Open Access Article
Open Access ArticleCarbide complexes as π-acceptor ligands†
Anders
Reinholdt
 ,
Johan E.
Vibenholt
,
Thorbjørn J.
Morsing
,
Johan E.
Vibenholt
,
Thorbjørn J.
Morsing
 ,
Magnus
Schau-Magnussen
,
Nini E. A.
Reeler
and
Jesper
Bendix
,
Magnus
Schau-Magnussen
,
Nini E. A.
Reeler
and
Jesper
Bendix
 *
*
Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100, Denmark. E-mail: bendix@kiku.dk; Tel: +45 35320101
First published on 7th July 2015
Abstract
The π-accepting character of a terminal carbide complex acting as a ligand is demonstrated experimentally and corroborates earlier theoretical predictions. As a result, coordination of a terminal ruthenium carbide complex to electron-rich metal centres is shown to provide a facile and versatile route to carbide-bridged heterometallic complexes. Synthesis, reactivity, spectroscopic and structural characterization are reported for heterobimetallic systems with auxiliary metals from groups 9–11: Rh(I), Ir(I), Pd(II), Pt(II), Ag(I), and Au(I) coordinated by [Ru(C)Cl2(PCy3)2] (RuC). This encompasses the first example of a homoleptic carbide-ligated transition metal complex: [{(Cy3P)2Cl2RuC}2Au]+. Kinetics of substitution on Pt(II) by RuC ranks the carbide complex as having intermediate nucleophilicity. The 13C-NMR signals from the carbide ligands are significantly more shielded in the bridged heterobimetallic complexes than in the parent terminal carbide complex. Structurally, RuC forms very shorts bonds to the heterometals, which supports the notion of the multiple bonded complex acting as a π-backbonding ligand. Reactions are reported where RuC displaces CO coordinated to Rh(I) and Ir(I). A strong trans influence exerted by RuC indicates it to be a stronger σ-donor than CO. The geometries around the carbide bridges resemble those in complexes of electron-rich metals with carbonyl or bridging nitride-complex-derived ligands, which establishes a link to other strong π-acceptor ligands.
1 Introduction
Mono-atomic carbon as a ligand is not common. Nevertheless, it has implications to all forms of life as it was recently shown to be present in nitrogenase,1–4 which is responsible for the conversion of atmospheric nitrogen into bioavailable ammonium. The most effective nitrogenase has an FeMo cofactor active site containing a six-coordinate interstitial carbon. Mono-atomic carbon ligands are also of relevance in large-scale industrial heterogeneous catalysed processes such as fuel synthesis by the Fischer–Tropsch process5,6 which encompasses the catalytic hydrogenation and polymerization of CO into alkanes and oxygenated compounds. Here terminal carbon ligands form on the surface of Fischer–Tropsch catalysts and possibly play a role in the formation of C–C bonds in the products.7Terminal carbide complexes are rational precursors for carbide-bridged complexes as the commodious, one-coordinate carbide ligand in an M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C: unit allows an incoming metal centre to approach and be ligated, but this approach is virtually unexplored. Generally, routes to heterometallic carbide-bridged systems are singular in the sense that they have a limited scope for generalization to other metals. However, one notable example of a versatile route to bimetallic carbide-bridged complexes is Templeton's and Hill's development of the (Tp*)(OC)2MC–M′ platform (M = Mo, W, M′ = Si, Ge, Sn, Pb,8 Fe,9 Ni,10 Au,11 Hg,12 Tp*− = hydridotris(3,5-dimethyl-pyrazol-1-yl)borate). Terminal carbides have been isolated for 2nd and 3rd row group 6 (ref. 13 and 14) and group 8 (ref. 15–19) transition metals. The known terminal carbides of molybdenum and tungsten are very sensitive to air and moisture and must be handled under inert atmospheres. Similarly, in bimetallic group 6 complexes, bridging carbide ligands are prone to associate with non-metals affording μ-CR ligands (R = H,20 R = PEt3,10 Et = ethyl).
C: unit allows an incoming metal centre to approach and be ligated, but this approach is virtually unexplored. Generally, routes to heterometallic carbide-bridged systems are singular in the sense that they have a limited scope for generalization to other metals. However, one notable example of a versatile route to bimetallic carbide-bridged complexes is Templeton's and Hill's development of the (Tp*)(OC)2MC–M′ platform (M = Mo, W, M′ = Si, Ge, Sn, Pb,8 Fe,9 Ni,10 Au,11 Hg,12 Tp*− = hydridotris(3,5-dimethyl-pyrazol-1-yl)borate). Terminal carbides have been isolated for 2nd and 3rd row group 6 (ref. 13 and 14) and group 8 (ref. 15–19) transition metals. The known terminal carbides of molybdenum and tungsten are very sensitive to air and moisture and must be handled under inert atmospheres. Similarly, in bimetallic group 6 complexes, bridging carbide ligands are prone to associate with non-metals affording μ-CR ligands (R = H,20 R = PEt3,10 Et = ethyl).
Contrarily, the terminal ruthenium carbide complex, [Ru(C)Cl2(PCy3)2] (RuC), is remarkably stable in air in which it may be handled and stored for years without apparent decomposition. Heppert's serendipitous discovery15 of the metathesis-facilitated route to RuC and Grubbs' rational extension16 based on phosphine-exchange reactions offered the terminal carbide readily, though with the requirement for rather complex organic reagents. This was circumvented in Johnson's elegant synthesis17 of RuC, which employed commonplace vinyl acetate in lieu of Feist's esters. The stability combined with straightforward 13C-labeling of the terminal carbide ligand17,21 simplifies attempts at a rational bottom-up approach to carbide-bridged systems and led us to investigate the reactivity of RuC as a ligand. Among the known reactions of RuC are oxidations to yield [Ru(CO)Cl2(PCy3)2], [Ru(CS)Cl2(PCy3)2],17 and [Ru(CSe)Cl2(PCy3)2].22 On reaction with strong acids, the protonated carbide associates with a phosphine to yield phosphonium alkylidenes, [(Cy3P)Cl2Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(PCy3)]X,19,23 X = BF4−, B(C6F5)4−, OTf− (OTf− = trifluoromethanesulfonate). Reactions of RuC with MeO2CC
CH(PCy3)]X,19,23 X = BF4−, B(C6F5)4−, OTf− (OTf− = trifluoromethanesulfonate). Reactions of RuC with MeO2CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCO2Me, MeOTf, and the tropylium ion yield [(Cy3P)2Cl2Ru
CCO2Me, MeOTf, and the tropylium ion yield [(Cy3P)2Cl2Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC2(CO2Me)2],21 [(Cy3P)2Cl2Ru
CC2(CO2Me)2],21 [(Cy3P)2Cl2Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe]OTf,18 and [(Cy3P)2Cl2Ru
CMe]OTf,18 and [(Cy3P)2Cl2Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH2C6H5]+,18 respectively. These protonations and alkylations demonstrate that RuC reacts as a nucleophile, though it appears a rather weak nucleophile, as it fails to react with even fairly reactive electrophiles such as MeI, MeCOCl, and C6H5CH2Br.21 Along these lines, Grubbs and co-workers reported the formation of heterometallic carbide-bridged complexes assembled from RuC and Pd(II) or Mo(0) with the argument that the ruthenium carbide functions as a σ-donating ligand towards these metal centres.16
CCH2C6H5]+,18 respectively. These protonations and alkylations demonstrate that RuC reacts as a nucleophile, though it appears a rather weak nucleophile, as it fails to react with even fairly reactive electrophiles such as MeI, MeCOCl, and C6H5CH2Br.21 Along these lines, Grubbs and co-workers reported the formation of heterometallic carbide-bridged complexes assembled from RuC and Pd(II) or Mo(0) with the argument that the ruthenium carbide functions as a σ-donating ligand towards these metal centres.16
In coordination chemistry, molecular complexes with mono-atomic carbon ligands are scarce in comparison with the numerous complexes with mono-atomic N, O, and F ligands. For the latter ligands, their oxidation state is generally not ambiguous, and they can be viewed as isoelectronic N3− (nitride), O2− (oxide) and F− (fluoride) ligands although some complexes of mono-atomic nitrogen are best considered nitrene (N−) complexes. Mono-atomic carbon ligands are often referred to as carbide ligands, implying C4−, by analogy with the above isoelectronic series. However, for carbon a quite clear dichotomy exists between the formulation as carbide or carbon (C0) ligands. Thus, based on the computational studies of charge density distributions in Fe(C)(CO)4, Frenking suggested a nomenclature for carbon-containing ligands that classifies RuC as a carbon complex.24 We do not dispute this result, but prefer in the following to denote RuC and the derived systems as carbide complexes in agreement with common usage and in line with their very strong resemblance in both structure and reactivity to established bona fide nitride complexes.
Computational studies of terminal carbide complexes of group 8 metals reveal metal carbide triple bonds that are polarized toward the metal24 and carbon-based lone pairs with a large degree of 2 s character.25 This corresponds well with the experimentally observed weak nucleophilic character exhibited by the carbide ligand in RuC. In addition to σ-donating properties, the presence of energetically low-lying unoccupied molecular orbitals with local π-symmetry suggests the suitability of the carbide moiety for coordination to electron-rich transition metals as a back-bonding ligand.26 Frenking compared metal carbon complexes with carbon monoxide in terms of their donor–acceptor interactions as ligands.27 Based on orbital energies, the metal carbon complexes were argued to be promising, possibly better σ-donating ligands than CO and comparably good π-accepting ligands. Thus, it was suggested that metal carbon complexes could potentially outcompete CO as a ligand toward transition metal centres and that the formation of homoleptic carbon-bridged complexes might be achieved, since homoleptic carbonyl complexes are stable and numerous.
The electronic structure of terminal carbide complexes has a counterpart in the coordination chemistry of the isoelectronic terminal nitride complexes. Based on the spatial and orbital relationships between CO and terminal nitride complexes, and corroborated by experimental data, Mayer and co-workers originally proposed that terminal nitrides can function as strong π-accepting ligands.28 In the following, we demonstrate the same to be the case for terminal carbides and thereby experimentally establish a link between terminal carbides, terminal nitrides, and CO as ligands.
2 Results and discussion
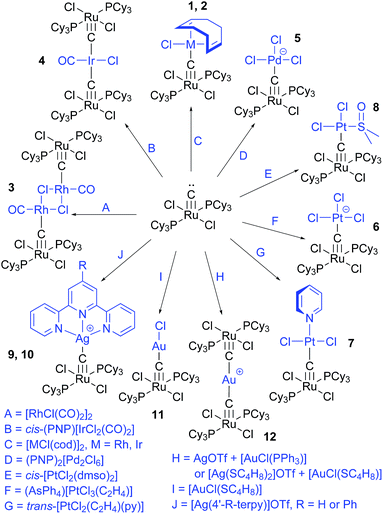
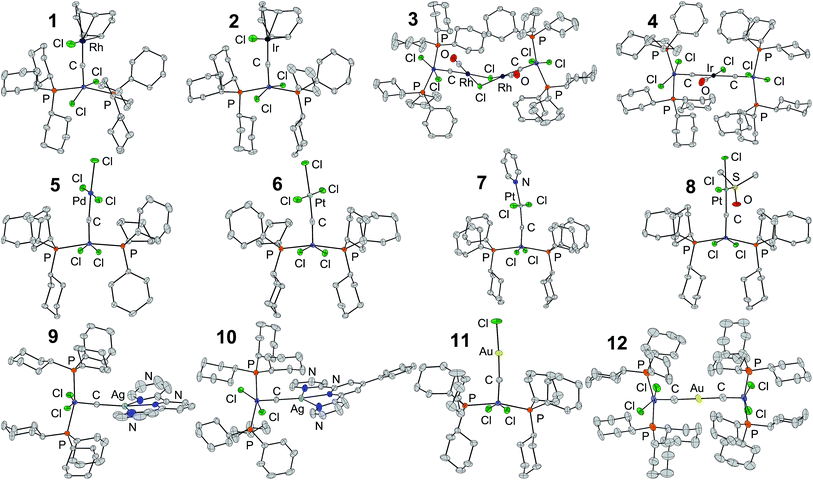
Carbide-bridged systems form smoothly (vide infra) when solutions of RuC in CH2Cl2 or CHCl3 are treated with complexes of electron-rich metals with displaceable ligands (Scheme 1). This furnishes a general route to the heterometallic carbide-bridged complexes [(Cy3P)2Cl2RuC–RhCl(cod)] (1), [(Cy3P)2Cl2RuC–IrCl(cod)] (2), [(Cy3P)2Cl2RuC–RhCl(CO)]2 (3), [{(Cy3P)2Cl2RuC}2IrCl(CO)] (4), (PNP)[(Cy3P)2Cl2RuC–PdCl3] (5), (AsPh4)[(Cy3P)2Cl2RuC–PtCl3] (6), [(Cy3P)2Cl2RuC–PtCl2(py)] (7), [(Cy3P)2Cl2RuC–PtCl2(dmso-S)] (8), [(Cy3P)2Cl2RuC–Ag(terpy)]OTf (9), [(Cy3P)2Cl2RuC–Ag(4′-Ph-terpy)]OTf (10) [(Cy3P)2Cl2RuC–AuCl] (11), and [{(Cy3P)2Cl2RuC}2Au]OTf (12).‡Fig. 1 depicts the molecular structures of complexes 1–12. The conformations near the heterometal carbide bonds demonstrate the requirement for space exerted by the bulky tricyclohexylphosphines in RuC. Thus, ligands on the heterometals lie in a plane nearly perpendicular to the phosphine–ruthenium bonds. This is readily apparent from inspection of the molecular structures of 1–10, whose heterometal centres have square planar ligand arrangements.The dimeric group 9 metal complexes, [RhCl(cod)]2 and [IrCl(cod)]2, undergo symmetric cleavage of their chloride-bridged cores upon reaction with RuC to form [(Cy3P)2Cl2RuC–RhCl(cod)] (1) and [(Cy3P)2Cl2RuC–IrCl(cod)] (2). On the other hand, [RhCl(CO)2]2 reacts differently from the cyclooctadiene complexes: the chloride-bridged core in [RhCl(CO)2]2 persists, and RuC displaces one CO ligand from each metal centre to form the tetranuclear structure, [(Cy3P)2Cl2RuC–RhCl(CO)]2 (3). The carbides are arranged trans with respect to the chloride bridges between the Rh centres. Mononuclear cis-(PNP)[IrCl2(CO)2] reacts with RuC to lose both a CO and a Cl− affording the neutral trans, bis complex, [{(Cy3P)2Cl2RuC}2IrCl(CO)] (4).
The reactivity of divalent group 10 metal centres resembles that of the monovalent group 9 metals. Thus, RuC cleaves the dichloride bridge in the anion of dimeric (PNP)2[Pd2Cl6] to form (PNP)[(Cy3P)2Cl2RuC–PdCl3] (5), and the same motif of reactivity is observed in the reaction of RuC with the anion of Zeise's salt, [PtCl3(C2H4)]−, to yield (AsPh4)[(Cy3P)2Cl2RuC–PtCl3] (6), whose anion is isostructural to the anion of 5. Reactions of RuC with trans-[PtCl2(C2H4)(py)], and cis-[PtCl2(dmso-S)2] result in displacement of ethene or dmso to yield trans-[(Cy3P)2Cl2RuC–PtCl2(py)] (7) and cis-[(Cy3P)2Cl2RuC–PtCl2(dmso-S)] (8), respectively. This shows that RuC is a good ligand towards Pt(II) as it outcompetes the soft ligands, C2H4 and dmso. An alternative route to 7 starts from 6, which reacts with pyridine to substitute the chloride trans to the carbide bridge. The kinetics of ligand substitution in cis-[PtCl2(dmso-S)2] to yield 8 was investigated (cf. ESI incl. Fig. S16†) and the reaction was found to occur with a second-order rate constant of k2 = 0.27(3) M−1 s−1, which is an intermediate rate for substitution in this class of systems ranking RuC comparable to thiocyanate and sulphite in terms of nucleophilicity.29,30
Among the simplest conceivable routes to carbide-bridged complexes of group 11 metals are reactions between RuC and simple silver salts such as AgOTf, but these reactions failed to give isolable products. On the other hand, triflate salts of the silver complexes, [Ag(terpy)]+ and [Ag(4′-Ph-terpy)]+ react with RuC to give [(Cy3P)2Cl2RuC–Ag(terpy)]OTf (9) and [(Cy3P)2Cl2RuC–Ag(4′-Ph-terpy)]OTf (10). The reactivity of RuC towards gold(I) complexes varies in a subtle manner: [AuCl(SC4H8)] (SC4H8 = tetrahydrothiophene) readily dissociates SC4H8 in favour of RuC to form [(Cy3P)2Cl2RuC–AuCl] (11). On the contrary, no reaction occurs between [AuCl(PPh3)] and RuC. Initial treatment with AgOTf generates AgCl and formal [Au(PPh3)]+ that reacts with RuC to form [(Cy3P)2Cl2RuC–AuPPh3]+ as characterized by NMR (vide infra). The presence of RuC renders PPh3 labile, and another RuC associates with the gold centre, yielding the homoleptic carbide-bridged complex, [{(Cy3P)2Cl2RuC}2Au]OTf (12). An alternative route to 12 uses [Ag(SC4H8)2]OTf and [AuCl(SC4H8)] to generate Au(I) with all labile ligands, which subsequently reacts with two equivalents of RuC. The existence of 12 indicates the stability of the gold carbide bond and confirms the predicted feasibility of formation of homoleptic carbide-ligated complexes, though it is achieved for much lower coordination numbers than the ones discussed by Frenking.27 The structural determination of the homoleptic complex suffered from disorder problems with triflate as the counterion, and consequently, the corresponding BF4− salt was prepared by the first route using AgBF4.
2.1 Spectroscopic evidence of π-backbonding
Some insight into the bonding situation can be gained from vibrational spectroscopy. Vaska's complex (trans-[IrCl(CO)(PPh3)2])31 and 4 belong to the same family of complexes, trans-[IrCl(CO)L2], which allows for a direct comparison of their solid state infrared (IR) carbonyl stretching frequencies. Within Vaska-like complexes, νCO/cm−1 increases in the order: L = PCy3 (1934)32 < PPh3 (1954)32 < P(CH2CH2(CF2)5CF3)3 (1975)33 < P(C6F5)3 (1994);34,35 in 4, νCO is 1990 cm−1 (cf. ESI Fig. S14†). The phosphines in Vaska-like complexes presumably function as π-accepting ligands,36,37 and in terms of π-accepting strength, this consequently ranks RuC on a par with the strongest π-accepting phosphines.Raman spectroscopy combined with isotopic labelling identifies the stretching frequencies that relate to the carbide ligands in RuC (12C, 13C: 1050, 1013 cm−1) and 11 (12C, 13C: 1145, 1103 cm−1) (cf. ESI Fig. S11 and S12†). Naively, the shift would suggest a strengthening of the Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bond upon coordination, which would contrast with RuC acting as a π-accepting ligand towards gold(I). However, the literature provides examples of increase in stretching frequencies of metal nitride multiple bonds upon coordination to main group element and transition metal fragments: the IR stretching frequency of the nitride ligand in (NBu4)[Os(N)O3] (1023 cm−1) increases on coordination to [AuPPh3]+ (1102 and 1088 cm−1) and cis-[Pt(PMe3)2]2+ (1088 and 1072 cm−1).38 Similarly, the stretching frequency of nitride in Re(N)Cl2(PMe2Ph)3 (1061 cm−1)39 increases on coordination to [AuCl] (1125 cm−1)40 and [BCl3] (1180 cm−1).41 These observations have been rationalized41 as the result of coupled vibrations in the Re
C bond upon coordination, which would contrast with RuC acting as a π-accepting ligand towards gold(I). However, the literature provides examples of increase in stretching frequencies of metal nitride multiple bonds upon coordination to main group element and transition metal fragments: the IR stretching frequency of the nitride ligand in (NBu4)[Os(N)O3] (1023 cm−1) increases on coordination to [AuPPh3]+ (1102 and 1088 cm−1) and cis-[Pt(PMe3)2]2+ (1088 and 1072 cm−1).38 Similarly, the stretching frequency of nitride in Re(N)Cl2(PMe2Ph)3 (1061 cm−1)39 increases on coordination to [AuCl] (1125 cm−1)40 and [BCl3] (1180 cm−1).41 These observations have been rationalized41 as the result of coupled vibrations in the Re![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–X moieties that shift νRe
N–X moieties that shift νRe![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N and νN–X to higher and lower wavenumbers, respectively. Based on this, it must be concluded that stretching frequencies of the Ru
N and νN–X to higher and lower wavenumbers, respectively. Based on this, it must be concluded that stretching frequencies of the Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds are unsuited as probes for π-backdonation from the metal fragments coordinated to RuC.
C bonds are unsuited as probes for π-backdonation from the metal fragments coordinated to RuC.
The facile labelling of the carbide ligand in RuC makes 13C-NMR a useful handle on the reactivity and electronic structure of the derived heterometallic systems. Signals from carbide bridges (δC = 345–434 ppm) and resonances from organic carbons and unreacted RuC (472 ppm) are easily discriminated by 13C{1H}-NMR (cf.Table 1). The upfield shift of the carbide resonance upon bridging demonstrates increased shielding, suggesting increased electron density around the carbide. This corresponds well with the notion that RuC functions as a π-accepting ligand. However, this view is too simplistic, as the least backbonding heterometal centres, Pt(II) and Pd(II), provide the largest shifts in δC. Rather, the internal shielding in the Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C moiety needs to be factored in, as is the case for carbonyl complexes.42
C moiety needs to be factored in, as is the case for carbonyl complexes.42
| Complex | δ C (ppm) | J C–M (Hz) |
|---|---|---|
| 1 | 411.7 | 59.1 |
| 2 | 387.6 | — |
| 3 (CRu) | 396.4 | 60.2 |
| 3 (CO) | 177.7 | 85.6 |
| 4 | 397.4 | — |
| 5 | 380.9 | — |
| 6 | 344.7 | 1395.5 |
| 7 | 350.3 | 1283.4 |
| 8 | 349.0 | 1333.8 |
| 9 | 433.5 | 187.0 |
| 10 | 433.1 | 187.8 |
| 11 | 395.3 | — |
| 12 | 395.3 | — |
Heterometal to carbide NMR coupling constants (Table 1) serve as fingerprints of coordination and handles on backbonding in the carbide-bridged complexes. For instance, the isostructural complexes, 1 and 2, are readily distinguished by 13C-NMR: the carbide bridge in the iridium complex yields a singlet at 387.6 ppm whereas the carbide bridge in the rhodium complex yields a doublet (411.7 ppm, JC–Rh = 59.4 Hz). The carbide bridge in 3 (397.4 ppm) yields a doublet with nearly the same coupling constant as in 1 (59.0 Hz), while the carbonyl ligand in 3 couples more strongly to Rh than the carbide (JC–Rh = 85.6 Hz). This stronger coupling parallels shorter bonds from rhodium to the carbonyl ligands than to the carbide ligands (vide infra). As expected, the carbide bridge signals from 6–8 display satellite peaks due to 195Pt (JC–Pt = 1283.4–1395.5 Hz), and those from 9 and 10 appear as broadened doublets due to coupling to both 107Ag and 109Ag (JC–Ag = 187.0–187.8 Hz).
2.2 Reactivity of heterometallic systems
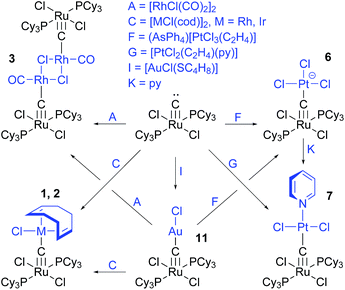
The stepwise formation of the homoleptic gold(I) complex, 12, through the intermediate, [(Cy3P)2Cl2RuC–AuPPh3]+, is clearly evident from 13C-NMR as the reaction mixture yields a doublet in the carbide range δ = 411.0 ppm, JC–P = 108.7 Hz. This coupling is significantly larger than typical coupling constants between the bridging carbide and the PCy3 ligands (5.6–7.5 Hz). The isolable product of the reaction, 12, yields a broad singlet (395.3 ppm) without discernible couplings to phosphorus, consistent with the absence of PPh3 in the final product.The combination of solid-state structures and 13C-NMR data also provides insight into the reactivity of the carbide-bridged systems (cf.Scheme 2). Compound 6 loses the chloride ligand on Pt(II) positioned trans to the bridging carbide when treated with pyridine. This alternative route to 7 shows that the larger structural trans influence of the carbide ligand compared to that of chloride (vide infra) is accompanied by a preference for trans substitution. Additionally, NMR reveals that 11 is suited as precursor for other systems, as it undergoes transmetallation with appropriate metal complexes. Hence, [RhCl(cod)]2, [IrCl(cod)]2, [RhCl(CO)2]2, and (AsPh4)[PtCl3(C2H4)] slowly react with 11 to form 1, 2, 3, and 6, respectively. The reaction with [RhCl(CO)2]2 first generates an intermediate (393.3 ppm, J = 60 Hz) that reacts further to yield 3.
2.3 Structural influence of the Ru![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e002.gif) C moiety
C moiety
Table 2 contains metrics for the carbide-bridged systems investigated here. To a first approximation, the geometries around the carbide bridges correspond to sp-hybridized C with a triple bond to Ru and a single bond to the heterometal: the carbide bridges are linear (172.9(2)–180°), and the short Ru–C triple bonds fall in the range 1.642(3)–1.698(3) Å corresponding to modest elongations of 0.6–4.0% of the triple bond in the precursor RuC. The assignment with carbide forming a triple and a single bond agrees with the suggestion by Hoffmann and co-workers43 that in the series M–X–M (M = transition metal, X = F−, O2−, N3−, and C4−), the tendency is for the X bridge to become increasingly asymmetric as its electronegativity decreases. However, counterexamples for carbide-containing homometallic, symmetric M–C–M bridges, e.g. M = Nb,44 Re,45 Fe,46–50 and Ru,51 exist.
| Complex | Ru–C–M (°) | Ru–C (Å) | C–M (Å) | Percentile rank (%) |
|---|---|---|---|---|
| a 4 and 12 crystallize with two crystallographically independent carbide bridges, with identical connectivity. | ||||
| 1 | 173.4(1) | 1.690(2) | 1.897(2) | 10.0 |
| 2 | 174.75(15) | 1.698(3) | 1.882(3) | 8.9 |
| 3 | 176.88(13) | 1.688(2) | 1.864(2) | 7.4 |
| 4 | 180 | 1.677(5) | 1.988(5) | 16.5 |
| 180 | 1.675(5) | 1.974(5) | 15.5 | |
| 5 | 173.50(15) | 1.668(2) | 1.892(2) | 0.5 |
| 6 | 174.4(2) | 1.691(3) | 1.873(3) | 4.2 |
| 7 | 172.9(2) | 1.679(3) | 1.882(3) | 4.9 |
| 8 | 177.6(1) | 1.682(2) | 1.919(2) | 6.9 |
| 9 | 177.23(15) | 1.642(3) | 2.082(3) | 17.6 |
| 10 | 176.5(2) | 1.651(3) | 2.072(3) | 13.4 |
| 11 | 175.4(2) | 1.664(3) | 1.921(3) | 2.3 |
| 12 | 173.6(6) | 1.679(10) | 1.960(10) | 9.1 |
| 175.4(6) | 1.655(9) | 1.974(9) | 14.3 | |
The only previously reported system belonging to the present class, which has been structurally characterized, namely Grubbs' trans-[(Cy3P)2Cl2RuC–PdCl2(SMe2)],16 provides a good basis for comparison with 5 as it contains RuC linearly coordinated to Pd(II). The Ru–C triple bond is 0.4% shorter than in 5, and the C–Pd single bond is 2.9% longer than in 5. The palladium carbide bond in 5 falls within the range of platinum carbide bond lengths spanned by the Pt(II) complexes 6–8. Compared with its most obvious congener, 6, the Pd–C bond in 5 is longer than the Pt–C bond by 0.019 Å (1.0%). This similarity between the Pd(II) and Pt(II) systems is mirrored by the Rh(I) and Ir(I) complexes 1 and 2. Here, bond distances that involve the carbide are identical within 3σ, underlining the geometric similarity between the rhodium and iridium complexes. On the other hand, the iridium carbide bonds in 4 are distinctly longer (0.092–0.106 Å) than those in 2. This is in part caused by the large structural trans influence of RuC (compared to Cl−, vide infra) and in part by the severe steric crowding from two RuC, Cl−, and CO in the ligand sphere of 4. Similarly, the gold carbide bonds in 12 are longer than those in 11. The shortening of the RuC–M bonds seen for 5d vs. 4d metals becomes more pronounced with increasing group number: in group 11, 9 and 10 have significantly longer Ag–C bonds than the Au–C bonds in 11 and 12.
Due to its unique nature, straight-forward structural analogues for comparisons with 12 do not exist. However, Hill and co-workers11 reported the tetrameric homoleptic carbide-bridged gold complex, [(Tp*)(OC)2W(μ3-C)Au]4. As opposed to the exclusive end-on binding mode in 12, the tetrameric gold complex has the tungsten carbide coordinated to Au(I) with both end-on and side-on binding modes, which enforces significantly bent carbide bridges (160.3(12)–167.7(12)°). The end on Au–C bonds are at 1.971(19)–2.03(2) Å, comparable to or slightly longer than the Au–C bonds in 11 and 12, and the side on Au–C bonds are longer (2.03(2)–2.14(2) Å).
In 12, carbide and gold(I) take on inverted roles compared to the homoleptic gold carbides studied by Schmidbaur and co-workers:52–58 the [(R3PAu)6C]2+ and [(R3PAu)5C]+ complexes contain carbide with a coordination sphere composed only of gold(I), whereas the coordination sphere of gold(I) in 12 is composed only of carbide.
On the heterometal centres in 1, 2, 5, and 6, chloride and the bridging carbide are positioned trans to the same ligands, which allows for a direct comparison of their trans influences. In 1, the average Rh–C distance (C belonging to cod) is 2.139 Å trans to Cl− and 2.305 Å trans to carbide. Equivalently, trans to Cl− and carbide the Ir–C distances in 2 are 2.134 Å and 2.291 Å, the Pd–Cl distances in 5 are 2.3079 Å and 2.3276(7) Å, and the Pt–Cl distances in 6 are 2.311 Å and 2.357(1) Å. Changing from chloride to carbide, the relative elongations of the trans bonds are 0.168 Å (7.8%) for 1, 0.156 Å (7.3%) for 2, 0.0198 Å (0.9%) for 5, and 0.046 Å (2.0%) for 6. This demonstrates that the structural trans influence of RuC is larger than that of Cl− in complexes of Rh(I), Ir(I), Pd(II), and Pt(II).
2.4 Structural credence to π-backbonding
The formation of complexes 1–12 demonstrates that RuC coordinates well to electron-rich 2nd and 3rd row transition metals. Thus, the preferred reactivity of RuC resembles that of π-accepting ligands. Structurally, this type of ligands is characterized by forming short ligand–metal bonds in complexes with electron-rich metals. Along these lines, the identification of {Os(N)}3+ and {Cr(N)}2+ as back-bonding ligands towards electron-rich metal centres was partially based on structural evidence.28,59The Cambridge Structural Database (CSD) allows a quantification of whether the metal-carbide bonds in 1–12 are short. Fig. 2 shows a histogram with all distances from carbon to Pd. For similar data for Rh, Ir, Pt, Ag, and Au, see ESI.† These diagrams and the percentile rank (Table 2) reveal that the metal-carbide bonds are at the very short end of the range of carbon–metal bonds (the shortest 0.5–17.6%) for all of these metals and characteristically similar to the respective metal-carbonyl bond lengths. This supports the notion that the ruthenium carbide acts as a π-accepting ligand.
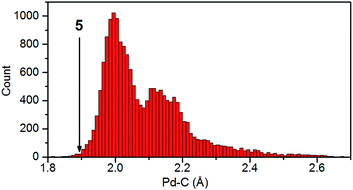 | ||
| Fig. 2 Pd–C distances from the CSD (v.1.16); the arrow indicates the position of the carbide–palladium bond in 5. | ||
A convincing juxtaposition of carbide complexes as ligands and CO as ligand requires kindred complexes of both ligand types. The existence of carbonyl analogues to 3, 4, 5, 6, 11, and 12 provides for such a comparison, and suggests that the RuC and CO moieties play the same role in the respective complexes. The availability of X-ray crystal structures of [RhCl(CO)2]2,60 [IrCl(CO)3],61 (Bu4N)[PdCl3(CO)],62 (Bu = butyl) (Bu4N)[PtCl3(CO)],63 and [AuCl(CO)]64 allows a direct comparison of the geometries of the carbonyl complexes and the structures of 1–12 (Table 3), and here it is relevant to note that 3 and 4 further allow a comparison of the RuC and CO ligands within the same complex. Though Au(CO)2+ is well established,65–68 its crystal structure has not been reported.
| a Carbide C. b Carbonyl C. c trans to C4−. d trans to CO. e trans to Cl. | |||
|---|---|---|---|
| 3 | [RhCl(CO) 2 ] 2 | ||
| Rh–Ca | 1.864(3) | Rh–C | 1.853(9) |
| Rh–Cb | 1.835(2) | Rh–C | 1.840(8) |
| Rh–Clc | 2.403(1) | Rh–Cl | 2.386(2) |
| Rh–Cld | 2.384(1) | Rh–Cl | 2.382(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 4 | [IrCl(CO) 3 ] | ||
| Ir–Ca | 1.988(5) | Ir–Cd | 2.04(5) |
| Ir–Ca | 1.974(5) | Ir–Cd | 1.974(8) |
| Ir–Cb | 1.785(11) | Ir–Ce | 1.915(7), 1.903(9) |
| Ir–Cl | 2.416(4) | Ir–Cl | 2.317(10), 2.369(2) |
| Ir–Ca | 1.988(5) | Ir–Cd | 2.04(5) |
| Ir–Ca | 1.974(5) | Ir–Cd | 1.974(8) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 5 | (Bu 4 N)[PdCl 3 (CO)] | ||
| Pd–C | 1.892(2) | Pd–C | 1.87(1) |
| Pd–Clc | 2.3276(7) | Pd–Cld | 2.283(2) |
| Pd–Cle | 2.3076(7) | Pd–Cle | 2.289(4) |
| Pd–Cle | 2.3081(7) | Pd–Cle | 2.295(3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 6 | (Bu 4 N)[PtCl 3 (CO)] | ||
| Pt–C | 1.873(3) | Pt–C | 1.825(6) |
| Pt–Clc | 2.357(1) | Pt–Cld | 2.289(7) |
| Pt–Cle | 2.309(1) | Pt–Cle | 2.289(2) |
| Pt–Cle | 2.313(1) | Pt–Cle | 2.295(2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 11 | [AuCl(CO)] | ||
| Au–C | 1.921(3) | Au–C | 1.93(2) |
| Au–Cl | 2.2630(10) | Au–Cl | 2.261(6) |
| C–Au–Cl | 177.27(10) | C–Au–Cl | 180 |
In 3 and [RhCl(CO)2]2, the Rh–C bonds are 0.6–1.6% shorter for CO than for RuC. The Rh–Cl bonds trans to CO are equal within three standard deviations, whereas the bond trans to RuC is longer by 0.8%, suggesting a larger structural trans influence of RuC than of CO. The RuC–Ir bonds in 4 fall within the range found for trans carbonyls in [IrCl(CO)3]. Contrarily, carbonyls trans to Cl form comparably short bonds in 4 and [IrCl(CO)3], particularly within 4, where the Ir–C bond lengths are much shorter for CO than for RuC (on average shorter by 0.196 Å). This can in part be ascribed to electronic effects, but also the steric bulk of the RuC units (vide supra) may contribute to the elongation of the RuC–Ir bonds in the trinuclear complex.
The Pd–CRu bond in 5 is likely longer than the Pd–CO bond in (Bu4N)[PdCl3(CO)], but the experimental uncertainty on the bond length in the carbonyl complex is too large to allow safe conclusions. In 5, the Pd–Cl bond trans to the carbide is elongated (0.0198 Å, 0.9%) compared to the Pd–Cl bonds trans to Cl−. Contrarily, all Pd–Cl bonds in (Bu4N)[PdCl3(CO)] are similar in length, again demonstrating a larger structural trans influence of RuC than of CO. 6 and (Bu4N)[PtCl3(CO)] show trends that parallel their Pd analogues with the Pt–CRu bond longer than the Pt–CO bond, but with RuC exhibiting a larger structural trans influence than chloride, whereas those of CO and chloride are comparable (see Table 3).
The strong similarity of RuC and CO as ligands is also borne out for coordination to Au(I) since [AuCl(CO)] and 11 have nearly identical geometries around gold: the Au–C bonds are identical within 3σ between the two systems, and the same applies to the Au–Cl bonds. The gold(I) centres are linear in 11 (177.0(1)°) and [AuCl(CO)] (180°).
Similarities between carbide and nitride complexes as ligands might be expected based on the isolobal relationship between M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C: and M
C: and M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N: moieties. A direct comparison is made possible by the fact that 1,59,69–722,71,73,748,59,75 and 11 (ref. 40) have nitride-bridged analogues, [LnMN–RhCl(cod)], [LnMN–IrCl(cod)], [LnMN–PtCl2(dmso-S)], and [LnMN–AuCl], where the ligand spheres only differ by RuC being replaced by a terminal nitride complex (Table 4). It has been argued that terminal nitride complexes act as strong π-accepting ligands28 binding readily to electron-rich metal centres. The nitride bridges are linear like the carbide bridges in 1–12. The rhodium and iridium nitride-bridged complexes display M–N bond lengths, which deviate significantly in both directions relative to the carbide-metal bond lengths in 1 and 2. The longer bonds are present in complexes of Cr nitrides, Re nitrides, and [Os(N)O3]−; the relatively short bonds are present in complexes of Os(VI) nitrides, which, notably, are among the most electrophilic nitride complexes. Conversely, the N–Pt bonds in the CrN–Pt complexes are slightly shorter than or equal within 3σ to their carbide analogue, 8. The N–Au bond in [(Me2PhP)3Cl2ReN–AuCl] is equal to those in 12 within 3σ, though longer than that in 11.
N: moieties. A direct comparison is made possible by the fact that 1,59,69–722,71,73,748,59,75 and 11 (ref. 40) have nitride-bridged analogues, [LnMN–RhCl(cod)], [LnMN–IrCl(cod)], [LnMN–PtCl2(dmso-S)], and [LnMN–AuCl], where the ligand spheres only differ by RuC being replaced by a terminal nitride complex (Table 4). It has been argued that terminal nitride complexes act as strong π-accepting ligands28 binding readily to electron-rich metal centres. The nitride bridges are linear like the carbide bridges in 1–12. The rhodium and iridium nitride-bridged complexes display M–N bond lengths, which deviate significantly in both directions relative to the carbide-metal bond lengths in 1 and 2. The longer bonds are present in complexes of Cr nitrides, Re nitrides, and [Os(N)O3]−; the relatively short bonds are present in complexes of Os(VI) nitrides, which, notably, are among the most electrophilic nitride complexes. Conversely, the N–Pt bonds in the CrN–Pt complexes are slightly shorter than or equal within 3σ to their carbide analogue, 8. The N–Au bond in [(Me2PhP)3Cl2ReN–AuCl] is equal to those in 12 within 3σ, though longer than that in 11.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–M′ complexes (M = Cr, Re, Os, M′ = Rh, Ir, Pt, Ag, Au)
N–M′ complexes (M = Cr, Re, Os, M′ = Rh, Ir, Pt, Ag, Au)
| Complex | M–N–M′ | M–N | N–M′ |
|---|---|---|---|
| a Crystallizes with two crystallographically independent but connectively identical nitride bridges. b dbm− = dibenzoylmethanoate. c H2salen = N,N′-bis(salicylidene)ethylenediamine. d acac− = acetylacetonate. | |||
| [(dbm)2CrN–RhCl(cod)]a,b,59 | 171.8(1) | 1.590(2) | 1.971(2) |
| 170.5(1) | 1.588(2) | 1.970(2) | |
| [(salen)CrN–RhCl(cod)]c,69 | 173.04(9) | 1.594(1) | 1.959(1) |
| [(Me2PhP)3Cl2ReN–RhCl(cod)]70 | 174.8(4) | 1.722(6) | 1.956(6) |
| [(Ph3As)2Cl3OsN–RhCl(cod)]71 | 176.1(9) | 1.675(9) | 1.86(1) |
| [(Ph3Sb)2Cl3OsN–RhCl(cod)]72 | 175.3(4) | 1.685(6) | 1.847(6) |
| [(Me2PhP)3Cl2ReN–IrCl(cod)]73 | 173.9(6) | 1.70(1) | 1.96(1) |
| (Ph4P)[O3OsN–IrCl(cod)]73 | 161.8(4) | 1.693(7) | 1.978(6) |
| [(Ph3As)2Cl3OsN–IrCl(cod)]71 | 176.2(9) | 1.712(8) | 1.816(8) |
| [(Ph3Sb)2Cl3OsN–IrCl(cod)]74 | 175.3(7) | 1.71(1) | 1.83(1) |
| [(dmso-O)(dbm)2CrN–PtCl2(dmso-S)]b,59 | 173.99(9) | 1.618(1) | 1.906(1) |
| [(acac)2CrN–PtCl2(dmso-S)]d,75 | 172.3(1) | 1.623(2) | 1.901(2) |
| [{(Me3SiCH2)2CpOsN}2Ag]BF4 (ref. 76) | 166.7(9) | 1.60(1) | 2.15(1) |
| 162.6(9) | 1.61(1) | 2.12(1) | |
| [(Me2PhP)3Cl2ReN–AuCl]40 | 173.8(1) | 1.674(2) | 1.969(2) |
| [(Me3SiCH2)2CpOsN–AuPPh3]BF4 (ref. 77) | 176.6(3) | 1.675(4) | 2.014(4) |
| [O3OsN–AuPPh3]38 | 168(1) | 1.69(2) | 2.02(2) |
When RuC reacts with a stoichiometric amount of [Au(PPh3)]OTf generated in situ from [AuCl(PPh3)] and AgOTf, [(Cy3P)2Cl2RuC–AuPPh3]OTf initially forms. This species turns out to be unstable with respect to phosphine exchange, and subsequently, another RuC displaces PPh3 from the Au(I) centre to yield 12. The phosphine-containing intermediate has nitride-bridged analogues, namely [(Me3SiCH2)2CpOsN–AuPPh3]BF4 (ref. 77) (Cp− = cyclopentadienide) and [O3OsN–AuPPh3].38 The Au–N bonds are longer than the Au–C bonds in 12. Additionally, the homoleptic nitride-bridged Ag(I)-complex, [{(Me3SiCH2)2CpOsN}2Ag]BF4,76 resembles 12 with respect to connectivity. The nitride bridges are, however, distinctly bent compared to the carbide bridges in 12, and the Ag–N bonds are longer than the Ag–C bonds in 9 and 10.
Complexes of carbides and nitrides clearly show similar reactivities towards electron-rich metal centres, and yield structurally very similar bridged products. This suggests that the terminal carbide and nitride moieties interact similarly with electron-rich metal centres, i.e. with π-backdonation from the metal centres into low-lying π*-orbitals of the multiply-bonded carbides and nitrides.
3 Conclusions
We have demonstrated the ability of the ruthenium carbide, [Ru(C)Cl2(PCy3)2] (RuC), to form linear carbide bridges to Rh(I), Ir(I), Pd(II), Pt(II), Ag(I), and Au(I). RuC binds readily to these low-valent metal centres, and the concomitant short bonds corroborate the view of the multiply bonded complex as a π-accepting ligand. The terminal carbide RuC forms similar complexes with closely matching geometries around the heterometals compared to the complexes formed by nitrides and carbon monoxide. The similarity in both structure and reactivity of RuC and strong π-accepting ligands yields further support to the π-acceptor nature of terminal carbides as ligands. The same conclusion derives from the large stretching frequency of CO in complex 4, which suggests significant π-backdonation from Ir(I) to the RuC moiety. Generally, the structural trans influence of RuC is large and similar to or surpassing that of CO. The formations of 3 and 4 in stoichiometric reactions proceed through substitution of CO by RuC, showing that RuC binds to Rh(I) and Ir(I) with competitive strength to CO. These findings in conjunction with the large trans influence of RuC correspond well with the suggestion that terminal carbide complexes of group 8 metals are stronger σ-donating ligands than CO.27 From the reactivity of 6 leading to substitution on Pt(II) in the position trans to the carbide ligand, a relatively high kinetic trans effect of the RuC moiety as a ligand can also be deduced.In summary, earlier computationally based predictions, that M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C: moieties should be able to outcompete CO as ligands and even form homoleptic metal complexes have been verified experimentally. Further studies extending this approach to molecular carbide complexes are ongoing.
C: moieties should be able to outcompete CO as ligands and even form homoleptic metal complexes have been verified experimentally. Further studies extending this approach to molecular carbide complexes are ongoing.
Acknowledgements
Dr Tom Vosch is acknowledged for experimental support. We thank the Danish Research Council for Independent Research for funding (Grant 12-125226).Notes and references
- T. Spatzal, M. Aksoyoglu, L. Zhang, S. L. A. Andrade, E. Schleicher, S. Weber, D. C. Rees and O. Einsle, Science, 2011, 334, 940 CrossRef CAS PubMed.
- K. M. Lancaster, M. Roemelt, P. Ettenhuber, Y. Hu, M. W. Ribbe, F. Neese, U. Bergmann and S. DeBeer, Science, 2011, 334, 974–977 CrossRef CAS PubMed.
- J. A. Wiig, Y. Hu, C. C. Lee and M. W. Ribbe, Science, 2012, 337, 1672–1675 CrossRef CAS PubMed.
- J. A. Wiig, C. C. Lee, Y. Hu and M. W. Ribbe, J. Am. Chem. Soc., 2013, 135, 4982–4983 CrossRef CAS PubMed.
- F. Fischer and H. Tropsch, Chem. Ber., 1926, 59, 830–831 CrossRef PubMed.
- F. Fischer and H. Tropsch, Chem. Ber., 1926, 59, 832–836 CrossRef PubMed.
- C. K. Rofer-DePoorter, Chem. Rev., 1981, 81, 447–474 CrossRef CAS.
- R. L. Cordiner, A. F. Hill, R. Shang and A. C. Willis, Organometallics, 2010, 30, 139–144 CrossRef.
- M. Etienne, P. S. White and J. L. Templeton, J. Am. Chem. Soc., 1991, 113, 2324–2325 CrossRef CAS.
- A. F. Hill, M. Sharma and A. C. Willis, Organometallics, 2012, 31, 2538–2542 CrossRef CAS.
- E. S. Borren, A. F. Hill, R. Shang, M. Sharma and A. C. Willis, J. Am. Chem. Soc., 2013, 135, 4942–4945 CrossRef CAS PubMed.
- A. L. Colebatch, R. L. Cordiner, A. F. Hill, K. T. H. D. Nguyen, R. Shang and A. C. Willis, Organometallics, 2009, 28, 4394–4399 CrossRef CAS.
- J. C. Peters, A. L. Odom and C. C. Cummins, Chem. Commun., 1997, 1995–1996, 10.1039/a704251e.
- A. E. Enriquez, P. S. White and J. L. Templeton, J. Am. Chem. Soc., 2001, 123, 4992–5002 CrossRef CAS PubMed.
- R. G. Carlson, M. A. Gile, J. A. Heppert, M. H. Mason, D. R. Powell, D. V. Velde and J. M. Vilain, J. Am. Chem. Soc., 2002, 124, 1580–1581 CrossRef CAS PubMed.
- A. Hejl, T. M. Trnka, M. W. Day and R. H. Grubbs, Chem. Commun., 2002, 2524–2525, 10.1039/B207903H.
- S. R. Caskey, M. H. Stewart, J. E. Kivela, J. R. Sootsman, M. J. A. Johnson and J. W. Kampf, J. Am. Chem. Soc., 2005, 127, 16750–16751 CrossRef CAS PubMed.
- M. H. Stewart, M. J. A. Johnson and J. W. Kampf, Organometallics, 2007, 26, 5102–5110 CrossRef CAS.
- P. E. Romero, W. E. Piers and R. McDonald, Angew. Chem., Int. Ed., 2004, 43, 6161–6165 CrossRef CAS PubMed.
- R. L. Miller, P. T. Wolczanski and A. L. Rheingold, J. Am. Chem. Soc., 1993, 115, 10422–10423 CrossRef CAS.
- S. R. Caskey, M. H. Stewart, M. J. A. Johnson and J. W. Kampf, Angew. Chem., Int. Ed., 2006, 45, 7422–7424 CrossRef CAS PubMed.
- Y. Mutoh, N. Kozono, M. Araki, N. Tsuchida, K. Takano and Y. Ishii, Organometallics, 2010, 29, 519–522 CrossRef CAS.
- M. L. Macnaughtan, M. J. A. Johnson and J. W. Kampf, J. Am. Chem. Soc., 2007, 129, 7708–7709 CrossRef CAS PubMed.
- Y. Chen, W. Petz and G. Frenking, Organometallics, 2000, 19, 2698–2706 CrossRef CAS.
- J. B. Gary, C. Buda, M. J. A. Johnson and B. D. Dunietz, Organometallics, 2008, 27, 814–826 CrossRef CAS.
- A. Krapp, K. K. Pandey and G. Frenking, J. Am. Chem. Soc., 2007, 129, 7596–7610 CrossRef CAS PubMed.
- A. Krapp and G. Frenking, J. Am. Chem. Soc., 2008, 130, 16646–16658 CrossRef CAS PubMed.
- T. J. Crevier, S. Lovell and J. M. Mayer, Chem. Commun., 1998, 2371–2372, 10.1039/a805277h.
- R. G. Wilkins, in Kinetics and Mechanism of Reactions of Transition Metal Complexes, Wiley-VCH Verlag GmbH & Co. KGaA, 1991, p. 236, DOI:10.1002/3527600825.ch4.
- U. Belluco, L. Cattalini, F. Basolo, R. G. Pearson and A. Turco, J. Am. Chem. Soc., 1965, 87, 241–246 CrossRef CAS.
- L. Vaska and J. W. DiLuzio, J. Am. Chem. Soc., 1961, 83, 2784–2785 CrossRef CAS.
- R. Brady, W. H. De Camp, B. R. Flynn, M. L. Schneider, J. D. Scott, L. Vaska and M. F. Werneke, Inorg. Chem., 1975, 14, 2669–2675 CrossRef CAS.
- M.-A. Guillevic, A. M. Arif, J. A. Gladysz and I. T. Horváth, Angew. Chem., Int. Ed. Engl., 1997, 36, 1612–1615 CrossRef CAS PubMed.
- L. Vaska and L. S. Chen, J. Chem. Soc. D, 1971, 1080–1081, 10.1039/c29710001080.
- M. Selke, W. L. Karney, S. I. Khan and C. S. Foote, Inorg. Chem., 1995, 34, 5715–5720 CrossRef CAS.
- F. Abu-Hasanayn, A. S. Goldman and K. Krogh-Jespersen, Inorg. Chem., 1994, 33, 5122–5130 CrossRef CAS.
- G. Pacchioni and P. S. Bagus, Inorg. Chem., 1992, 31, 4391–4398 CrossRef CAS.
- W.-H. Leung, J. L. C. Chim and W.-T. Wong, J. Chem. Soc., Dalton Trans., 1996, 3153–3154, 10.1039/dt9960003153.
- J. Chatt, C. D. Falk, G. J. Leigh and R. J. Paske, J. Chem. Soc. A, 1969, 2288–2293, 10.1039/J19690002288.
- G. Beuter, U. Englert and J. Strähle, Z. Naturforsch., B: J. Chem. Sci., 1988, 43, 145–148 CAS.
- R. Dantona, E. Schweda and J. Strähle, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1984, 39, 733–735 Search PubMed.
- C. Bach, H. Willner, F. Aubke, C. Wang, S. J. Rettig and J. Trotter, Angew. Chem., Int. Ed. Engl., 1996, 35, 1974–1976 CrossRef CAS PubMed.
- R. A. Wheeler, M. H. Whangbo, T. Hughbanks, R. Hoffmann, J. K. Burdett and T. A. Albright, J. Am. Chem. Soc., 1986, 108, 2222–2236 CrossRef CAS PubMed.
- A. Caselli, E. Solari, R. Scopelliti and C. Floriani, J. Am. Chem. Soc., 2000, 122, 538–539 CrossRef CAS.
- R. D. Young, A. F. Hill, G. E. Cavigliasso and R. Stranger, Angew. Chem., Int. Ed., 2013, 52, 3699–3702 CrossRef CAS PubMed.
- D. Mansuy, J. P. Lecomte, J. C. Chottard and J. F. Bartoli, Inorg. Chem., 1981, 20, 3119–3121 CrossRef CAS.
- V. L. Goedken, M. R. Deakin and L. A. Bottomley, J. Chem. Soc., Chem. Commun., 1982, 607–608, 10.1039/c39820000607.
- L. Galich, A. Kienast, H. Hückstädt and H. Homborg, Z. Anorg. Allg. Chem., 1998, 624, 1235–1242 CrossRef CAS.
- G. Rossi, V. L. Goedken and C. Ercolani, J. Chem. Soc., Chem. Commun., 1988, 46–47, 10.1039/c39880000046.
- A. Kienast, L. Galich, K. S. Murray, B. Moubaraki, G. Lazarev, J. D. Cashion and H. Homborg, J. Porphyrins Phthalocyanines, 1997, 1, 141–157 CrossRef CAS.
- E. Solari, S. Antonijevic, S. Gauthier, R. Scopelliti and K. Severin, Eur. J. Inorg. Chem., 2007, 2007, 367–371 CrossRef PubMed.
- F. Scherbaum, A. Grohmann, B. Huber, C. Krüger and H. Schmidbaur, Angew. Chem., Int. Ed. Engl., 1988, 27, 1544–1546 CrossRef PubMed.
- F. Scherbaum, A. Grohmann, G. Müller and H. Schmidbaur, Angew. Chem., Int. Ed. Engl., 1989, 28, 463–465 CrossRef PubMed.
- O. Steigelmann, P. Bissinger and H. Schmidbaur, Angew. Chem., Int. Ed. Engl., 1990, 29, 1399–1400 CrossRef PubMed.
- H. Schmidbaur, B. Brachthäuser, O. Steigelmann and H. Beruda, Chem. Ber., 1992, 125, 2705–2710 CrossRef CAS PubMed.
- F. P. Gabbaï, A. Schier, J. Riede and H. Schmidbaur, Chem. Ber., 1997, 130, 111–114 CrossRef PubMed.
- J.-H. Jia and Q.-M. Wang, J. Am. Chem. Soc., 2009, 131, 16634–16635 CrossRef CAS PubMed.
- J.-H. Jia, J.-X. Liang, Z. Lei, Z.-X. Cao and Q.-M. Wang, Chem. Commun., 2011, 47, 4739–4741 RSC.
- J. Bendix, C. Anthon, M. Schau-Magnussen, T. Brock-Nannestad, J. Vibenholt, M. Rehman and S. P. A. Sauer, Angew. Chem., Int. Ed., 2011, 50, 4480–4483 CrossRef CAS PubMed.
- L. Walz and P. Scheer, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1991, 47, 640–641 CrossRef.
- A. H. Reis, V. S. Hagley and S. W. Peterson, J. Am. Chem. Soc., 1977, 99, 4184–4186 CrossRef CAS.
- B. P. Andreini, D. Belli Dell'Amico, F. Calderazzo, M. G. Venturi and G. Pelizzi, J. Organomet. Chem., 1988, 354, 369–380 CrossRef CAS.
- D. R. Russell, P. A. Tucker and S. Wilson, J. Organomet. Chem., 1976, 104, 387–392 CrossRef CAS.
- P. G. Jones, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1982, 823–824 CAS.
- H. Willner and F. Aubke, Inorg. Chem., 1990, 29, 2195–2200 CrossRef CAS.
- M. Adelhelm, W. Bacher, E. G. Höhn and E. Jacob, Chem. Ber., 1991, 124, 1559–1561 CrossRef CAS PubMed.
- Q. Xu, Y. Imamura, M. Fujiwara and Y. Souma, J. Org. Chem., 1997, 62, 1594–1598 CrossRef CAS.
- H. Willner, J. Schaebs, G. Hwang, F. Mistry, R. Jones, J. Trotter and F. Aubke, J. Am. Chem. Soc., 1992, 114, 8972–8980 CrossRef CAS.
- J. Vibenholt, M. Magnussen, C. Anthon and J. Bendix, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2010, 66, m177–m179 CAS.
- S. Schwarz and J. Strähle, Z. Anorg. Allg. Chem., 2003, 629, 493–496 CrossRef CAS PubMed.
- D. Morrogh, M. Galceran Mestres, E. Niquet, C. F. Barboza da Silva, A. Santos Saez, S. Schwarz and J. Strähle, Z. Anorg. Allg. Chem., 2005, 631, 1113–1118 CrossRef CAS PubMed.
- S. Schwarz, M. Galceran Mestres, E. Niquet, C. F. Barboza da Silva and J. Strähle, Z. Naturforsch., B: J. Chem. Sci., 2004, 59, 167–173 CAS.
- S. Schwarz, E. Niquet, A. Santos Saez, M. Cots Pascual and J. Strähle, Z. Anorg. Allg. Chem., 2003, 629, 2479–2484 CrossRef CAS PubMed.
- D. Morrogh, S. Schwarz, C. Maichle-Mössmer and J. Strähle, Z. Anorg. Allg. Chem., 2006, 632, 801–806 CrossRef CAS PubMed.
- E. D. Hedegaard, M. Schau-Magnussen and J. Bendix, Inorg. Chem. Commun., 2011, 14, 719–721 CrossRef CAS PubMed.
- R. W. Marshman, J. M. Shusta, S. R. Wilson and P. A. Shapley, Organometallics, 1991, 10, 1671–1676 CrossRef CAS.
- C. M. Lutz, S. R. Wilson and P. A. Shapley, Organometallics, 2005, 24, 3350–3353 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional crystallographic details for 1–12, synthetic procedures, spectral data (NMR, Raman, IR), metrical data, and kinetic investigations. CCDC 1403006–1403017. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02077h |
| ‡ Cod = 1,5-cyclooctadiene, PNP+ = bis(triphenylphosphoranylidene)iminium, py = pyridine, dmso = dimethylsulfoxide, terpy = 2,2′:6′,2′′-terpyridine, 4′-Ph-terpy = 4′-phenyl-2,2′:6′,2′′-terpyridine. |
| This journal is © The Royal Society of Chemistry 2015 |