Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixed valence mono- and hetero-metallic grid catenanes†
Chandan
Giri
a,
Filip
Topić
a,
Massimo
Cametti
*b and
Kari
Rissanen
*a
aUniversity of Jyvaskyla, Department of Chemistry, Nanoscience Center, P. O. Box. 35, FI-40014 University of Jyvaskyla, Finland. E-mail: kari.t.rissanen@jyu.fi
bDepartment of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Politecnico di Milano, Via L. Mancinelli 7, 20131 Milano, Italy. E-mail: massimo.cametti@polimi.it
First published on 30th June 2015
Abstract
Here, we report on the multicomponent self-assembly and single crystal X-ray diffraction study of a series of three interlocked mixed valence mono- and hetero-metallic [2]-catenanes made of [2 × 2] metallo-grids. They show unique structural features and highlight the essential roles of both the Cu(II)/Cu(I) pair and of the conformationally adaptable organic ligands for achieving catenation of grids.
Introduction
In the last half century, the scientific community has heavily focused on the design and synthesis of structurally demanding molecular architectures following the desire for complexity, aesthetic beauty, topological novelty and, most importantly, novel emerging properties.1 To this day, differently interlocked and/or entangled species, such as rotaxanes,1a catenanes,1j,k knots1h and other complex structures1c,d,f,i have been vigorously pursued. In particular, catenanes3 consist of two or more mechanically interlocked macro- or metallocycles, which cannot be separated without breaking a bond. The first discovery of a metal complex [2]-catenane self-assembled in one step was reported by Fujita and co-workers in 1994,4 yet exploration into this new field remained mostly limited to [M2L2]2 [2]-catenane species (M = metal, L = ligand). Only very recently have some examples of more complex catenanes been reported.5 For instance, Clever et al. reported on a dimeric [M2L4]2 interpenetrated coordination cage, which forms by the assembly of Pd(II) metal ions with a simple ditopic pyridyl ligand.5a Notably, the same group, by using a very similar but slightly shorter ligand, was able to achieve double ([M2L4]2) and triple ([M2L2]3) catenation in a stepwise process triggered by halide anions.5b A fully organic [3]-catenane was, instead, obtained by Nitschke et al., by employing an elegant but intricate self-assembly procedure based on a metal ion template and utilizing five different precursor molecules.5c An increase in the number of catenated species can be achieved, as demonstrated in the dynamic systems comprised of poly-catenanes (up to [7]-catenane) based on a tetrahedrally-shaped cage framework.5d Leigh et al. have recently demonstrated the preparation of a Solomon link,5e a pentafoil knot5f and a Star of David catenane5g in one5e,f or two steps,5g through careful ligand design while also taking advantage of iron(II) and anion (chloride5f or sulfate5g) templating effects. Finally, the assembly of a two-dimensional metal–organic network possessing Borromean links has recently been reported by Hardie et al.5hIn the pursuit of the construction of chemical objects of considerable structural complexity, multicomponent self-assembly has been proven to be a very successful technique,6 as demonstrated by many of the examples described above. It relies on the designed organization of carefully selected sub-components, which are linked together through the simultaneous formation of covalent and/or coordinative bonds, ultimately yielding the desired superstructure.
An assembly constituted by arrays of linear molecules held together and shaped into a discrete crisscrossed two-dimensionally ordered framework is usually termed a molecular grid or, when metal ions interconnect a set of organic ligands, a metallogrid.1e Although grid-like systems7 have become relatively common over the last decade,1e and their potential for applications has been demonstrated,1i,t,2 a higher-level organization of grids as components remains quite rare.1e,g In the context of interlocked systems, only two notable examples involving metallogrids can be found, namely that of the so far only homometallic grid catenane by Thompson et al.14 and the recent elegant use of a metallogrid as a scaffold in the synthesis of a Solomon link, reported by Leigh et al.1b To the best of our knowledge, no examples of catenanes formed by heterometallic grids7a,1m,n have been reported to this date.
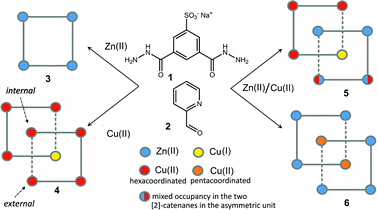
Here, we report on the isolation in the solid state of three [2]-catenane species where the macrocyclic ring units are made of [2 × 2] metal grids. These metal–organic architectures are formally created by the self-assembly of 32 components (8 × 1, 16 × 2 and 8 × metal, with 2 = 2-formylpyridine and metal = Zn(II) or Cu(II)/(I)) and they spontaneously precipitate as crystalline solids from a H2O/DMF mixture. The first species, 4 (Scheme 1), is a mono-metallic mixed valence Cu(II)/Cu(I) grid catenane, while the other two, 5 and 6 (Scheme 1), are bi-metallic Cu/Zn complexes. Among the eight metal ions that constitute the nodes of the two concatenated grid units, six of them can be considered as external while the remaining two can be viewed as internal with respect to the whole assembly (Scheme 1). Interestingly, in all cases, at least one of the internal copper atoms displays different coordination geometry with respect to the other external ones, along with a conspicuous conformational change of the organic ligands that fill its coordination sphere. Importantly, this conformational change renders the ligand's NH moieties available to establish additional hydrogen bonding (HB) interactions, which, as it will be shown, are important to allow for the catenation of the two [2 × 2] grids in 4 and 5. In addition to this, while in the case of 4 and 5 we observed a tetrahedral coordination geometry for one of the internal copper ions, which led us to assume a Cu(II)-to-Cu(I) transformation,9 in 6, both internal copper ions are instead found to be penta-coordinated. Indeed, the versatility of the coordination of the Cu(II)/Cu(I) couple appears to be fundamental for the formation of the [2]-catenane species, since the use of Zn(II) salts only led to more trivial [2 × 2] grid-like structures, such as 3. To the best of our knowledge, structures 4–6 represent a unique set of [2]-catenane systems including the heterometallic grid catenanes 5 and 6, which are the first of their kind.
 | ||
| Scheme 1 The chemical formulae of components 1 and 2 and a schematic representation of grid 3 and the [2]-catenanes 4–6. | ||
Results and discussion
Compound 1 (Scheme 1) can be easily synthesized by the addition of hydrazine to the sodium salt of dimethyl 5-sulfoisophthalate in MeOH.10The slow evaporation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1, 2-formylpyridine, 2, and Zn(II) acetate in a 2
1 mixture of 1, 2-formylpyridine, 2, and Zn(II) acetate in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF mixture produced good quality yellow single crystals of 3 after approximately one week. Their X-ray diffraction analysis revealed the formation of the [2 × 2] grid shown in Fig. 1.
DMF mixture produced good quality yellow single crystals of 3 after approximately one week. Their X-ray diffraction analysis revealed the formation of the [2 × 2] grid shown in Fig. 1.
 | ||
| Fig. 1 (a) Ball and stick and (b) space-filling model of 3. (c) Schematic representation of 3. C: gray, H: turquoise, N: blue; O: red, S: yellow, Zn: violet. | ||
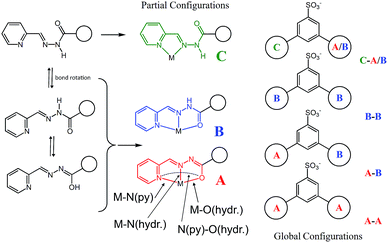
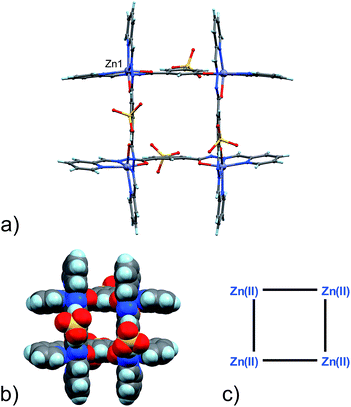
Four Zn(II) cations are held in place in a square grid-like arrangement by four ditopic ligands, each generated by Schiff base condensation of 1 with two equivalents of 2, and each interacting in a tridentate fashion with two metals. The latter ions are found to be in an octahedral environment, coordinated by four nitrogen and two oxygen atoms. As far as the ligand is concerned, it is important to note that tridentate coordination can be attained by different configurations, depending on the degree of ligand deprotonation. In Scheme 2, the possible configurations for the metal binding site are shown. While both configurations A and B lead to a tridentate NNO coordination mode, deprotonation occurs in A. On the other hand, configuration C results in a NN bidentate coordination. On the whole, each ligand can be found in a combination of A–A, A–B, B–B and A/B–C global configurations, either symmetric or not, all of which are chemically feasible and have been observed in metal complexes with similar ligands.11 In particular, coordinating C–O bond lengths are expected to show a change upon (de)protonation viz., C–O− (A) vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (B), with the latter being shorter than the former. However, in grid 3, due to high symmetry and possible positional disorder, the relevant C–O distance, but also C–N and N–N distances, are found to be averaged out and thus the exact configuration status of the ligand in the grid-like structure cannot be directly determined by the X-ray diffraction data. Component 1 is expected to be a sodium salt and we were unable to find and model any metallic counter-ions in the lattice. This may be simply due to the high symmetry and disorder present in the structure, which prevented us from clearly identifying and differentiating between counterions and solvent. However, we notice that if all ligands adopted configuration A–B, with each ligand being mono-deprotonated, 3 would be neutral on the whole, with no additional counter-cations needed to balance the charge. Indeed, this would represent a quite simple and appealing hypothesis that we are poised to believe as real, yet unconfirmed.
O (B), with the latter being shorter than the former. However, in grid 3, due to high symmetry and possible positional disorder, the relevant C–O distance, but also C–N and N–N distances, are found to be averaged out and thus the exact configuration status of the ligand in the grid-like structure cannot be directly determined by the X-ray diffraction data. Component 1 is expected to be a sodium salt and we were unable to find and model any metallic counter-ions in the lattice. This may be simply due to the high symmetry and disorder present in the structure, which prevented us from clearly identifying and differentiating between counterions and solvent. However, we notice that if all ligands adopted configuration A–B, with each ligand being mono-deprotonated, 3 would be neutral on the whole, with no additional counter-cations needed to balance the charge. Indeed, this would represent a quite simple and appealing hypothesis that we are poised to believe as real, yet unconfirmed.
Packing of the [2 × 2]-grids in 3 can be interpreted as directed by steric factors and by the polarity of the hydrophilic sulfonate groups (Fig. S5, ESI†). [2 × 2] grids of this kind are quite common in the literature and have also been obtained with ligands of similar chemical structures.7
It is well known that changing the identity of the metal ions can results in a complete change of coordination geometry and, hence, of the final complex architecture.12 As to our system, interestingly, the replacement of Zn(II) ions with Cu(II) ions led to a quite surprising outcome. Indeed, the slow evaporation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1, 2 and Cu(OAc)2 in a 2
1 mixture of 1, 2 and Cu(OAc)2 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O/DMF solution produced good quality single crystals of [2]-catenane 4, whose X-ray determined structure is shown in Fig. 2.
1 mixture of H2O/DMF solution produced good quality single crystals of [2]-catenane 4, whose X-ray determined structure is shown in Fig. 2.
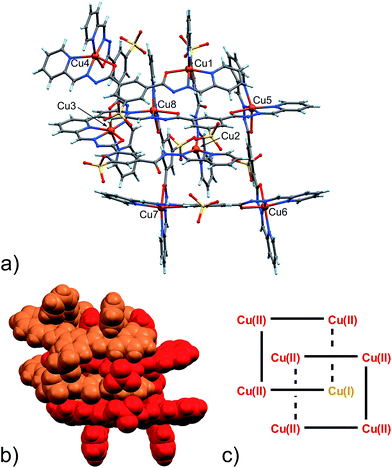 | ||
| Fig. 2 (a) Ball and stick and (b) space-filling model of 4. (c) Schematic representation of 4. C: gray, H: turquoise, N: blue; O: red, S: yellow, Cu: orange. | ||
As clearly seen,13 [2 × 2] grids are formed also in this case. However, two grids are interlocked with each other to form a [2]-catenane superstructure. The two concatenated grids are not symmetrical and one is slightly larger than the other, with the distances between adjacent copper ions in the 8.359–8.665 Å and 8.549–9.054 Å range for the smaller and larger grid, respectively. The concatenation leads to a rotation of the two grids with respect to each other of approximately 40°. The detailed inspection of its structural elements reveals that not all of the copper ions adopt the same coordination geometry. Indeed, one of the two internal copper ions is found to be tetra-coordinated, while the other seven are in octahedral environments. The two ligands that bind this “anomalous” copper ion display a non-symmetrical structure with one of their binding sites having a different configuration (C in Scheme 2), and acting as a NN bidentate ligand. This coordination motif, as well as the hydrogen bonding around the internal Cu(II) atoms, implies that deprotonation is not occurring on those sites of the ligands. Since tetrahedral coordination of Cu(II) with NN-bidentate ligands is not common and the crystallization conditions are known to induce reduction of Cu(II),9 we propose that a Cu(II)-to-Cu(I) transformation is occurring in this case.
The coordination geometry of most of the other Cu(II) atoms shows the characteristic distortion from an ideal octahedron due to the Jahn–Teller effect (Table S7, ESI†). This feature, while confirming the oxidation state of Cu ions as +2, will also become important later.
As far as charge state is concerned, also in this case, as in the previously described Zn(II)-grid 3, we were not able to find and model any additional counter-cations in the lattice (neither Na+, nor Cu2+/Cu+ ions), possibly implying the neutrality of the whole structure. If that were the case, the presence of a lower valence state for the Cu(I) ion would be therefore counterbalanced, for example, by one of the eight ligands forming the [2]-catenanes adopting a fully protonated state.
Inter-macrocycle interactions, together with the necessity of efficient packing, are usually responsible for the formation of concatenated architectures. In this case, the two ligand sites that act as NN-bidentate do not make use of their carbonyl groups in the coordination with the metal and two additional inter-grid NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C interactions are thus established (N24A–H24A⋯O11H (2.933(8) Å, ang. 167°) + C17A–H17A⋯O11H (3.184(10), ang. 133°) (bifurcated); N9B–H9B⋯O22G (2.833(9) Å, ang. 164°) + C17B–H17B⋯O22G (3.160(10) Å, ang. 140°) (bifurcated)). This reminded us of the well-known Vögtle–Hunter type of [2]-catenane,14 where inter-macrocyclic interactions between amidic NH and carbonyl groups were first observed. Notably, the ligand C-type partial configuration is stabilized by the presence of weak intramolecular hydrogen bonds (C26A–H26A⋯O22A (2.751(10) Å, ang. 126°), C7B–H7B⋯O11B (2.796(13) Å, ang. 119°)). Additional structural stabilization is surely provided by the abundant water forming a network of hydrogen bonds, which, however, remain undefined due to the less-than-ideal data quality and a large degree of disorder.
C interactions are thus established (N24A–H24A⋯O11H (2.933(8) Å, ang. 167°) + C17A–H17A⋯O11H (3.184(10), ang. 133°) (bifurcated); N9B–H9B⋯O22G (2.833(9) Å, ang. 164°) + C17B–H17B⋯O22G (3.160(10) Å, ang. 140°) (bifurcated)). This reminded us of the well-known Vögtle–Hunter type of [2]-catenane,14 where inter-macrocyclic interactions between amidic NH and carbonyl groups were first observed. Notably, the ligand C-type partial configuration is stabilized by the presence of weak intramolecular hydrogen bonds (C26A–H26A⋯O22A (2.751(10) Å, ang. 126°), C7B–H7B⋯O11B (2.796(13) Å, ang. 119°)). Additional structural stabilization is surely provided by the abundant water forming a network of hydrogen bonds, which, however, remain undefined due to the less-than-ideal data quality and a large degree of disorder.
The [2]-catenane units pack with direct interactions by means of N–H⋯O hydrogen bonds, supported by weaker C–H⋯O hydrogen bonds, with sulfonate groups, forming centrosymmetric pairs. The same can be observed for the other, symmetrically independent catenane species.
To the best of our knowledge, there exists only one example of [2]-catenane systems made by interlocked grids.8 In that case, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co(II)/Co(III) metal distribution within the grid was observed due to the oxidation of Co(II) to Co(III) by air. The ligand employed displayed two different conformations in the grid structure, however, no configuration change was observed, making our system unique.
1 Co(II)/Co(III) metal distribution within the grid was observed due to the oxidation of Co(II) to Co(III) by air. The ligand employed displayed two different conformations in the grid structure, however, no configuration change was observed, making our system unique.
Intrigued by the unexpected grid concatenation observed in 4 and by the different behaviour of Zn(II) and Cu(II) under these experimental conditions, we wondered if the process could be selective and we tried to crystallize a mixture of 1 and 2 with Cu(II) and Zn(II) salts in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ratio. After several crystallization attempts, we were able to obtain good quality single crystals of two species, 5 and 6 (Scheme 1). They are both heterometallic species and while displaying strong similarities with 4, are still substantially different. Such mixed valence heterometallic [2]-catenane species made of grid units are unprecedented.
0.5 ratio. After several crystallization attempts, we were able to obtain good quality single crystals of two species, 5 and 6 (Scheme 1). They are both heterometallic species and while displaying strong similarities with 4, are still substantially different. Such mixed valence heterometallic [2]-catenane species made of grid units are unprecedented.
With the assignment of metal identity being of exceeding importance in this work, we devoted particular attention to establishing a set of criteria15 that could be used to properly distinguish Zn(II) from Cu(II) in 5 (Fig. 3). First of all, the internal metal ion that displays a tetrahedral coordination environment was unambiguously assigned as Cu(I). As far as discriminating Zn(II) from Cu(II) ions, the observation of Jahn–Teller effect – present in d9 electronic configuration as in Cu(II) ions but not in d10 Zn(II) ions – was very helpful. Indeed, in 5,13 four metal ions display such an effect, which leads to the elongation of the metal–ligand bond distances in the apical coordination positions. For example, the mean apical Cu(II)–N(py) and Cu(II)–O(hydr.) distances in the catenane unit of 5 discussed here of 2.26 and 2.32 Å, respectively, can be compared to the respective equatorial distances of 2.06 and 2.05 Å.
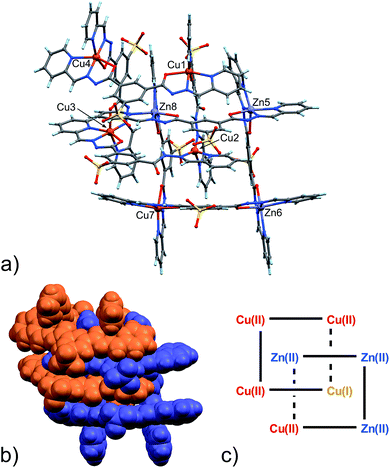 | ||
| Fig. 3 (a) Ball and stick and (b) space-filling model of 5. (c) Schematic representation of 5. C: gray, H: turquoise, N: blue; O: red, S: yellow, Zn: violet, Cu: orange. | ||
The availability of monometallic Zn(II) grid 3 and the Cu(II)/Cu(I) [2]-catenane 4 provided a solid ground for the comparison with 5, aimed at the discrimination between copper and zinc centres. Also, an analysis of the CSD for Zn(II) and Cu(II) complexes with similar ligands provided additional confirmation on the validity of the criteria chosen and allowed us to evaluate the effect of the strain due to the [2]-catenane complex formation on the coordination geometries around the metal ions.16 Indeed, in simple Cu(II) and Zn(II) metal complexes, the M–N(hydr) distance (Scheme 2) seems to be the clearest indicator of the metal atom type, with the average distances for Zn(II) being larger – in cases of both A and B type configuration – than those for Cu(II). Furthermore, we considered the N(py)–O(hydr) distance (Scheme 2) as revealing for the presence of Jahn–Teller effect. Indeed, its mean values are larger for Zn complexes than for Cu ones and distributed over a narrower interval (4.087–4.357 Å, to be compared with the 3.850–4.539 Å interval found in the case of Cu-complexes, see ESI† – CSD search).
In the [2]-catenane systems, strain due to concatenation is surely present and the divergence between the average Cu(II) and Zn(II) coordination environments is less profound.17 However, it has been always possible to determine the Zn(II)/Cu(II) quite confidently (Table S8, ESI†).
Packing in 5 is essentially very similar to that found in 4, showing interdigitation of the neighbouring catenane units (Fig. S9 in ESI†). However, an additional solvated Zn(II) ion is found in the lattice. The latter ion, present as a DMF/water solvate, balances the overall charge. This feature again confirms the considerable adaptability of the ligand, which can adjust to the environment in terms of global charge distribution.
In any case, given the central importance of determining the metal composition in 5, we sought additional confirmation of the Zn/Cu ratio in 5. Energy-dispersive X-ray spectroscopy (EDS) and atomic absorption spectrophotometry (AAS) analyses made on the same single crystals analysed by X-ray diffraction brought in comforting results. EDS data show a Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio = 0.63 on average of multiple measurements (see ESI†). This ratio is in perfect agreement with the Zn
Cu ratio = 0.63 on average of multiple measurements (see ESI†). This ratio is in perfect agreement with the Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu ratio provided by the metric analysis on the X-ray diffraction data, also taking into account the presence of an additional Zn(II) ion (with 0.4 occupancy factor, total 6.4
Cu ratio provided by the metric analysis on the X-ray diffraction data, also taking into account the presence of an additional Zn(II) ion (with 0.4 occupancy factor, total 6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 Zn
10 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu equals to 0.64) in the lattice (see ESI†).18 Moreover, the AAS of Cu–Zn catenane 5 gives 5.87 (±0.01%) for Cu and 3.63 (±0.07%) for Zn, giving a ratio of 0.62 ± 0.01, in accordance with the EDS and the X-ray analysis made on 5.
Cu equals to 0.64) in the lattice (see ESI†).18 Moreover, the AAS of Cu–Zn catenane 5 gives 5.87 (±0.01%) for Cu and 3.63 (±0.07%) for Zn, giving a ratio of 0.62 ± 0.01, in accordance with the EDS and the X-ray analysis made on 5.
An important aspect that is common to both 4 and 5 pertains to the observed necessity of a change in the coordination geometry of, at least, one the internal metal ions in order to produce the concatenated structures. Steric factors can be involved, however, we must stress that the tetra-coordination exerted on the Cu(I) ion by the C-type configuration ligands frees two carbonyl groups per [2]-catenane unit, and they can then be involved in stabilizing inter-grid interactions. In the case of structures 4 and 5, each grid unit is connected to the concatenated one by two such NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C HB interactions.
C HB interactions.
From the data above, it might seem that the concatenation process has a slight preference for the incorporation of copper over zinc, however, the attainment – by a slightly modified crystallization procedure – and characterization of 6 disproves this hypothesis. Indeed, the crystal structure of 6, shown in Fig. 4, features a [2]-catenane structure having six Zn(II) metals and only two Cu(II) ions.13 Again, similarly to the situation found in 4 and 5, the catenation leads to a change in the configuration of the ligands involved in the coordination of the internal Cu(II) ions. This strongly suggests that the catenation happens in the presence of at least one internal copper atom and a change in the configuration of the ligands bound to it. In the case of 6, the symmetrical grids require both of the internal Cu(II) ions to be penta-coordinated (Table S9, ESI†), whereas in the structures of 4 and 5, only one tetra-coordinated internal Cu(I) suffices for catenation. Confident assignment of the metal identity was achieved by a careful metric analysis of the data in relation to 4 and 5,19 and by the comparison with published structures of NNO–NN penta-coordinated Cu(II) complexes (ESI†).20
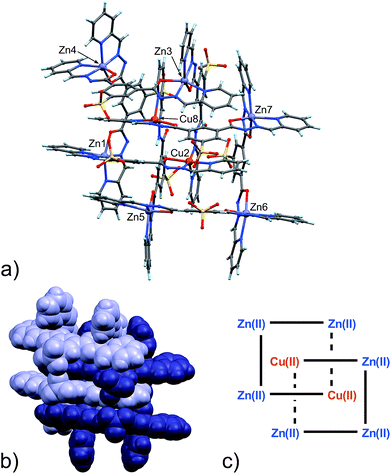 | ||
| Fig. 4 (a) Ball and stick and (b) space-filling models of 6. (c) Schematic representation of 6. C: gray, H: turquoise, N: blue; O: red, S: yellow, Zn: violet, Cu: orange. | ||
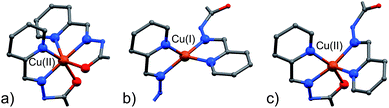
A visual summary of the three different environments that the Cu(II/I) metal ions adopt in the structurally similar [2]-catenanes 4–6 is shown in Fig. 5. Hexa- and penta-coordinated Cu(II), and tetra-coordinated Cu(I) are indeed observed.
Twelve Na+ counter-cations can be located and quite appropriately modelled in the structure (six for each [2]-catenane adduct, there are two of them in the asymmetric unit),21 therefore, on average, each grid must be triply negative and the four ligands wholly bear 11 negative charges.
Interestingly, in this case, neither of the non-coordinating carbonyl groups (C10H![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O11H, C10B
O11H, C10B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O11B), freed by the change of configuration of the ligand, are involved in inter-grid interactions as in 4 and 5, but instead participate in binding to Na+ ions mediated by water molecules.
O11B), freed by the change of configuration of the ligand, are involved in inter-grid interactions as in 4 and 5, but instead participate in binding to Na+ ions mediated by water molecules.
It is important to add here that the crystals of 6 were obtained by a slightly modified crystallization procedure, that is, by ethanol vapour diffusion into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF solution. Since the organic components of the [2]-catenanes are quite adaptable both in terms of their conformation and configuration, the combination of ligand and copper ion gives access to a large configurational and conformational space, and the crystallization of a different structure does not come as a surprise. Clearly, the difference in the experimental procedure (evaporation vs. anti-solvent diffusion) presented a significant enough perturbation to lead to the formation of a completely different structure in 6, with all the distinguishing features as already highlighted above. As a final comment, we note that complexes 4–6 were obtained in yields close to 30%, a figure which is far from optimal, and in striking variance with other reported systems, which can be obtained in significantly higher yields, and sometimes even quantitatively.5a,b
DMF solution. Since the organic components of the [2]-catenanes are quite adaptable both in terms of their conformation and configuration, the combination of ligand and copper ion gives access to a large configurational and conformational space, and the crystallization of a different structure does not come as a surprise. Clearly, the difference in the experimental procedure (evaporation vs. anti-solvent diffusion) presented a significant enough perturbation to lead to the formation of a completely different structure in 6, with all the distinguishing features as already highlighted above. As a final comment, we note that complexes 4–6 were obtained in yields close to 30%, a figure which is far from optimal, and in striking variance with other reported systems, which can be obtained in significantly higher yields, and sometimes even quantitatively.5a,b
Conclusion
Structural complexity is sought by chemists to refine their synthetic capabilities and to discover new properties. This work describes three examples of [2]-catenane systems made of interlocked metal–organic grids obtained by multicomponent self-assembly, and characterized in the solid state by single crystal X-ray diffraction. These unique metal complex systems are either mono- (4) or hetero-metallic (5 and 6) and display multivalence copper (4 and 5). To the best of our knowledge, 5 and 6 represent the first catenated heterometallic grids reported to date. Their apparent heterogeneity should not be misleading. Indeed, all the [2]-catenane systems possess common features. For example, at least one of the internal metal ions is copper, which displays a different coordination geometry with respect to the other external ones, along with a conspicuous conformational change of the organic ligand involved. The role of the Cu(II)/Cu(I) metal pair, especially in terms of its versatility, is here highlighted as a key factor responsible for the formation of such species. We would also like to emphasize that the presented work provides a simple route to obtain octanuclear complexes, some of which are non-symmetric, and with defined metal composition, which would be otherwise unaccessible. This feature stems from the particular environment surrounding the internal metal ions within the framework and from the catenation event. The possibility to lay a given metal ion into a precise position in such a complex structure could have indeed a large impact in the development of functional materials.Further research is aimed at verifying the degree of control that can be attained over the systems in terms of overall charge distribution in the [2]-catenanes and final Zn/Cu ratio within the hetero-metallic species. Also, structural modifications of components 1 and 2 have been envisaged in order to improve control over metal identities and complex stoichiometry. Extensive crystallization trial experiments with a wider range of d-block metal salts and concentrations are currently ongoing in our laboratories.
Acknowledgements
The authors kindly acknowledge the financial support from the Academy of Finland (K. R. grant no.'s 263256, 265328), the Programma per Giovani Ricercatori “Rita Levi Montalcini” – 2009 (M. C.), the University of Jyväskylä and NGS-NANO (F. T., Ph.D. fellowship) and ICDD (2015 Ludo Frevel Crystallography Scholarship Award to F. T.). Dr Francesco Basoli is gratefully acknowledged for the EDS analyses and Mr T. Mäkitalo for initial studies on compound 1.Notes and references
- (a) M. Xue, Y. Yang, X. Chi, X. Yan and F. Huang, Chem. Rev., 2015 DOI:10.1021/cr5005869; (b) J. E. Beves, J. J. Danon, D. A. Leigh, J.-F. Lemonnier and I. J. Vitorica-Yrezabal, Angew. Chem., Int. Ed., 2015, 54, 7555–7559 CrossRef CAS PubMed; (c) S. F. M. van Dongen, S. Cantekin, J. A. A. W. Elemans, A. E. Rowan and R. J. M. Nolte, Chem. Soc. Rev., 2014, 43, 99–122 RSC; (d) J.-C. Chambron and J.-P. Sauvage, New J. Chem., 2013, 37, 49–57 RSC; (e) J. G. Hardy, Chem. Soc. Rev., 2013, 42, 7881–7899 RSC; (f) C. J. Bruns and J. Fraser Stoddart, Top. Curr. Chem., 2012, 323, 19–72 CrossRef CAS PubMed; (g) A. R. Stefankiewicz, G. Rogez, J. Harrowfield, A. N. Sobolev, A. Madalan, J. Huuskonen, K. Rissanen and J.-M. Lehn, Dalton Trans., 2012, 13848–13855 RSC; (h) R. S. Forgan, J.-P. Sauvage and J. F. Stoddart, Chem. Rev., 2011, 111, 5434–5464 CrossRef CAS PubMed; (i) J. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh and R. T. McBurney, Angew. Chem., Int. Ed., 2011, 50, 9260–9327 CrossRef CAS PubMed; (j) G. Gil-Ramírez, D. A. Leigh and A. J. Stephens, Angew. Chem., Int. Ed., 2015, 54, 6110–6150 CrossRef PubMed; (k) N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658–4683 RSC; (l) E. Breuning, M. Ruben, J.-M. Lehn, F. Renz, Y. Garcia, V. Ksenofontov, P. Güttlich, E. Wegelius and K. Rissanen, Angew. Chem., Int. Ed., 2000, 39, 2504–2507 CrossRef CAS; (m) L. H. Uppadine and J.-M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 240–243 CrossRef CAS PubMed; (n) A. R. Stefankiewicz, J. Harrowfield, A. Madalan, K. Rissanen, A. N. Sobolev and J.-M. Lehn, Dalton Trans., 2011, 12320–12332 RSC.
- T. Matsumoto, G. N. Newton, T. Shiga, H. Oshio, S. Hayami, Y. Matsui, H. Okamoto, R. Kumai and Y. Murakami, Nat. Commun., 2014, 5, 3865, DOI:10.1038/ncomms4865.
- Term proposed by E. Wasserman, J. Am. Chem. Soc., 1960, 82, 4433–4434 ( J. Am. Chem. Soc. , 1961 , 83 , 3789–3795 ) CrossRef CAS , from Latin catena, catenae, f. (“chain”).
- (a) M. Fujita, F. Ibukuro, H. Hagihara and K. Ogura, Nature, 1994, 367, 720–723 CrossRef CAS; (b) M. Fujita, Acc. Chem. Res., 1999, 32, 53–61 CrossRef CAS.
- (a) S. Freye, J. Hey, A. Torras-Galán, D. stalke, R. Herbst-Irmer, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2012, 51, 2191–2194 CrossRef CAS PubMed; (b) R. Zhu, J. Lübben, B. Dittrich and G. H. Clever, Angew. Chem., Int. Ed., 2015, 54, 2796–2800 CrossRef CAS PubMed; (c) C. S. Wood, T. K. Ronson, A. M. Belenguer, J. J. Holstein and J. R. Nitschke, Nat. Chem., 2015, 7, 354–358 CrossRef CAS PubMed; (d) S. P. Black, A. R. Stefankiewicz, M. M. J. Smulders, D. Satler, C. A. Schalley, J. R. Nitschke and J. K. M. Sanders, Angew. Chem., Int. Ed., 2013, 52, 5749–5752 CrossRef CAS PubMed; (e) J. E. Beves, C. J. Campbell, D. A. Leigh and R. G. Pritchard, Angew. Chem., Int. Ed., 2013, 52, 6464–6467 CrossRef CAS PubMed; (f) J.-F. Ayme, J. E. Beves, D. A. Leigh, R. T. McBurney, K. Rissanen and D. Schultz, Nat. Chem., 2012, 4, 15–20 CrossRef CAS PubMed; (g) D. A. Leigh, R. G. Pritchard and A. J. Stephens, Nat. Chem., 2014, 6, 978–982 CrossRef CAS PubMed; (h) F. L. Thorp-Greenwood, A. N. Kulak and M. J. Hardie, Nat. Chem., 2015, 7, 526–531 CrossRef CAS PubMed.
- (a) S. Zarra, D. M. Wood, D. A. Roberts and J. R. Nitschke, Chem. Soc. Rev., 2015, 44, 419–432 RSC; (b) Q. Gan, T. K. Ronson, D. A. Vosburg, J. D. Thoburn and J. R. Nitschke, J. Am. Chem. Soc., 2015, 137, 1770–1773 CrossRef CAS PubMed; (c) D. M. Wood, W. Meng, T. K. Ronson, A. R. Stefankiewicz, J. K. M. Sanders and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 3988–3992 CrossRef CAS PubMed; (d) P. D. Frischmann, V. Kunz, V. Stepanenko and F. Würthner, Chem.–Eur. J., 2015, 21, 2766–2769 CrossRef CAS PubMed; (e) D.-H. Ren, D. Qiu, C.-Y. Pang, Z. Li and Z.-G. Gu, Chem. Commun., 2015, 51, 788–791 RSC; (f) W. J. Ramsay and J. R. Nitschke, J. Am. Chem. Soc., 2014, 136, 7038–7043 CrossRef CAS PubMed; (g) S. Li, J. Huang, T. R. Cook, J. B. Pollock, H. Kim, K.-W. Chi and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 2084–2087 CrossRef CAS PubMed; (h) S. Yi, V. Brega, B. Captain and A. E. Kaifer, Chem. Commun., 2012, 48, 10295–10297 RSC.
- (a) X. Bao, W. Liu, L. L. Mao, S. D. Jiang, J. L. Liu, Y. C. Chen and M. L. Tong, Inorg. Chem., 2013, 52, 6233–6235 CrossRef CAS PubMed; (b) M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J. M. Lehn, Angew. Chem., Int. Ed., 2004, 43, 3644–3662 CrossRef CAS PubMed.
- T. S. M. Abedin, L. K. Thompson and D. O. Miller, Chem. Commun., 2005, 5512–5514 RSC.
- Under the experimental conditions, viz. H2O/DMF, it is known that Cu(II) can be reduced to Cu(I), see R. T. Pflaum, A. I. Popov and N. C. Goodspeed, Anal. Chem., 1955, 2, 253–255 CrossRef.
- Synthesis and characterization of compound 1 was previously reported ( D. Nissen, V. Rossbach and H. Zahn, J. Appl. Polymer Sci., 1974, 18, 1953–1968 CrossRef CAS ). Additional characterization can be found in the ESI†.
- Structural data are available for metal complexes similar to those reported here and where different configurations are displayed by the ligand. As to Cu(II) complexes, some examples are: B. A. D. Neto, B. F. L. Viana, T. S. Rodrigues, P. M. Lalli, M. N. Eberlin, W. A. da Silva, H. C. B. de Oliveira and C. C. Gatto, Dalton Trans., 2013, 11497–11506 RSC; R. N. Patel, D. K. Patel, V. P. Sondhiya, K. K. Shukla, Y. Singh and A. Kumar, Inorg. Chim. Acta, 2013, 405, 209–217 CrossRef CAS; M. R. Bermejo, R. Pedrido, A. M. Gonzalez-Noya, M. J. Romero, M. Vazquez and L. Sorace, New J. Chem., 2003, 27, 1753–1759 RSC; A. Datta, N.-T. Chuang, Y.-S. Wen, J.-H. Huang and S.-C. Sheu, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, 67, m1388 Search PubMed; R. N. Patel, A. Singh, K. K. Shukla, V. P. Sondhiya, D. K. Patel, Y. Singh and R. Pandey, J. Coord. Chem., 2012, 65, 1381–1397 CrossRef , some of the structures reported show 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand
1 ligand![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu(II) complexes where the two ligands have different configurations; see also ref. 20.
Cu(II) complexes where the two ligands have different configurations; see also ref. 20. - (a) N. J. Hurley, J. J. Hayward, J. M. Rawson, M. Murrie and M. Pilkington, Inorg. Chem., 2014, 53, 8610–8623 CrossRef CAS PubMed; (b) S. Sharma, G. J. E. Davidson and S. J. Loeb, Chem. Commun., 2008, 582–584 RSC; (c) C. Giri, F. Topić, P. Mal and K. Rissanen, Dalton Trans., 2014, 17889–17892 RSC; (d) Comprehensive Coordinatioon Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier Ltd., 2003 Search PubMed.
- The asymmetric unit actually contains two symmetry-independent moieties. Given their structural similarity, for the sake of conciseness, we have described in the text only one of the two [2]-catenanes, while the reader could find the figures of the other in the ESI†.
- (a) C. A. Hunter, J. Am. Chem. Soc., 1992, 114, 5303–5311 CrossRef CAS; (b) F. Vögtle, S. Meier and R. Hoss, Angew. Chem., Int. Ed., 1992, 31, 1619–1622 CrossRef.
- The applied criteria for the Cu(II)/Zn(II) discrimination based on metal–ligand bond distances in 5 can be summarized as follows: (i) metal ions having a coordination number equal to 4 were assigned as Cu(I). (ii) For metal ions with a coordination number equal to 6: the metal ion is assigned as Cu(II) (a) if one M–N(hydr) bond is between 1.90 and 2.00 Å and the other one is around 2.00–2.10Å, also (b) if one N(py)⋯O(hydr) distance is at least 0.3 Å longer than the other and (c) if the longer M–O(hydr) and the M–N(py) are opposite to each other. The metal ion was assigned as Zn(II) (a) if the M–N(hydr) bonds are between 2.0 and 2.1 Å long, and (b) if both M–O(hydr) bonds are of similar length, between 2.0 and 2.2 Å, and (c) if both M–N(py) bonds are between 2.1 and 2.3 Å in length, and finally (d) if the difference between the two N(py)⋯O(hydr) distances is less than 0.2 Å.
- Cambridge Structural Database (CSD), Version 5.35, November 2013, see ESI† for more details on the CSD search criteria.
- As a practical example, see Zn5 or Zn13 in 5. In the case of Zn5, the M–N(hydr) distances might point to Cu(II) but the absence of a Jahn–Teller effect, reflected in M–N(py) and M–N(hydr) distances, strongly points to Zn(II). For Zn13, on the other hand, the M–O(hydr) and N(py)⋯O(hydr) distances might suggest a Cu(II), whereas M–N(py) and M–N(hydr) are more indicative of Zn(II). Since the M–N(hydr) distance, based on CSD analysis (see ESI†), seems to be a relatively robust indicator of the metal atom type, the final assignment was based on this. The low resolution of the crystal structure of 5 as compared to 4 and 6 certainly complicated the assignment and reduced its reliability.
- EDS analyses on 3 and 4 were also made to test the technique on these materials and the results confirm the presence of only Zn and Cu atoms for 3 and 4, respectively (see ESI†).
- This structure is also the best one in terms of data quality among the three [2]-catenanes species obtained.
- (a) S. Roy, T. N. Mandal, A. K. Barik, S. Gupta, M. S. El Fallah, J. Tercero, R. J. Butcher and S. K. Kar, Dalton Trans., 2009, 8215–8226 RSC; (b) H. Grove, T. L. Kelly, L. K. Thompson, L. Zhao, Z. Xu, T. S. M. Abedin, D. O. Miller, A. E. Goeta, C. Wilson and J. A. K. Howard, Inorg. Chem., 2004, 43, 4278–4288 CrossRef CAS PubMed; (c) C. J. Matthews, K. Avery, Z. Xu, L. K. Thompson, L. Zhao, D. O. Miller, K. Biradha, K. Poirier, M. J. Zaworotko, C. Wilson, A. E. Goeta and J. A. K. Howard, Inorg. Chem., 1999, 38, 5266–5276 CrossRef CAS.
- We have located and modelled 5.75 Na+ ions, which compares well with the expected 6 Na+ ions per catenane unit.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the CSD database search, additional crystallographic information on 3–6 with relevant tables, EDS analysis and EDS plots. CCDC 1054509–1054512. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc01851j |
| This journal is © The Royal Society of Chemistry 2015 |