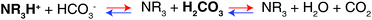Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
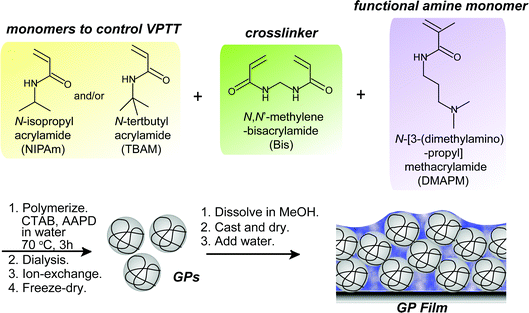
Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles†
Mengchen
Yue
,
Yu
Hoshino
 * and
Yoshiko
Miura
* and
Yoshiko
Miura
Department of Chemical Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: yhoshino@chem-eng.kyushu-u.ac.jp
First published on 28th July 2015
Abstract
Herein we revealed the design rationale of thermally responsive gel particle (GP) films that reversibly capture and release large amounts of CO2 over a narrow temperature range (30–75 °C). The pKa value of ammonium ions in the GPs at both the CO2 capture temperature (30 °C) and release temperature (75 °C) is found to be the primary factor responsible for the stoichiometry of reversible CO2 capture by the amines in the GP films. The pKa values can be tuned by the properties of GPs such as volume phase transition temperature (VPTT), size, swelling ratio, and the imprinted microenvironment surrounding the amines. The optimal GP obtained according to the design rationale showed high capture capacity (68 mL CO2 per g dry GPs, 3.0 mmol CO2 per g dry GPs), although the regeneration temperature was as low as 75 °C. We anticipate that GP films that reversibly capture other acidic and basic gases in large amounts can also be achieved by the pKa tuning procedures.
Introduction
The accumulation of CO2 in the atmosphere due to burning of fossil fuels is considered the main cause of global climate change.1 Meanwhile, CO2 is expected to be a carbon source for the production of liquid fuels, such as methanol and dimethyl ether, through the use of regenerative energy.2 Thus, the development of energy-efficient CO2 separation and recovery processes for point sources, such as fossil fuel power plants, is essential not only for minimizing climate change but also for the future use of carbon-based energy.Conventional processes that recover CO2 from the high humidity exhaust gases of power plants use aqueous solutions of ethanolamine as a CO2 absorbent. CO2 in the exhaust gases is selectively captured by the absorbent at a low temperature (∼40 °C) and then recovered by heating the solution, typically above 140 °C.3–5 Although the amine solution exhibits a high CO2 absorption capacity, the high-energy consumption of the heating process limits its use in the environmentally friendly process.4–8
Porous solid adsorbents that can be regenerated under relatively mild conditions, such as metal–organic frameworks (MOFs),9,10 zeolites,9,10 and silica with physically or chemically supported amine polymers,9–13 have recently been developed as alternatives to the aqueous amine solution. However, solid CO2 sorbents are rarely capable of efficiently capturing CO2 from highly humid gases because the adsorption of water molecules competes with that of CO2 on the pore surfaces. Furthermore, the capillaries and pores of the sorbents are easily blocked by the liquefied water, preventing CO2 from diffusing into the pores.9–12 Thus, the development of sorbents that absorb/adsorb large amount of CO2, even in humid environments, and desorb CO2 at low temperatures (<100 °C) is of great importance.
It has been reported that poly-N-isopropylacrylamide (pNIPAm) undergoes a reversible volume phase transition from a hydrophilic swollen state to a hydrophobic collapsed state at temperatures below and above, respectively, the volume phase transition temperature (VPTT) of ∼32 °C.14 The phase transition is induced by the entropy-driven dissociation of water from pNIPAm chains after heating above the VPTT.14 The temperature responsive pNIPAm-based functional hydrogels have been widely used as materials to reversibly capture targets, such as dyes,15,16 drugs,17,18 peptides,19 proteins,20,21 nucleotides,22 cells,23 and protons,24 in aqueous media via the volume phase transition. The multipoint interactions are reversibly switched on/off by varying the volume density of hydrophobic functional groups,17,25,26 the number of charged functional groups,22,27 and/or the rigidity of the polymer chains28 at temperatures above and below the VPTT.
Recently, we reported that hydrogel films composed of temperature-responsive microgel particles (GPs) consisting of NIPAm and N-[3-(dimethylamino)propyl]methacrylamide (DMAPM) reversibly absorbed and released CO2via a volume phase transition during cooling (30 °C) and heating (75 °C) cycles, respectively.29 Below the VPTT, amines in the swollen GPs were capable of forming ion pairs with absorbed bicarbonate ions. Above the VPTT, shrinkage of the GPs triggered CO2 desorption.27 The GP films showed faster CO2 capture and release rate29 than the conventional bulk hydrogel films due to the fast thermal responsibility of the GPs.30
However, guidelines to design GP films that reversibly absorb CO2 with high capacity and stoichiometry have not been revealed.
In the case of reversible CO2 absorption by the blood of animals, the pKa value of the Brønsted acid and base in the hemoglobin plays a crucial role for the control of CO2 solution equilibrium: The pKa value of the ammonium and imidazolium cations decreases due to the drastic conformational change of the hemoglobin caused by oxygen binding to the heme. The shift of the pKa triggers the release of H+ into the blood, leading to the release of CO2 efficiently from lungs (Bohr effect).31,32
Inspired by the Bohr effect of hemoglobin, we hypothesized that the pKa value of the protonated amines (ammonium ions) within GPs (pKa value of GPs) at the CO2 capture temperature (30 °C) and release temperature (75 °C) would contribute to the reversible capture efficiency of CO2.
In this study, in order to clarify the design rationale, we prepared a series of GPs with a variety of compositions using different polymerization conditions. The effects of the physical and chemical properties of GPs, such as VPTT, size, swelling ratio, on pKa values and the reversible CO2 capture stoichiometry against amines were systematically investigated. pKa values of the ammonium ions in the GPs were also tuned by the “microenvironment-imprinting” strategy as we described in the recent report.24 The reversible CO2 capture capacity was maximized based on the design rational revealed in this study. Humidified gas (60 °C) consisting of 10% CO2 and 90% N2, which is comparable to the post-combustion gas of fire power plants after wet desulfurization process, was used as feed gas.6,33 The CO2 was captured at 30 °C and released at 75 °C under the same atmosphere (10% CO2, 90% N2, 60 °C water moisture).
Experimental
Preparation of GPs
A series of GPs containing NIPAm, a functional tertiary amine monomer DMPAM, and a crosslinker N,N′-methylenebisacrylamide (Bis) were synthesized by precipitation polymerization as reported (Scheme 1).29 The amount of Bis was varied to prepare GPs with different degrees of crosslinking and swelling ratios. GPs with the same composition but different pKa values were synthesized via a “microenvironment-imprinting” strategy by adding HCl or NaOH into the monomer solution.24 Larger GPs were obtained by decreasing the concentration of surfactant, while smaller GPs were prepared from solutions with a lower total monomer concentration. To decrease the VPTT of GPs, a more hydrophobic monomer, N-tert-butyl acrylamide (TBAm), was incorporated into the GPs. Details of the polymerization conditions of the GPs are summarized in Table 1. Polymerization process is described in ESI.†| GPs | Feed ratio | Polymerization media | CTAB (mM) | AAPD (mM) | Concentration (mM) | |||
|---|---|---|---|---|---|---|---|---|
| NIPAm (mol%) | DMAPM (mol%) | Bis (mol%) | TBAM (mol%) | |||||
| D5B2 | 93 | 5 | 2 | 0 | Water | 2.0 | 2.5 | 312 |
| D5B0 | 95 | 5 | 0 | 0 | Water | 2.0 | 2.5 | 312 |
| D5B5 | 90 | 5 | 5 | 0 | Water | 2.0 | 2.5 | 312 |
| D5B10 | 85 | 5 | 10 | 0 | Water | 2.0 | 2.5 | 312 |
| D5B2T40 | 53 | 5 | 2 | 40 | Water | 2.0 | 2.5 | 312 |
| D5B10-1/4HCl | 85 | 5 | 10 | 0 | 1/4 eq. HCl | 2.0 | 2.5 | 312 |
| D5B2-1/2HCl | 93 | 5 | 2 | 0 | 1/2 eq. HCl | 2.0 | 2.5 | 312 |
| D5B2-1/1HCl | 93 | 5 | 2 | 0 | 1/1 eq. HCl | 2.0 | 2.5 | 312 |
| D5B2-1/2NaOH | 93 | 5 | 2 | 0 | 1/2 eq. NaOH | 2.0 | 2.5 | 312 |
| D5B2-L | 93 | 5 | 2 | 0 | Water | 0.16 | 2.5 | 312 |
| D5B2-S | 93 | 5 | 2 | 0 | Water | 2.0 | 0.625 | 78 |
| D5B2-1/2NaOH-S | 93 | 5 | 2 | 0 | 1/2 eq. NaOH | 2.0 | 0.625 | 78 |
| D30B2 | 68 | 30 | 2 | 0 | Water | 2.0 | 2.5 | 312 |
| D30B2T40 | 28 | 30 | 2 | 40 | Water | 2.0 | 2.5 | 312 |
| D55B2 | 43 | 55 | 2 | 0 | Water | 2.0 | 2.5 | 312 |
| D55B2T43 | 0 | 55 | 2 | 43 | Water | 2.0 | 2.5 | 312 |
Quantification of hydrodynamic diameters, VPTTs, and swelling ratios of GPs
The hydrodynamic diameters and VPTT of GPs were measured by dynamic light scattering (DLS) as described.27,29 The swelling ratio is defined as the ratio of the diameters at 30 °C (D30) and 75 °C (D75).Quantification of amine content and pKa of ammonium ions in GPs
The amine content and pKa of amines in GPs was quantified by acid–base titration under N2 purging as described.27,29Measurement of pKa shift curve during heating process
Since the apparent pKa can be approximated as the pH value at the half-neutralization point, the temperature dependent pKa shift of the GP solution was measured with the pH probe as follows. The half-neutralized GP solution was prepared by adding 0.5 eq. of HCl to the GP solution. Then, under a N2 atmosphere with stirring the pH value and temperature of the solution were recorded by the pH meter every 3 s during the heating process.Preparation of GP films
The lyophilized GPs were dissolved in methanol. The hydrogel films were then prepared by casting the methanol solutions containing 120 mg of GPs on the inner bottom surface of a stainless steel container with a surface area of 120 cm2. After completely evaporating the methanol, 4 mL of water was added per gram of GPs.Measurement of CO2 absorption capacity of GP films
The CO2 absorption capacities of the GP films are quantified as illustrated in Scheme S1.† 10% CO2 (90% N2, 10 mL min−1) gas was passed through 60 °C water to generate saturated water vapor. The resulting 60 °C water–saturated gas mixture was channeled into a stainless steel container with the GP film on the inner surface, and then to a gas chromatograph after condensing the water moisture at 5 °C.More detailed information about materials and experimental process are shown in ESI.†
Results and discussion
Effect of pKa values of GPs on the reversible CO2 capture stoichiometry of GP films
The dynamic and reversible transition of the pKa values of Brønsted acid and base groups in proteins plays an important role in their functions, such as molecule/ion transfer, enzymatic reactions, and molecular recognition.32,34 The pKa values are significantly influenced by the microenvironment around the functional groups, such as hydrophobicity, hydrogen bonding, and distance to the neighboring charges.32,34–38 Thus, the pKa value can be further dynamically shifted by the conformational change of polypeptides around the functional groups, which induces a microenvironment change.In the case of CO2 transfer through the bloodstream of animals, the pKa value of the ammonium and imidazolium cations in/on the hemoglobin decreases due to the drastic conformational change of the polypeptides caused by oxygen binding to the heme. The shift of the pKa value triggers the release of H+ into the blood, lowers the pH of the blood, and drives the CO2 efficiently from the lungs (Bohr effect).32,38
Inspired by the function of hemoglobin, we expected that the pKa value of the protonated amines (ammonium ions) within GPs (pKa value of GPs) at the CO2 capture temperature (30 °C) and release temperature (75 °C) would contribute to the reversible capture efficiency of CO2.
To clarify the effect of the pKa value of GPs on the reversible CO2 capture stoichiometry of GP films in the temperature swing process from 30 °C to 75 °C, a series of GPs with the same composition but different pKa values were prepared by the “microenvironment-imprinting” strategy.24,39 Imprinting is a method to create polymers with specific microstructures that show strong affinity to target molecules and ions by crosslinking the polymer networks in the presence of targets.40,41 It has been reported that pNIPAm-based acrylic acids (AAc) containing GPs with different pKa values can be prepared by the “proton-imprinted” strategy.24 The GPs polymerized at a pH below the pKa of AAc showed a much higher pKa value at the collapsed state than those prepared at a high pH because stronger proton-affinity sites were incorporated into the relatively hydrophobic microenvironment around the protonated AAc. Note that the GPs were polymerized at the collapsed state in the temperature above the VPTT.
We anticipated that amine-containing GPs with different pKa values could also be prepared via the “microenvironment-imprinting” strategy. Thus, the GPs were polymerized in the presence of acid (1 or 1/2 eq. of HCl against the amine monomer DMAPM) or base (1/2 eq. of NaOH). All GPs were polymerized in the aqueous monomer solution consisting of 5 mol% DMAPM, 2 mol% Bis and 93 mol% NIPAm at the temperature above the VPTT of the growing GPs (70 °C).
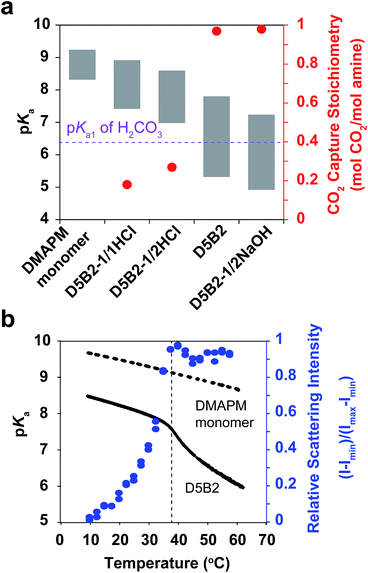
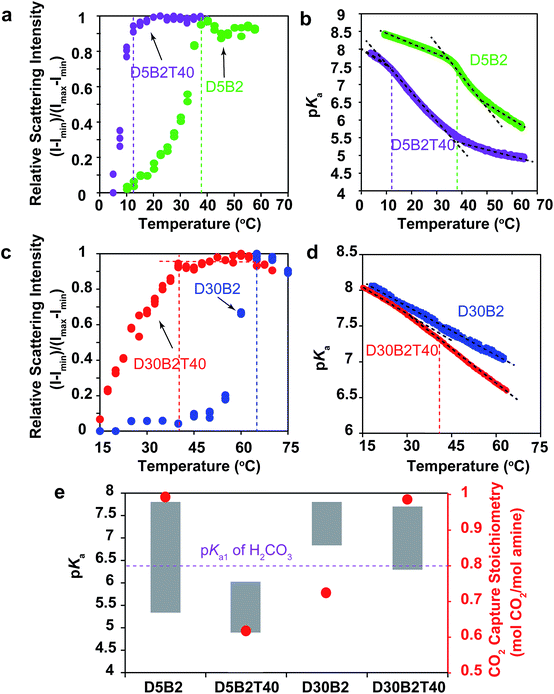
The VPTTs of the GPs were determined by monitoring the scattering intensity of the GP solutions during the heating process (Fig. S1a in ESI†). Despite the different polymerization conditions, all four GPs, D5B2-1/1HCl (1 eq. HCl), D5B2-1/2HCl (1/2 eq. HCl), D5B2-1/2NaOH, (1 eq. NaOH), and D5B2 (without HCl or NaOH), show similar VPTTs at about 38 °C. Similar swelling ratios in the range of 2.3–2.7 are also observed (Fig. S1b in ESI†), indicating the VPTTs and swelling ratios of GPs are independent of the polymerization conditions.
The apparent pKa values of the GPs at 30 °C and 75 °C were determined by acid–base titration of the GP solutions and are plotted as the top and bottom, respectively, of the gray bars in Fig. 1a.27 All GPs show lower pKa values than the amine monomer DMAPM, at both temperatures, indicating the relatively low dielectric constant and high steric hindrance around the amine groups in the GPs, as well as the high electrostatic repulsion between neighboring charges.42,43 Moreover, in accordance with our expectation, the pKa values of D5B2-1/1HCl and D5B2-1/2HCl at 75 °C (7.4 and 7.0, respectively) were significantly higher than those of D5B2 and D5B2-1/2NaOH (5.3 and 4.9, respectively).
The large difference in pKa values can be explained by the “microenvironment-imprinting” effect. When the GPs are polymerized in a proton-rich solution (in the presence of HCl), the protonated DMAPM is imprinted in the polar and hydrophilic microenvironment of the GPs (D5B2-1/1HCl and D5B2-1/2HCl), resulting in higher pKa values than those of the GPs polymerized in the proton-poor solution (D5B2 and D5B2-1/2NaOH). Although there is variation in the GP diameters (Fig. S1b in ESI†), the large difference in the pKa values (>2) at 75 °C cannot be a result of the size differences because the smallest GP, D5B2 (74 nm), and the largest GP, D5B2-1/2NaOH (136 nm), show a pKa difference of only 0.4 (5.3–4.9).
Fig. 1a also shows that the pKa difference between 30 °C and 75 °C (ΔpKa) of all GPs (1.5–2.5) is larger than that of the monomer (0.9). For better understanding of the temperature dependent pKa difference, the apparent pKa values of D5B2 and the monomer DMAPM, were recorded during the heating process. Fig. 1b shows that a sharp pKa transition of D5B2 occurs at a temperature around its VPTT, and a steep pKa decrease continues over a wide temperature range above the VPTT. However, this sharp pKa transition is not observed in the case of the monomer, indicating that the sharp pKa decrease was induced by a volume phase transition of GPs, during which the increased hydrophobicity and steric hindrance, and the decreased dielectric constant at high temperature make the amine groups more difficult to be protonated.42
In Fig. 1a, the ΔpKa values between 75 °C and 30 °C of the GPs polymerized with HCl, D5B2-1/1HCl (1.5) and D5B2-1/2HCl (1.6), are significantly lower than those of D5B2 (2.5) and D5B2-1/2NaOH (2.3), although the swelling ratios are similar (2.3–2.7). We hypothesize that in comparison with D5B2 and D5B2-1/2NaOH, which were polymerized with 0 and 0.5 eq. of NaOH, respectively, the protonated DMAPM groups (ammoniums) within D5B2-1/1HCl and D5B2-1/2HCl are relatively distant from the hydrophobic pNIPAm moieties and backbones in the collapsed GPs. This is because the protonated amine groups are relatively polar and hydrophilic. In other words, the amine groups of the GPs prepared with HCl are located in a “pseudo-swollen” microenvironment analogous to the swollen state even at the collapsed state. After the volume phase transition from the collapsed to the swollen state, swelling has less effect on the amines within the “pseudo-swollen” GPs. As a result, the ΔpKa values of the GPs prepared with HCl are less than those of the GPs prepared without HCl.
The reversible CO2 capture stoichiometries of the GP films are presented as red plots in Fig. 1a. D5B2-1/1HCl and D5B2-1/2HCl show very low reversible CO2 capture stoichiometries (0.18 and 0.27 mol CO2 per mol amine, respectively), while the values for D5B2 and D5B2-1/2NaOH are both ∼0.97 mol CO2 per mol amine.
The difference in the stoichiometry can be interpreted by the acid–base theory as follows. It has been reported that the tertiary amines in GPs and CO2 form ammonium ions (RN3H+) and bicarbonate ions (HCO3−) via base-catalyzed reactions.44,45 In aqueous media containing RN3H+ and HCO3−, there is always an equilibrium between their corresponding conjugated base–acid, as described in Scheme 2.
When the pKa of NR3H+ is above the pKa1 of H2CO3, which indicates that NR3H+ holds a proton more tightly than H2CO3 does, the proton will move from the stronger acid, H2CO3, to the stronger base, NR3. As a result, more R3N will be consumed and more CO2 will be captured, as presented by the blue arrows in Scheme 2. In contrast, when the pKa of NR3H+ is below the pKa1 of H2CO3, more CO2 will be released, as indicated by the red arrows in Scheme 2.46
The pKa1 value of carbonic acid (H2CO3) is in the range of 6.35 ± 0.05 at both 30 °C and 75 °C, as determined by Harned and Davis,47 however, the pKa values of the amine-containing thermal responsive GPs dramatically depend on the temperature, as presented in Fig. 1. The high pKa values of D5B2-1/1HCl and D5B2-1/2HCl at 75 °C (7.4 and 7.0, respectively) are higher than the pKa1 of H2CO3 (6.35) and inhibit the release of CO2. As a result, the reversible CO2 capture stoichiometries of D5B2-1/1HCl and D5B2-1/2HCl are low (0.18 and 0.27 mol CO2 per mol amine, respectively), although the pKa values at 30 °C (8.9 and 8.6, respectively) are high enough to capture CO2 efficiently. For D5B2 and D5B2-1/2NaOH, the high reversible CO2 capture stoichiometries (0.97 mol CO2 per mol amine for both) are achieved due to the low pKa values at 75 °C (5.3 and 4.9, respectively), which are below the pKa1 of H2CO3, and meanwhile, the high pKa values at 30 °C (7.8 and 7.2, respectively), which are above the pKa1 of H2CO3.
Effect of ΔpKa values of GPs on the reversible CO2 capture stoichiometry of GP films
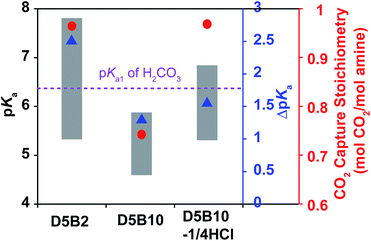
Despite the low pKa values of D5B2 and D5B2-1/2NaOH at 75 °C (Fig. 1a), their larger ΔpKa values between 30 °C and 75 °C (2.5 and 2.3, respectively) than those of D5B2-1/1HCl and D5B2-1/2HCl (1.5 and 1.6, respectively) may also be responsible for their high reversible CO2 capture stoichiometries.In order to distinguish the effects of the pKa value and the ΔpKa on the reversible CO2 capture stoichiometry of the GP films, a GP with a smaller ΔpKa than D5B2 was prepared by increasing the Bis cross-linker to 10 mol% into the GP that contains 5 mol% DMAPM (D5B10), since it has been reported that the crosslink degree influences the ΔpKa of the GPs.24
The pKa values of D5B2 and D5B10 at 30 °C and 75 °C are plotted as the top and bottom of the gray bars in Fig. 2. The ΔpKa of D5B10 (1.4) is apparently less than that of D5B2 (2.5). However the pKa value of D5B10 (5.9) at 30 °C is below the pKa1 of H2CO3. Thus the possible reason for the lower reversible CO2 capture stoichiometry of D5B10 than D5B2 (0.74 and 0.97 mol CO2 per mol amine, respectively), the smaller ΔpKa of D5B10 than D5B2 and/or the low pKa value of D5B10 at 30 °C which is below the pKa1 of H2CO3, still cannot be distinguished.
To identify the main factor that governs the reversible CO2 capture stoichiometry, we designed another GP with a greater pKa value than D5B10 and a lower ΔpKa than D5B2, using the “microenvironment-imprinting” strategy described above. The GPs were prepared by polymerizing D5B10 in the presence of an appropriate amount of HCl (D5B10-1/4HCl).
Fig. 2 shows the pKa values and ΔpKa of D5B10-1/4HCl. The pKa values at 30 °C and 75 °C of D5B10-1/4HCl (6.8 and 5.3, respectively) are higher than those of D5B10 (5.9 and 4.5, respectively), but the ΔpKa of D5B10-1/4HCl (1.5) is comparable to that of D5B10 (1.4). D5B10-1/4HCl shows a much higher CO2 capture stoichiometry (0.97 mol CO2 per mol amine) than D5B10 (0.74 mol CO2 per mol amine), despite the similar ΔpKa values.
On the other hand, as displayed in Fig. 2, the pKa value of D5B10-1/4HCl at 75 °C (5.3) is similar to that of D5B2 (5.3), while the ΔpKa of D5B10-1/4HCl (1.5) is much less than that of D5B2 (2.5). However, both GPs exhibit comparably high reversible CO2 capture stoichiometries (0.97 mol CO2 per mol amine), even though the ΔpKa values are much different.
We conclude from these results that the ΔpKa has a minor influence on the reversible CO2 capture stoichiometry, and a high reversible CO2 capture stoichiometry is possible for GPs that exhibit reduced ΔpKa values as long as the pKa values lie within the appropriate range. The pKa value of the GPs must be tuned above the pKa1 of H2CO3 (6.35) at the CO2 capture temperature (30 °C) and below the pKa1 of H2CO3 (6.35) at the CO2 release temperature (75 °C) in order to achieve a high reversible CO2 capture stoichiometry.
Effect of VPTT of GPs on the reversible CO2 capture stoichiometry of GP films
As shown in Fig. 1b, the thermal responsive volume phase transition of the GPs leads to a sharp pKa transition. In addition, the pKa value of the GPs has been revealed essential for the reversible CO2 capture stoichiometry. Therefore, it is interesting to observe the effect of the VPTT of GPs on the reversible CO2 capture stoichiometry of the GP films.A GP with a lower VPTT than D5B2 was synthesized by polymerizing 40 mol% of TBAm, which is more hydrophobic than NIPAm, together with 5 mol% DMAPM and 2 mol% Bis (D5B2T40). The relative scattering intensity of the GP solutions is shown in Fig. 3a. Compared with D5B2 (VPTT = 38 °C), the VPTT of D5B2T40 is only 12 °C. The higher intra-particle hydrophobic interaction of the GPs containing 40 mol% TBAm causes the entropy-driven collapse of D5B2T40 to occur at a lower temperature.48,49
Fig. 3b shows the temperature dependent pKa values of D5B2 and D5B2T40. Similar to D5B2, D5B2T40 shows a sharp pKa transition at a temperature around its VPTT (12 °C) and a steep pKa decrease continues over a wide temperature range above the VPTT. As a result, the pKa values of D5B2T40 at 30 °C and 75 °C (6.0 and 4.8, respectively) are both low. The lower reversible CO2 capture stoichiometry of D5B2T40 (0.60 mol CO2 per mol amine) than that of D5B2 (0.97 mol CO2 per mol amine), can be explained by the low pKa value of D5B2T40 at 30 °C (6.0), which is below the pKa1 of H2CO3.
Besides D5B2 and D5B2T40, another pair of GPs with different VPTTs were prepared by increasing the feed ratio of DMAPM to 30 mol% (D30B2 and D30B2T40).48,49Fig. 3c shows that the VPTT of D30B2 is above 60 °C. To lower the VPTT, 40 mol% of TBAm was incorporated into the 30 mol% DMAPM-containing GP (D30B2T40). In comparison, the VPTT of D30B2T40 is only 40 °C (Fig. 3c).
In Fig. 3d, the pKa value of D30B2 is linearly dependent on temperature, and no obvious sharp transition is observed in the temperature range due to the high VPTT. However, the pKa value of D30B2T40 exhibits a transition from the temperature around its VPTT (40 °C), and a steeper pKa decrease than that of D30B2 is observed at the temperatures above the VPTT. As a result, D30B2T40 has lower pKa values at higher temperatures (>40 °C) and a larger ΔpKa between 30 °C and 75 °C than D30B2 (Fig. 3e).
The degree of pKa transition of D30B2T40 (Fig. 3d) induced by the phase transition is less significant than D5B2 and D5B2T40 (Fig. 3b). This may be a result of the increased intra-GP charge repulsion within D30B2T40 caused by the smaller amine–amine distance.
The pKa values at 30 °C and 75 °C of D30B2 (7.8 and 6.8, respectively) and D30B2T40 (7.7 and 6.3, respectively) are shown in Fig. 3e. The reversible CO2 capture stoichiometry (0.72 mol CO2 per mol amine) of D30B2 is quite low; however, that of D30B2T40 is much higher at 0.97 mol CO2 per mol amine. The improved stoichiometric efficiency can be explained by the relatively low pKa value of D30B2T40 at 75 °C (6.3), which is below the pKa1 of H2CO3. The VPTT of D30B2T40, which is close to 30 °C, induces a sharp pKa transition and a steep pKa decrease over a wide temperature range above the VPTT, lowers the pKa value at 75 °C, and consequently improves the reversible CO2 capture stoichiometry.
In summary, the results in Fig. 3 lead to a comparable conclusion to those of Fig. 1 and 2: the pKa values of the GPs at 30 °C and 75 °C govern the reversible CO2 capture stoichiometry. However, the VPTT of the GPs also plays a crucial role. The GPs with a much lower VPTT than the CO2 capture temperature (30 °C) show low reversible CO2 capture stoichiometries due to the low pKa value. Meanwhile, to achieve a high reversible CO2 capture stoichiometry, the VPTT should be above and as close to 30 °C as possible to generate a large pKa transition over a wide temperature range above the VPTT.
However, a VPTT above and close to 30 °C is not enough for a high CO2 capture stoichiometry if the pKa values at 30 °C and 75 °C lie at an improper level. For example, in Fig. 1, though the VPTTs of D5B2-1/1HCl and D5B2-1/2HCl are both 38 °C, the high pKa values at 75 °C (7.4 and 7.0, respectively) considerably restrict efficient CO2 release (0.18 and 0.27 mol CO2 per mol amine, respectively).
Effect of GP size on the reversible CO2 capture stoichiometry of GP films
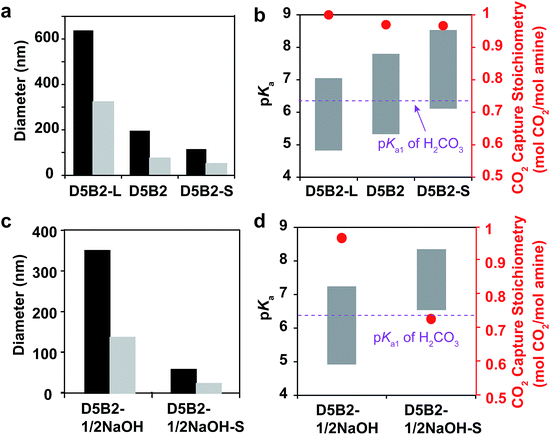
We have reported that the GP films exhibited larger CO2 capture capacities than conventional bulk hydrogel films due to the small dimensions of GPs, which lead to a fast response and fast ion diffusion.29 Herein, the effect of GP size on the reversible CO2 capture stoichiometry is discussed.Larger GPs can be prepared by increasing the concentration of monomer or by decreasing the concentration of surfactant.50 In this study, GPs with the same composition (5 mol% DMAPM and 2 mol% Bis) but different size were designed. A GP with a larger hydrodynamic diameter (D5B2-L) than D5B2 was prepared by decreasing the concentration of surfactant, while a smaller GP (D5B2-S) was synthesized using a monomer solution with a lower total concentration.
The diameters of the GPs at 30 °C and 75 °C are shown in Fig. 4a. As expected, D5B2-L (638 nm at 30 °C and 322 nm at 75 °C) has a larger diameter than D5B2 (196 nm at 30 °C and 74 nm at 75 °C), and D5B2-S has the smallest diameter (115 nm at 30 °C and 49 nm at 75 °C). The pKa values of the GPs at 30 °C and 75 °C are presented as the top and bottom, respectively, of the bars in Fig. 4b. It can be seen clearly that the pKa values of the GPs at both temperatures increase with the decreasing diameter.
The amine groups located at the exterior of the GPs are expected to show higher pKa values than those of the interior groups because of the high dielectric constant of water, as well as the reduced steric hindrance. In the meantime, the amine groups of the exterior of the smaller GP account for a much larger percentage than those of the larger GPs. Therefore, smaller GPs show higher pKa values than the larger GPs.
Fig. 4b also shows that the three GPs exhibit similar, high reversible CO2 capture stoichiometries (0.96–1.0 mol CO2 per mol amine), despite the difference in GP diameters and pKa values.
Another pair of GPs showing different pKa ranges from those of the GPs in Fig. 4a and b was polymerized using the “microenvironment-imprinting” strategy by adding 0.5 eq. of NaOH to the monomer solution (D5B2-1/2NaOH and D5B2-1/2NaOH-S). D5B2-1/2NaOH-S, having a smaller diameter than D5B2-1/2NaOH, was prepared by lowering the total monomer concentration.
Fig. 4c and d show the diameters and pKa values, respectively, of the GPs at 30 °C and 75 °C. In good agreement with the results in Fig. 4a, the diameters of D5B2-1/2NaOH-S (83 nm at 30 °C and 57 nm at 75 °C) are much smaller than the diameters of D5B2-1/2NaOH (715 nm at 30 °C and 349 nm at 75 °C). The pKa values of the smaller GP are also higher than those of the larger GP. However, the reversible CO2 capture stoichiometry of D5B2-1/2NaOH-S (0.74 mol CO2 per mol amine) is lower than that of D5B2-1/2NaOH (0.98 mol CO2 per mol amine), as shown in Fig. 4d.
The different results from Fig. 4b and d could also be ascribed to the different pKa values of the GPs. The high pKa values of D5B2-L, D5B2, D5B2-S, and D5B2-1/2NaOH at 30 °C (7.0, 7.8, 8.5, and 7.2, respectively), which are above the pKa1 of H2CO3 (6.35), and the low pKa values at 75 °C (4.8, 5.3, 6.1, and 4.9, respectively), which are below the pKa1 of H2CO3, result in high reversible CO2 capture stoichiometries. However, the pKa values of D5B2-1/2NaOH-S at 30 °C and 75 °C are 8.4 and 6.6, respectively. Its high pKa value at 75 °C (6.6), which is above the pKa1 of H2CO3, restricts the efficient release of CO2, resulting in a low reversible CO2 capture stoichiometry (0.74 mol CO2 per mol amine).
In general, the results of Fig. 4 lead to the conclusion that the reversible CO2 capture stoichiometry of GPs can be improved by regulating their pKa values at both 30 °C and 75 °C through varying their diameter. The smaller diameter leads to a higher pKa value.
Effect of swelling ratio of GP on the reversible CO2 capture stoichiometry of GP films
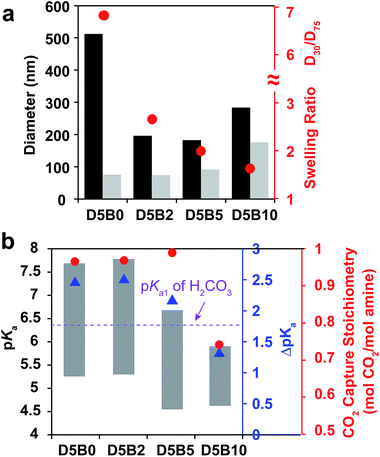
The swelling ratio of thermal responsive GPs plays an important role in their function such as the reversible target binding.26,51,52 The swelling ratio of GPs is typically controlled by the crosslink degree.53 Herein, we prepared a series of GPs containing 5 mol% of DMAPM with 0, 2, 5, and 10 mol% Bis crosslinker (D5B0, D5B2, D5B5, and D5B10, respectively) to investigate the effect of the swelling ratio on the reversible CO2 capture stoichiometry of GPs.The diameters at 30 °C and 75 °C, and the swelling ratios of the GPs are plotted in Fig. 5a. It is noteworthy that GP can be obtained without Bis crosslinker (D5B0), possibly due to the slight self-crosslinking.54 As expected, the swelling ratios of D5B2 (2.7), D5B5 (2.0), and D5B10 (1.6) decrease with the increasing crosslink degree. The diameters of the GPs at 75 °C increase slightly with the increasing amount of Bis crosslinker, indicating that Bis influences the nucleation process during polymerization, as observed by Pelton's group.50
Fig. 5b shows the pKa values of the GPs at 30 °C and 75 °C, and the ΔpKa values. The ΔpKa values of D5B2 (2.5), D5B5 (2.1), and D5B10 (1.3) between 30 °C and 75 °C show a positive correlation with the swelling ratios of the GPs (2.7, 2.0, and 1.6, respectively). This suggests that from 30 °C to 75 °C the microenvironmental changes, such as the change of dielectric constant and polymer density, around the less cross-linked amines are greater than those of the highly cross-linked amines in the GPs. At 30 °C, the pKa values of the swollen GPs, D5B2 (7.8), D5B5 (6.7), and D5B10 (5.9), decrease with the reduction in the swelling ratio, indicating the GP with a smaller swelling ratio is in a relatively collapsed state at 30 °C compared to those with higher swelling ratios, because swelling of the GP is inhibited by the high crosslink degree. The pKa value of D5B2 at 30 °C (7.8) is similar to that of D5B0 (7.7), although D5B0 exhibits a much larger swelling ratio, indicating that D5B2 is also fully swollen at 30 °C.
In Fig. 5b, D5B0, D5B2, and D5B5 exhibit similar reversible CO2 capture stoichiometries (0.97–1.0 mol CO2 per mol amine), while that of D5B10 (0.74 mol CO2 per mol amine) is much lower. The difference in the reversible CO2 capture stoichiometries can also be explained by their pKa values. The higher pKa values of D5B0, D5B2, and D5B5 at 30 °C (7.7, 7.8, and 6.7, respectively), which are above the pKa1 of H2CO3, and the lower pKa values at 75 °C (5.2, 5.3, and 4.4, respectively), which are below the pKa1 of H2CO3, lead to high reversible CO2 capture stoichiometries. However, the pKa value of D5B10 at 30 °C (5.9) is too low and the basicity is too weak to capture CO2 efficiently.
Conclusively, the swelling ratio (D30/D75), which can be controlled by crosslink degree, is an important factor to tune the ΔpKa and consequently the pKa values of GPs to improve the reversible CO2 capture stoichiometry.
Design rationale of GP film with large reversible CO2 capture stoichiometry
The above discussion has revealed the design rationale of GPs that show large reversible CO2 capture stoichiometry within a narrow temperature range of 30 °C to 75 °C. The pKa values of the GPs at 30 °C and 75 °C are the principal factors that govern the reversible CO2 capture stoichiometry. Higher pKa values at 30 °C, above the pKa1 of H2CO3 (6.35), and lower pKa values at 75 °C, below the pKa1 of H2CO3, enable high reversible CO2 capture stoichiometries of GP films. This is because the GPs are capable of capturing CO2 efficiently at 30 °C due to the stronger basicity of the amine than HCO3−, and then releasing CO2 sufficiently at 75 °C because of the weaker basicity. High reversible CO2 capture stoichiometry can be achieved for GPs that exhibit a smaller ΔpKa, as long as the pKa values lie within the appropriate range.The pKa value of the GPs can be readily adjusted to the desired level by varying the VPTT of the GPs above and close to the CO2 capture temperature (30 °C in this study), because the volume phase transition of GPs always brings out a large pKa transition throughout a wide temperature range above the VPTT. The pKa value of the GPs can also be tuned by controlling the size of the GPs since smaller GPs show higher pKa values. Another method is to regulate the GP swelling ratio, which influences ΔpKa and consequently the pKa value of GPs. Finally, the imprinted microenvironment around the amine groups in the GPs can also influence the pKa values of the GPs because the GPs synthesized in the presence of a large amount of protons exhibit higher pKa values. The inter-relationship between these factors is illustrated by Scheme 3.
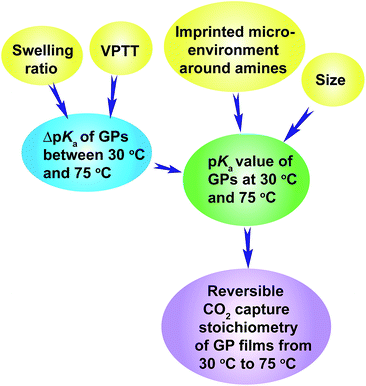 | ||
| Scheme 3 Illustration of the design rationale for GPs that show large reversible CO2 capture stoichiometry. | ||
Optimization of GPs to maximize reversible CO2 capture capacity
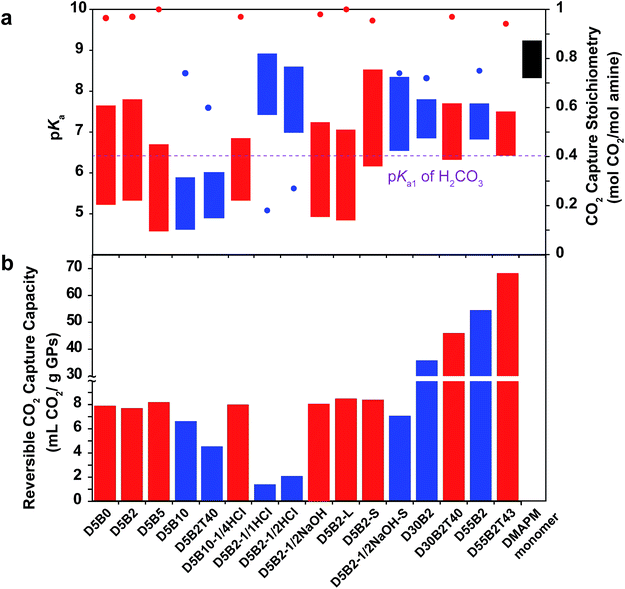
In order to improve the CO2 capture capacity by GP films, the ratio of functional amine monomer, DMAPM, was maximized to 55 mol% (GPs with a higher DMAPM concentration precipitated during polymerization process). However, D55B2 exhibited a low CO2 capture stoichiometry (0.73 mol CO2 per mol amine) due to its high pKa value at 75 °C (6.8) (Fig. 6a). According to the design rationale described above, highly efficient CO2 capture cycles could be achieved if the pKa value of the 55 mol% DMAPM-containing GP at 75 °C is below the pKa1 of H2CO3 (6.35).Since the VPTT of D55B2 is greater than 75 °C, one approach to reducing the pKa value of the 55 mol% DMAPM-containing GP at 75 °C is to lower its VPTT. Thus, 43 mol% TBAm was incorporated into the 55 mol% DMAPM-containing GP (D55B2T43).
In accordance with the design rationale, the pKa value of D55B2T43 at 75 °C decreases to about 6.35 (Fig. 6a) due to the reduced VPTT via the incorporation of TBAm. Consequently, a high reversible CO2 capture stoichiometry (0.93 mol CO2 per mol amine) is obtained. As a result of the high amine content and the high stoichiometric efficiency, as shown in Fig. 6b, D55B2T43 shows the largest reversible CO2 capture capacity (68 mL CO2 per g dry GPs, 3.0 mmol CO2 per g dry GPs) in this study.
Though the GP D55B2T43 showed high CO2 capture capacity (68 mL CO2 per g dry GPs, 3.0 mmol CO2 per g dry GPs), taking into account of water and support that are necessary for the GP films, the CO2 capture capacity of the GP film is lower than the best adsorbents.55 However the GP films can be used directly to capture CO2 from desulfurized post-combustion exhausted gas, without pre-treatment of the exhausted gas to remove water vapor inside, nor to decrease the temperature of the water vapor. Furthermore, the low regeneration temperature of the GP films (75 °C) enables the utilization of abundant and low cost waste heat (<100 °C) of factories as an energy source. Consequently the energy consumption of the GP films might be lowered.
Fig. 6a summarizes the pKa values and the reversible CO2 capture stoichiometries of the GPs studied in this paper. Overall, it can be concluded that the GPs with pKa values at 30 °C above the pKa1 of H2CO3, and pKa values at 75 °C below the pKa1 of H2CO3 (red) generally show larger reversible CO2 capture stoichiometries than other GPs (blue).
Reversibility of the optimized GPs as CO2 absorbent in wet environment
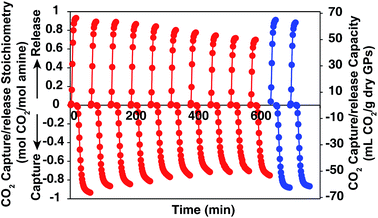
In order to show the cycle stability of the GP film, the reversible CO2 capture-release by D55B2T43 film was carried out for 10 cycles. The result is shown in Fig. 7 red plots. The reversible CO2 capture capacity decreased gradually from 0.93 mol CO2 per mol amine of the 1st cycle to 0.71 mol CO2 per mol amine of the 10th cycle. The reason is that the GP film partly dried out, as can be seen in Fig. 2S in ESI.† However, by supplying 4 mL water per g GPs to the dried part of the GP film after 10 CO2 release/capture cycles, the reversible CO2 capture capacity can be completely recovered (Fig. 7 blue plots).We believe the problem of cycle stability can be solved by tuning mass balance of water carefully by engineering the CO2 capture processes, such as reactor shape and operation condition optimization.
Conclusions
Inspired by the efficient CO2 transport mechanism of hemoglobin, known as the Bohr effect, here we revealed the design rationale of amine-functionalized thermo-responsive gel particle (GP) films that reversibly capture and release large amounts of CO2 efficiently from a model of the exhaust gas of fire power plants over a narrow temperature range (30–75 °C). Appropriate pKa values of the GPs at the CO2 capture and release temperatures (30 °C and 75 °C, respectively) are essential for the reversible CO2 capture stoichiometry of the GPs films. At 30 °C, a high pKa value, above the pKa1 of H2CO3, is required for efficient CO2 capture. Simultaneously, a low pKa value at 75 °C, below the pKa1 of H2CO3, is required for the efficient release of CO2.The pKa value of the GPs can be readily adjusted to the desired level via four methods. (1) Controlling the VPTT of the GPs above and close to 30 °C – the phase transition induces a large pKa transition over a wide temperature range above the VPTT. (2) Controlling the size of the GPs – smaller GPs show higher pKa values. (3) Controlling the swelling ratio, which influences ΔpKa, and consequently the pKa value of the GPs. (4) Controlling the imprinted microenvironment of the GPs – the GPs synthesized in the presence of a large amount of protons exhibit higher pKa values.
We successfully designed and acquired the GP, D55B2T43, which exhibited a large reversible CO2 capture capacity (68 mL CO2 per g dry GPs, 3.0 mmol CO2 per g dry GPs) as well as a high reversible CO2 capture stoichiometry (0.93 mol CO2 per mol amine), by optimizing the pKa value of GPs containing a maximum amount of amine-monomer. We believe GPs with an even larger CO2 capture capacity could be designed by incorporating co-monomers that are more hydrophobic than TBAm into the tertiary amine-containing GPs, and/or by using more hydrophobic tertiary amine monomers such as N-[3-(diethylamino)propyl] methacrylamide. The advantages of amine functionalized GP films, such as the large capture capacity, the low regeneration temperature (75 °C), and the unique ability to work in wet environments, would enable the use of GP films as energy efficient CO2 absorbents for the exhaust gas of fire power plants. We anticipate that GP films that can reversibly capture other acidic and basic gases with a large capacity can also be achieved by the same strategy inspired by the Bohr effect of hemoglobin.
Acknowledgements
The financial support from NEDO (11B10002d), MEXT (25107726; Innovative Areas of “Fusion Materials”) and JST-ALCA is greatly appreciated.References
- Climate Change 2007: The Physical Science Basis, ed. S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller, The Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, New York, 2007 Search PubMed.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, Beyond Oil and Gas: The Methanol Economy, Wiley-VCH, Weinheim, Germany, 2nd edn, 2009 Search PubMed.
- G. T. Rochelle, Science, 2009, 325, 1652 CrossRef CAS PubMed.
- T. Fout and J. T. Murphy, DOE/NETL’s Carbon Capture R&D Program for Existing Coal-Fired Power Plants; DOE/NETL 2009/1356, National Energy Technology Laboratory, Pittsburgh, PA, 2009 Search PubMed.
- D. Aaron and C. Tsouris, Sep. Sci. Technol., 2005, 40, 321 CrossRef CAS PubMed.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef PubMed.
- Y. Park, D. Shin, Y. N. Jang and A.-H. A. Park, J. Chem. Eng. Data, 2012, 57, 40 CrossRef CAS.
- H. Y. Huang and R. T. Yang, Ind. Eng. Chem. Res., 2003, 42, 2427 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796 CrossRef CAS PubMed.
- K. B. Lee, M. G. Beaver, H. S. Caram and S. Sircar, Ind. Eng. Chem. Res., 2008, 47, 8048 CrossRef CAS.
- A. Goeppert, M. Czaun, R. B. May, G. K. S. Prakash, G. A. Olah and S. R. Narayanan, J. Am. Chem. Soc., 2011, 133, 20164 CrossRef CAS PubMed.
- R. Serna-Guerrero, E. Da’na and A. Sayari, Ind. Eng. Chem. Res., 2008, 47, 9406 CrossRef CAS.
- P. J. E. Harlick and A. Sayari, Ind. Eng. Chem. Res., 2007, 46, 446 CrossRef CAS.
- I. Nishio, S. Sun, G. Swislow and T. Tanaka, Nature, 1979, 281, 208 CrossRef CAS PubMed.
- T. Tanaka, C. Wang, V. Pande, A. Y. Grosberg, A. English, S. Masamune, H. Gold, R. Leavy and K. King, Faraday Discuss., 1995, 101, 201 RSC.
- I. Lynch and K. A. Dawson, J. Phys. Chem. B, 2004, 108, 10893 CrossRef CAS.
- M. J. Serpe, K. A. Yarmey, C. M. Nolan and L. A. Lyon, Biomacromolecules, 2005, 6, 408 CrossRef CAS PubMed.
- C. A. Kavanagh, Y. A. Rochev, W. M. Gallagher, K. A. Dawson and A. K. Keenan, Pharmacol. Ther., 2004, 102, 1 CrossRef CAS PubMed.
- Y. Hoshino, W. W. Haberaecker III, T. Kodama, Z. Zeng, Y. Okahata and K. J. Shea, J. Am. Chem. Soc., 2010, 132, 13648 CrossRef CAS PubMed.
- S. H. Lee, Y. Hoshino, A. Randall, Z. Zeng, P. Baldi, R. A. Doong and K. J. Shea, J. Am. Chem. Soc., 2012, 134, 15765 CrossRef CAS PubMed.
- Y. Yonamine, Y. Hoshino and K. J. Shea, Biomacromolecules, 2012, 13, 2952 CrossRef CAS PubMed.
- K. Nagase, J. Kobayashi, A. Kikuchi, Y. Akiyama, H. Kanazawa and T. Okano, Biomacromolecules, 2008, 9, 1340 CrossRef CAS PubMed.
- Y. Akiyama, A. Kikuchi, M. Yamato and T. Okano, Langmuir, 2004, 20, 5506 CrossRef CAS.
- Y. Hoshino, R. C. Ohashi and Y. Miura, Adv. Mater., 2014, 26, 3718 CrossRef CAS PubMed.
- T. Oya, Science, 1999, 286, 1543 CrossRef CAS.
- K. Yoshimatsu, B. K. Lesel, Y. Yonamine, J. M. Beierle, Y. Hoshino and K. J. Shea, Angew. Chem., Int. Ed., 2012, 51, 2405 CrossRef CAS PubMed.
- Y. Hoshino, K. Imamura, M. Yue, G. Inoue and Y. Miura, J. Am. Chem. Soc., 2012, 134, 18177 CrossRef CAS PubMed.
- Y. Hoshino, M. Nakamoto and Y. Miura, J. Am. Chem. Soc., 2012, 134, 15209 CrossRef CAS PubMed.
- M. Yue, Y. Hoshino, Y. Ohshiro, K. Imamura and Y. Miura, Angew. Chem., Int. Ed., 2014, 53, 2654 CrossRef CAS PubMed.
- I. Lynch and K. A. Dawson, J. Phys. Chem. B, 2003, 107, 9629 CrossRef CAS.
- J. V. Kilmartin, J. J. Breen, G. C. K. Roberts and C. Ho, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 1246 CrossRef CAS.
- D. Voet and J. G. Voet, Biochemistry, Wiley, New York, USA, 4th edn, 1995 Search PubMed.
- J. Wilcox, R. Haghpanah, E. C. Rupp, J. He and K. Lee, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 479 CrossRef CAS PubMed.
- M. Eigen, Angew. Chem., Int. Ed., 1964, 3, 1 CrossRef PubMed.
- H. Li, A. D. Robertson and J. H. Jensen, Proteins, 2005, 61, 704 CrossRef CAS PubMed.
- D. W. Urry, D. C. Gowda, S. Q. Peng, T. M. Parker and R. D. Harris, J. Am. Chem. Soc., 1992, 114, 8717 CrossRef.
- W. Kühlbrandt, Nature, 2000, 406, 569 CrossRef PubMed.
- V. K. Rastogi and M. E. Girvin, Nature, 1999, 402, 263 CrossRef CAS PubMed.
- Y. Hoshino, T. Kodama, Y. Okahata and K. J. Shea, J. Am. Chem. Soc., 2008, 130, 15242 CrossRef CAS PubMed.
- K. Mosbach and O. Ramström, Nat. Biotechnol., 1996, 14, 163 CrossRef CAS PubMed.
- G. Wulff, Chem. Rev., 2002, 12, 1 CrossRef PubMed.
- H. Feil, Y. H. Bae, J. Feijen and S. W. Kim, Macromolecules, 1992, 25, 5528 CrossRef CAS.
- M. H. Kwok, Z. Li and T. Ngai, Langmuir, 2013, 29, 9581 CrossRef CAS PubMed.
- P. D. Vaidya and E. Y. Kenig, Chem. Eng. Technol., 2007, 30, 1467 CrossRef CAS PubMed.
- L. D. Terrence and Y. N. Nguyen, Ind. Eng. Chem. Fundam., 1980, 19, 260 Search PubMed.
- J. Mcmurry, Organic Chemistry, Thomson Higher Education, Belmont, CA, 7th edn, 2008 Search PubMed.
- H. S. Harned and R. Davis Jr., J. Am. Chem. Soc., 1943, 65, 2030 CrossRef CAS.
- J. D. Debord and L. A. Lyon, Langmuir, 2003, 19, 7662 CrossRef CAS.
- S. Nayak and L. A. Lyon, Angew. Chem., Int. Ed., 2005, 44, 7686 CrossRef CAS PubMed.
- W. McPhee, K. C. Tam and R. Pelton, J. Colloid Interface Sci., 1993, 156, 24 CrossRef CAS.
- M. Nakamoto, Y. Hoshino and Y. Miura, Biomacromolecules, 2014, 15, 541 CrossRef CAS PubMed.
- H. Tokuyama and Y. Kato, Colloids Surf., B, 2008, 67, 92 CrossRef CAS PubMed.
- I. Varga, T. Gilanyi, R. Meszaros, G. Filipcsei and M. Zrinyi, J. Phys. Chem. B, 2001, 105, 9071 CrossRef CAS.
- X. Hu, Z. Tong and L. A. Lyon, Langmuir, 2011, 27, 4142 CrossRef CAS PubMed.
- G. Qi, L. Fu and E. P. Giannelis, Nat. Commun., 2014, 5, 5796 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc01978h |
| This journal is © The Royal Society of Chemistry 2015 |