bera, A. Maji, S. K. Sahoo and D. Maiti, Angew. Chem., Int. Ed., 2015 DOI:10.1002/anie.201503112.
- During the preparation of our manuscript, two impressive reports on meta-selective C–H functionalizations via Pd/norbornene catalysis appeared: (a) X.-C. Wang, W. Gong, L.-Z. Fang, R.-Y. Zhu, S. Li, K. M. Engle and J.-Q. Yu, Nature, 2015, 519, 334 CrossRef CAS PubMed; (b) Z. Dong, J. Wang and G. Dong, J. Am. Chem. Soc., 2015, 137, 5887 CrossRef CAS PubMed.
- For other Pd-catalyzed meta-C–H functionalizations: (a) Y.-H. Zhang, B.-F. Shi and J.-Q. Yu, J. Am. Chem. Soc., 2009, 131, 5072 CrossRef CAS PubMed; (b) J. Cornella, M. Righi and I. Larrosa, Angew. Chem., Int. Ed., 2011, 50, 9429 CrossRef CAS PubMed; (c) M. Ye, G.-L. Gao and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 6964 CrossRef CAS PubMed; (d) M. Ye, G.-L. Gao, A. J. F. Edmunds, P. A. Worthington, J. A. Morris and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19090 CrossRef CAS PubMed; (e) L. Zhou and W. Lu, Organometallics, 2012, 31, 2124 CrossRef CAS; (f) Y.-N. Wang, X.-Q. Guo, X.-H. Zhu, R. Zhong, L.-H. Cai and X.-F. Hou, Chem. Commun., 2012, 48, 10437 RSC; (g) F. Dai, Q. Gui, J. Liu, Z. Yang, X. Chen, R. Guo and Z. Tan, Chem. Commun., 2013, 49, 4634 RSC; (h) X. Cong, H. Tang, C. Wu and X. Zeng, Organometallics, 2013, 32, 6565 CrossRef CAS; (i) J. Luo, S. Preciado and I. Larrosa, J. Am. Chem. Soc., 2014, 136, 4109 CrossRef CAS PubMed.
- For other types of metal-catalyzed regiodivergent and/or sequential C–H functionalizations: (a) N. P. Grimster, C. Gauntlett, C. R. A. Godfrey and M. J. Gaunt, Angew. Chem., Int. Ed., 2005, 44, 3125 CrossRef CAS PubMed; (b) E. M. Beck, N. P. Grimster, R. Hatley and M. J. Gaunt, J. Am. Chem. Soc., 2006, 128, 2528 CrossRef CAS PubMed; (c) D. R. Stuart, E. Villemure and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 12072 CrossRef CAS PubMed; (d) E. M. Beck, R. Hatley and M. J. Gaunt, Angew. Chem., Int. Ed., 2008, 47, 3004 CrossRef CAS PubMed; (e) T. W. Lyons, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 4455 CrossRef CAS PubMed; (f) D. Katayev, M. Nakanishi, T. Burgi and E. P. Kundig, Chem. Sci., 2012, 3, 1422 RSC; (g) A. M. Wagner, A. J. Hickman and M. S. Sanford, J. Am. Chem. Soc., 2013, 135, 15710 CrossRef CAS PubMed; (h) J. D. Dooley, S. Reddy Chidipudi and H. W. Lam, J. Am. Chem. Soc., 2013, 135, 10829 CrossRef CAS PubMed; (i) Y. Su, H. Zhou, J. Chen, J. Xu, X. Wu, A. Lin and H. Yao, Org. Lett., 2014, 16, 4884 CrossRef CAS PubMed; (j) N. Schroder, F. Lied and F. Glorius, J. Am. Chem. Soc., 2015, 137, 1448 CrossRef PubMed.
- For more selected examples of sequential C–H functionalizations: (a) S. Yanagisawa, K. Ueda, H. Sekizawa and K. Itami, J. Am. Chem. Soc., 2009, 131, 14622 CrossRef CAS PubMed; (b) K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2010, 49, 6169 CrossRef CAS PubMed; (c) W. R. Gutekunst and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 19076 CrossRef CAS PubMed; (d) H. Wang, G. Li, K. M. Engle, J.-Q. Yu and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6774 CrossRef CAS PubMed; (e) H. J. Kim, M. J. Ajitha, Y. Lee, J. Ryu, J. Kim, Y. Lee, Y. Jung and S. Chang, J. Am. Chem. Soc., 2014, 136, 1132 CrossRef CAS PubMed; (f) A. D. Yamaguchi, K. M. Chepiga, J. Yamaguchi, K. Itami and H. M. Davies, J. Am. Chem. Soc., 2015, 137, 644 CrossRef CAS PubMed.
- For remote C–H functionalizations: (a) R. Breslow, Acc. Chem. Res., 1980, 13, 170 CrossRef CAS; (b) H. Schwarz, Acc. Chem. Res., 1989, 22, 282 CrossRef CAS; (c) S. Das, C. D. Incarvito, R. H. Crabtree and G. W. Brudvig, Science, 2006, 312, 1941 CrossRef CAS PubMed; (d) J.-J. Li, R. Giri and J.-Q. Yu, Tetrahedron, 2008, 64, 6979 CrossRef CAS PubMed; (e) D. G. Pintori and M. F. Greaney, J. Am. Chem. Soc., 2010, 133, 1209 CrossRef PubMed; (f) E. E. Stache, C. A. Seizert and E. M. Ferreira, Chem. Sci., 2012, 3, 1623 RSC; (g) W. A. Nack, G. He, S.-Y. Zhang, C. Lu and G. Chen, Org. Lett., 2013, 15, 3440 CrossRef CAS PubMed; (h) J. Schranck, A. Tlili and M. Beller, Angew. Chem., Int. Ed., 2014, 53, 9426 CrossRef CAS PubMed.
- For remote-selective ortho-C–H bond functionalization: (a) T. Saget and N. Cramer, Angew. Chem., Int. Ed., 2013, 52, 7865 ( Angew. Chem. , 2013 , 125 , 8019 ) CrossRef CAS PubMed; (b) W. B. Cross, E. G. Hope, Y.-H. Lin, S. A. Macgregor, K. Singh, G. A. Solan and N. Yahya, Chem. Commun., 2013, 49, 1918 RSC; (c) For a reverse selectivity, see: M. Lafrance, S. I. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 14570 CrossRef CAS PubMed.
- (a) J.-J. Li, T.-S. Mei and J.-Q. Yu, Angew. Chem., Int. Ed., 2008, 47, 6452 CrossRef CAS PubMed; (b) G. He, C. Lu, Y. Zhao, W. A. Nack and G. Chen, Org. Lett., 2012, 14, 2944 CrossRef CAS PubMed; (c) T.-S. Mei, D. Leow, H. Xiao, B. N. Laforteza and J.-Q. Yu, Org. Lett., 2013, 15, 3058 CrossRef CAS PubMed; (d) A. Rodriguez, J. Albert, X. Ariza, J. Garcia, J. Granell, J. Farras, A. La Mela and E. Nicolas, J. Org. Chem., 2014, 79, 9578 CrossRef CAS PubMed; (e) X. Ye and X. Shi, Org. Lett., 2014, 16, 4448 CrossRef CAS PubMed; (f) J. Han, P. Liu, C. Wang, Q. Wang, J. Zhang, Y. Zhao, D. Shi, Z. Huang and Y. Zhao, Org. Lett., 2014, 16, 5682 CrossRef CAS PubMed.
- For π-coordination of nitrile group in C–H functionalizations: (a) P. Gandeepan and C. H. Cheng, Chem.–Asian J., 2015, 10, 824 CrossRef CAS PubMed; (b) F. Kakiuchi, M. Sonoda, T. Tsujimoto, N. Chatani and S. Murai, Chem. Lett., 1999, 28, 1083 CrossRef; (c) W. Li, Z. Xu, P. Sun, X. Jiang and M. Fang, Org. Lett., 2011, 13, 1286 CrossRef CAS PubMed; (d) J.-C. Wan, J.-M. Huang, Y.-H. Jhan and J.-C. Hsieh, Org. Lett., 2013, 15, 2742 CrossRef CAS PubMed.
- For reviews and selected recent examples of Pd-catalyzed C–H olefinations: (a) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS PubMed; (b) J. Le Bras and J. Muzart, Chem. Rev., 2011, 111, 1170 CrossRef CAS PubMed; (c) L. Zhou and W. Lu, Chem.–Eur. J., 2014, 20, 634 CrossRef CAS PubMed; (d) K. J. Stowers, K. C. Fortner and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 6541 CrossRef CAS PubMed; (e) C. Huang, B. Chattopadhyay and V. Gevorgyan, J. Am. Chem. Soc., 2011, 133, 12406 CrossRef CAS PubMed; (f) P. Gandeepan and C. H. Cheng, J. Am. Chem. Soc., 2012, 134, 5738 CrossRef CAS PubMed; (g) R. Shi, L. Lu, H. Zhang, B. Chen, Y. Sha, C. Liu and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 10582 CrossRef CAS PubMed; (h) Y. Shang, X. Jie, J. Zhou, P. Hu, S. Huang and W. Su, Angew. Chem., Int. Ed., 2013, 52, 1299 CrossRef CAS PubMed; (i) A. Deb, S. Bag, R. Kancherla and D. Maiti, J. Am. Chem. Soc., 2014, 136, 13602 CrossRef CAS PubMed.
- (a) K. M. Engle, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137 CrossRef CAS PubMed; (b) G.-J. Cheng, Y.-F. Yang, P. Liu, P. Chen, T.-Y. Sun, G. Li, X.-H. Zhang, K. N. Houk, J.-Q. Yu and Y.-D. Wu, J. Am. Chem. Soc., 2014, 136, 894 CrossRef CAS PubMed.
- Selected examples: (a) A. R. Dick, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 2300 CrossRef CAS PubMed; (b) H.-Y. Thu, W.-Y. Yu and C.-M. Che, J. Am. Chem. Soc., 2006, 128, 9048 CrossRef CAS PubMed; (c) V. S. Thirunavukkarasu, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2008, 47, 9462 CrossRef CAS PubMed; (d) Y. Tan and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 3676 CrossRef CAS PubMed; (e) S.-J. Lou, D.-Q. Xu and Z.-Y. Xu, Angew. Chem., Int. Ed., 2014, 53, 10330 CrossRef CAS PubMed.
- The hydrogenation after the methylation was necessary to avoid the resulting substrate with a double bond acting as an olefin coupling partner in the following meta-selective olefination step.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and data for new compounds. CCDC 1056709 and 1406595. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01737h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |