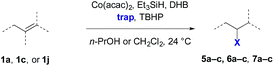Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer†
Xiaoshen
Ma
and
Seth B.
Herzon
*
Department of Chemistry, Yale University, New Haven, Connecticut 06520, USA. E-mail: seth.herzon@yale.edu
First published on 21st August 2015
Abstract
Classical methods for alkene hydrogenation typically reduce less-substituted or more-strained alkenes, or those in proximity to a directing group, most rapidly. Here we describe a cobalt-mediated hydrogenation protocol that provides complementary selectivities in the reduction of several classes of olefins and alkynes. The selectivity of this reduction derives from a hydrogen atom transfer mechanism, which favors the generation of the more stable alkylradical intermediate. We also report the first alkene hydrobromination, hydroiodination, and hydroselenylation by a hydrogen atom transfer process.
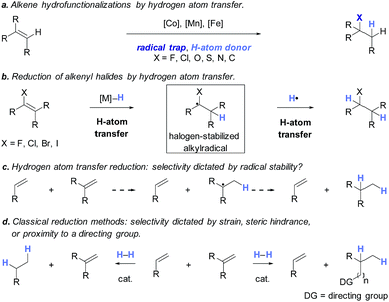
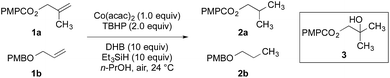
The application of transition metal hydrides as hydrogen atom donors to alkenes has been intensively studied.1,2 Early reports employed stoichiometric amounts of metal hydrides and activated alkenes.3–13 Recently a range of exceptionally useful alkene hydrofunctionalization reactions have been recorded by Mukaiyama, Carreira, Boger, Baran, and others using cobalt-, manganese-, and iron-based catalysts (Scheme 1a).14–42 Reports from our laboratory and Shenvi and co-workers detail methods for the reduction of alkenyl halides43,44 and unactivated alkenes44 by hydrogen atom transfer. Although prior examples of metal-catalyzed hydrogen atom transfer reduction had been described,45,46 these were the first to proceed with unactivated alkenes as substrates under mild conditions. In the reduction of alkenyl halides, the halogen substituent is thought to control selectivity by biasing the first hydrogen atom transfer toward the generation of a stabilized α-haloalkylradical intermediate (Scheme 1b). This mechanism avoids alkylmetal intermediates, which can lead to hydrodehalogenation products.47 Shenvi and co-workers subsequently reported a practical method for alkene isomerization and cycloisomerization by hydrogen atom transfer.48
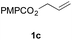
The rates of hydrogen atom transfer to alkenes depend upon the stability of the resulting alkylradical intermediate.49,50 These data and our alkenyl halide reduction led us to test whether the factors governing hydrogen atom transfer to alkenes could be exploited to obtain non-classical selectivities in alkene hydrogenation (Scheme 1c). Such selectivity would complement traditional approaches, which rely on the higher reactivity of strained and less-hindered alkenes51 or functional group coordination (Scheme 1d).52 However, for this approach to be successful, the steric encumbrance of the catalyst needs to be minimized to allow the radical-stabilizing effect to dominate.53
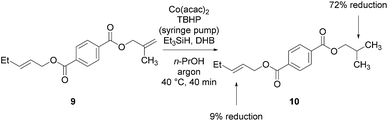
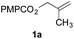
To facilitate analysis, 2-methylallyl 4-methoxybenzoate (1a) and an equimolar amount of allyl 4-methoxybenzyl ether (1b) were employed as substrates (Table 1). After some experimentation, we found that treatment of a solution of 1a and 1b in n-propanol with Co(acac)2 (1.0 equiv.), TBHP (2.0 equiv.), DHB (10 equiv.), and triethylsilane (10 equiv.) formed the products 2a and 2b in 71% and 14% yields after 135 min (5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 ratio of 2a
1.0 ratio of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b, entry 1). We attempted to improve the selectivity by decreasing the reaction temperature, but low conversion was observed (entry 2). Alternatively, when the amount of Co(acac)2 and TBHP were reduced to 50 mol%, the conversion of 1a was 72%, but only 31% of 2a was obtained, suggesting decomposition of the alkylradical (entry 3). The reactions in entries 1 and 2 were conducted under air in a flask sealed with a septum and pierced with a 16-gauge needle. Conducting the hydrogenation in an open flask enhanced the rate (30 vs. 135–180 min) but diminished selectivity (63% and 17% yield of 2a and 2b, respectively, entry 4). Interestingly, reducing the amount of Co(acac)2 and TBHP to 25 mol% decreased the conversion of 1b but the major product was the alcohol 3 (69%, entry 5). The basis for the difference in product selectivity between entries 4 and 5 is not known, but the production of 3 is consistent with earlier reports describing the formal Markovnikov hydration of alkenes by Mukaiyama and co-workers.15,19 We posited that higher selectivity and yields could be achieved under an inert atmosphere, as the catalyst would be less activated and the alkylradical intermediate would be less likely to undergo decomposition. When the reaction was conducted under argon, useful selectivities were observed (4.9
2b, entry 1). We attempted to improve the selectivity by decreasing the reaction temperature, but low conversion was observed (entry 2). Alternatively, when the amount of Co(acac)2 and TBHP were reduced to 50 mol%, the conversion of 1a was 72%, but only 31% of 2a was obtained, suggesting decomposition of the alkylradical (entry 3). The reactions in entries 1 and 2 were conducted under air in a flask sealed with a septum and pierced with a 16-gauge needle. Conducting the hydrogenation in an open flask enhanced the rate (30 vs. 135–180 min) but diminished selectivity (63% and 17% yield of 2a and 2b, respectively, entry 4). Interestingly, reducing the amount of Co(acac)2 and TBHP to 25 mol% decreased the conversion of 1b but the major product was the alcohol 3 (69%, entry 5). The basis for the difference in product selectivity between entries 4 and 5 is not known, but the production of 3 is consistent with earlier reports describing the formal Markovnikov hydration of alkenes by Mukaiyama and co-workers.15,19 We posited that higher selectivity and yields could be achieved under an inert atmosphere, as the catalyst would be less activated and the alkylradical intermediate would be less likely to undergo decomposition. When the reaction was conducted under argon, useful selectivities were observed (4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), but the conversion of 1a was incomplete (81%, entry 6). Warming to 50 °C increased conversion with only a minor decrease in selectivity (4.4
1.0), but the conversion of 1a was incomplete (81%, entry 6). Warming to 50 °C increased conversion with only a minor decrease in selectivity (4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 ratio of 2a
1.0 ratio of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b, entry 7). Slow addition of TBHP (syringe pump) provided a 91% yield of 2a with 4.6
2b, entry 7). Slow addition of TBHP (syringe pump) provided a 91% yield of 2a with 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 selectivity (entry 8). As the conditions of entry 1 provided the highest selectivity and those of entry 8 afforded the highest yield, both were employed in the investigation of the scope (referred to as conditions A and B, respectively). Other hydrogen atom donors were ineffective. Reduction using manganese tris(dipivaloylmethane)44 was non-selective (see Table S3†).
1.0 selectivity (entry 8). As the conditions of entry 1 provided the highest selectivity and those of entry 8 afforded the highest yield, both were employed in the investigation of the scope (referred to as conditions A and B, respectively). Other hydrogen atom donors were ineffective. Reduction using manganese tris(dipivaloylmethane)44 was non-selective (see Table S3†).
| Entry | Variation from above | Time | Conv. 1a | Yield 2a | Conv. 1b | Yield 2b |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
|---|---|---|---|---|---|---|---|
a Reactions employed 250 μmol each of 1a and 1b. Conversions and yields were determined by 1H NMR spectroscopy using mesitylene or 1,3,5-trimethoxybenzene as an internal standard.
b The ratio of 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b could not be determined due to the absence of 2a and/or 2b in the 1H NMR spectrum of the unpurified product mixture.
c 69% of 3 was isolated after purification by flash-column chromatography. 2b could not be determined due to the absence of 2a and/or 2b in the 1H NMR spectrum of the unpurified product mixture.
c 69% of 3 was isolated after purification by flash-column chromatography.
|
|||||||
| 1 | None | 135 min | >95% | 71% | 14% | 14% | 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 2 | 0 °C | 300 min | <5% | <1% | 7% | <1% | —b |
| 3 | Co(acac)2, TBHP (50 mol% each) | 180 min | 72% | 31% | 18% | <1% | —b |
| 4 | Open flask | 30 min | >95% | 63% | 56% | 17% | 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 5 | Co(acac)2, TBHP (25 mol% each), open flask | 180 min | 75% | 25% (69% of 3)c | 6% | <1% | —b |
| 6 | Argon | 360 min | 81% | 69% | 33% | 14% | 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 7 | Argon, 50 °C | 120 min | >95% | 80% | 25% | 18% | 4.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 8 | TBHP (1.0 equiv., slow addition), argon, 40 °C | 60 min | >95% | 91% | 28% | 20% | 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
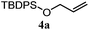
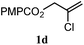
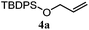
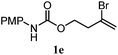
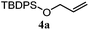
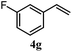
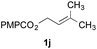
The experiments in Table 2 establish the relative reactivity of several alkene and alkene–alkyne pairs. For each substrate pair, the condition affording higher selectivities is shown (for complete data, see Table S2†). These data show that useful levels of selectivity can be obtained for eight pairs of unsaturated substrates. For example, entries 1 and 2 show that 2,2-disubstituted alkenes are reduced selectively over α-olefins, and that allylic substituents such as esters, bulky silyl ethers, or alkyl ethers do not significantly influence selectivity. The results in entries 3 and 4 show that bromo- and chloroalkenes are reduced more readily than α-olefins, which reflects the additional stabilization afforded by the halogen.54 It is noteworthy that reduction of the bromoalkene 1e is complete within 20 min while ∼2 h are required to achieve conversion of the dialkyl-substituted alkene 1a. The cyclic 2,2-disubstituted alkene 1f was also reduced with comparable selectivity over the α-olefin 4a (entry 5). In accord with these data and the mechanistic hypothesis shown in Scheme 1, 2, 2-disubstituted alkenes are reduced more readily than 1,2-disubstituted alkenes (entries 6 and 7). Heterogeneous hydrogenation catalysts typically reduce alkynes faster than alkenes,51 but this reduction method provides high levels of selectivity for 2,2-disubstituted alkenes over internal alkynes (entry 8). Trisubstituted alkenes are reduced with modest selectivity over α-olefins (entries 11 and 12), but are reduced with higher selectivities over trans- or cis-1,2-disubstituted alkenes (entries 13 and 14, respectively). Styrenyl and terminal arylalkynes undergo rapid decomposition to unidentified products (entries 15 and 16) and fluoroalkenes react slowly under these conditions (entry 17). To confirm that these conditions are effective in a polyfunctional setting, we evaluated the reduction of the diene 9 (Scheme 2). These conditions resulted in 72% reduction of the 2,2-disubstituted alkene and 9% reduction of the 1,2-disubstituted alkene (8.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 selectivity).
1.0 selectivity).
| Entry | Target substrate | Conditions and yielda | Competition substrate | Conversionb | Yieldb | Ratio of reduction products |
|---|---|---|---|---|---|---|
| a Yields refer to purified products isolated by flash-column chromatography, unless otherwise noted. Condition A: Co(acac)2 (1 equiv.), TBHP (1–8 equiv.), 1,4-DHB (10 equiv.), Et3SiH (10 equiv.), n-PrOH (0.3 M), air, 24 °C. Condition B: Co(acac)2 (1 equiv.), TBHP (0.97–1.28 equiv., slow addition), 1,4-DHB (10 equiv.), Et3SiH (10 equiv.), n-PrOH (0.3 M), argon, 40 °C. The amount of TBHP varies among substrates, see the ESI. b Determined by 1H NMR spectroscopy using mesitylene as an internal standard. c Competition substrate was converted to unidentified products. d Ratios are calculated as the yield of the target substrate versus the conversion of the competition substrate. e Conversion determined by 19F NMR with hexafluorobenzene as an internal standard. f Decomposition was observed. | ||||||
| 1 |
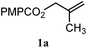
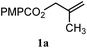
|
A: 79b% |
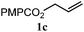
|
17% | 14% | 5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 2 |
|
A: 86% |
|
17% | 12% | 7.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 3 |
|
A: 79% |
|
15% | 15% | 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 4 |
|
A: 71% |
|
5% | —c | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0d 1.0d |
| 5 |
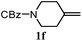
|
A: 78% |
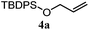
|
17% | 17% | 4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 6 |
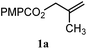
|
B: 96% |
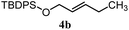
|
11% | 11% | 8.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 7 |
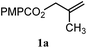
|
A: 70% |
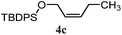
|
14% | 8% | 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 8 |
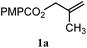
|
B: 93% |
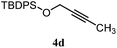
|
12% | —c | 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0d 1.0d |
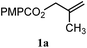
| 9 |
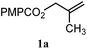
|
B: 89% |
|
30% | —c | 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0d 1.0d |
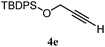
| 10 |
|
B: 90% |
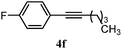
|
22e% | —c | 4.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0d 1.0d |
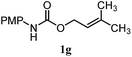
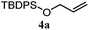
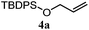
| 11 |
|
A: 92% |
|
46% | 46% | 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
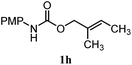
| 12 |
|
A: 95% |
|
64% | 64% | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 13 |
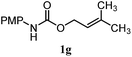
|
A: 86% |
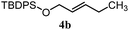
|
22% | 18% | 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
| 14 |
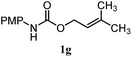
|
A: 90% |
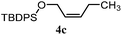
|
35% | 28% | 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
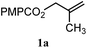
| 15 |
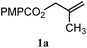
|
A: 95% |
|
93e% | —f | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0d 1.0d |
| 16 |
|
A: 55% |
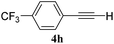
|
>95e% | —f | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7d 1.7d |
| 17 |
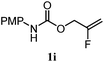
|
A: 62% |
|
82% | 83% | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
To benchmark these data, the relative reactivity of six classes of unsaturated substrates were examined under heterogeneous conditions (Table 3, for additional conditions, see Table S3†). As expected, the less-hindered alkene (or alkyne) was reduced preferentially. Thus, whereas classical hydrogenation conditions typically favor reaction of the most accessible (least-substituted) alkene, the hydrogen atom transfer reduction we have developed reverses this well-established trend.
| Co(acac)2 | H2/Pd–C | ||
|---|---|---|---|
| a For heterogeneous hydrogenation conditions, R = PMPCO2 (see the ESI). | |||
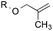
|
5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.4 4.4 |
|
|
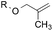
8.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9 |
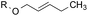
|
|
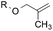
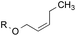
8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
|
|
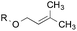
4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.0 8.0 |
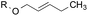
|
|
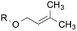
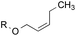
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
|
|
7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
|
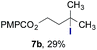
Finally, we extended these studies toward the first hydrobromination, hydroiodination, and hydroselenation reactions that proceed by hydrogen atom transfer (Table 4). These experiments find important precedent in the work of Carreira and co-workers, who developed the first hydrochlorination of alkenes by hydrogen atom transfer.32 Here we evaluated a range of bromine, iodine, and selenium atom donors under our hydrogen atom transfer conditions (Table S4†). We found that addition of p-toluenesulfonyl bromide, diiodomethane, or Se-phenyl 4-methylbenzenesulfonoselenoate formed the desired hydrofunctionalization products. The hydrobromination and hydroselenation reactions provided high yields of products for α-, 2,2-, and trisubstituted olefins, but the hydroiodination of α- and trisubstituted alkenes did not proceed to completion. Application of the hydrobromination reaction to alkenyl halides formed the geminal dihalides 8a and 8b in high yield.
| Substrate | Products | ||
|---|---|---|---|
| a Yields refer to purified products isolated by flash-column chromatography. Hydrobromination: Co(acac)2 (1 equiv.), TBHP (1 equiv.), 1,4-DHB (3.75 equiv. for unfunctionalized alkenes, omitted for alkenyl halides), Et3SiH (10 equiv.), tosyl bromide (2.5 equiv.), n-PrOH (0.3 M) for unfunctionalized alkenes, DCM (0.3 M) for alkenyl halides, argon, 24 °C. Hydroiodination: Co(acac)2 (1 equiv.), TBHP (1 equiv.), 1,4-DHB (3.75 equiv.), Et3SiH (10 equiv.), diiodomethane (15 equiv.), DCM (0.3 M), argon, 24 °C. Hydroselenation: Co(acac)2 (1 equiv.), TBHP (1 equiv.), 1,4-DHB (3.75 equiv.), Et3SiH (10 equiv.), Se-phenyl 4-methylbenzenesulfonoselenoate (2.5 equiv.), n-PrOH (0.3 M), argon, 24 °C. | |||
|
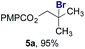
|
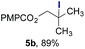
|
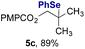
|
|
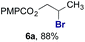
|
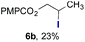
|
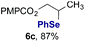
|
|
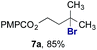
|
|
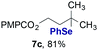
|
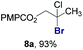
|
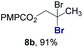
|
||
Conclusions
In summary, we have shown that hydrogen atom transfer reduction provides selectivities that complement classical methods in the reduction of several alkene and alkene–alkyne pairs. In addition, we have described the first hydrobromination, hydroiodination, and hydroselenation of alkenes that proceed by hydrogen atom transfer. We believe that these methods constitute useful additions to the burgeoning area of practical hydrogen atom transfer reactions.Acknowledgements
Financial support from the National Science Foundation (CHE-1151563) is gratefully acknowledged.Notes and references
- D. C. Eisenberg and J. R. Norton, Isr. J. Chem., 1991, 31, 55–66 CrossRef CAS PubMed.
- A. Gansäuer, L. Shi, M. Otte, I. Huth, A. Rosales, I. Sancho-Sanz, N. Padial and J. E. Oltra, Hydrogen Atom Donors: Recent Developments. Radicals in Synthesis III, Springer, Berlin Heidelberg, 2012 Search PubMed.
- R. L. Sweany and J. Halpern, J. Am. Chem. Soc., 1977, 99, 8335–8337 CrossRef CAS.
- J. A. Roth and M. Orchin, J. Organomet. Chem., 1979, 182, 299–311 CrossRef CAS.
- R. L. Sweany, D. S. Comberrel, M. F. Dombourian and N. A. Peters, J. Organomet. Chem., 1981, 216, 37–63 CrossRef.
- T. E. Nalesnik, J. H. Freudenberger and M. Orchin, J. Mol. Catal., 1982, 16, 43–49 CrossRef CAS.
- J. A. Roth, P. Wiseman and L. Ruszala, J. Organomet. Chem., 1982, 240, 271–275 CrossRef CAS.
- F. Ungvary and L. Marko, Organometallics, 1982, 1, 1120–1125 CrossRef CAS.
- J. W. Connolly, Organometallics, 1984, 3, 1333–1337 CrossRef CAS.
- J. F. Garst, T. M. Bockman and R. Batlaw, J. Am. Chem. Soc., 1986, 108, 1689–1691 CrossRef CAS.
- M. J. Thomas, T. A. Shackleton, S. C. Wright, D. J. Gillis, J. P. Colpa and M. C. Baird, J. Chem. Soc., Chem. Commun., 1986, 312–314 RSC.
- B. Wassink, M. J. Thomas, S. C. Wright, D. J. Gillis and M. C. Baird, J. Am. Chem. Soc., 1987, 109, 1995–2002 CrossRef CAS.
- J. Choi, M. E. Pulling, D. M. Smith and J. R. Norton, J. Am. Chem. Soc., 2008, 130, 4250–4252 CrossRef CAS PubMed.
- A. Nishinaga, T. Yamada, H. Fujisawa, K. Ishizaki, H. Ihara and T. Matsuura, J. Mol. Catal., 1988, 48, 249–264 CrossRef CAS.
- T. Mukaiyama, S. Isayama, S. Inoki, K. Kato, T. Yamada and T. Takai, Chem. Lett., 1989, 449–452 CrossRef CAS.
- S. Inoki, K. Kato, T. Takai, S. Isayama, T. Yamada and T. Mukaiyama, Chem. Lett., 1989, 515–518 CrossRef CAS.
- S. Isayama and T. Mukaiyama, Chem. Lett., 1989, 569–572 CrossRef CAS.
- S. Isayama and T. Mukaiyama, Chem. Lett., 1989, 573–576 CrossRef CAS.
- S. Isayama and T. Mukaiyama, Chem. Lett., 1989, 1071–1074 CrossRef CAS.
- K. Kato, T. Yamada, T. Takai, S. Inoki and S. Isayama, Bull. Chem. Soc. Jpn., 1990, 63, 179–186 CrossRef CAS.
- S. Isayama, Bull. Chem. Soc. Jpn., 1990, 63, 1305–1310 CrossRef CAS.
- K. Kato and T. Mukaiyama, Chem. Lett., 1992, 1137–1140 CrossRef CAS.
- P. Magnus, A. H. Payne, M. J. Waring, D. A. Scott and V. Lynch, Tetrahedron Lett., 2000, 41, 9725–9730 CrossRef CAS.
- J. Waser and E. M. Carreira, J. Am. Chem. Soc., 2004, 126, 5676–5677 CrossRef CAS PubMed.
- J. Waser and E. M. Carreira, Angew. Chem., Int. Ed., 2004, 43, 4099–4102 CrossRef CAS PubMed.
- J. Waser, J. C. González-Gómez, H. Nambu, P. Huber and E. M. Carreira, Org. Lett., 2005, 7, 4249–4252 CrossRef CAS PubMed.
- J. Waser, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2005, 127, 8294–8295 CrossRef CAS PubMed.
- T. Tokuyasu, S. Kunikawa, K. J. McCullough, A. Masuyama and M. Nojima, J. Org. Chem., 2005, 70, 251–260 CrossRef CAS PubMed.
- J. Waser, B. Gaspar, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2006, 128, 11693–11712 CrossRef CAS PubMed.
- B. Gaspar and E. M. Carreira, Angew. Chem., Int. Ed., 2007, 46, 4519–4522 CrossRef CAS PubMed.
- B. Gaspar, J. Waser and E. M. Carreira, Synthesis, 2007, 3839–3845 CAS.
- B. Gaspar and E. M. Carreira, Angew. Chem., Int. Ed., 2008, 47, 5758–5760 CrossRef CAS PubMed.
- B. Gaspar and E. M. Carreira, J. Am. Chem. Soc., 2009, 131, 13214–13215 CrossRef CAS PubMed.
- T. Taniguchi, N. Goto, A. Nishibata and H. Ishibashi, Org. Lett., 2010, 12, 112–115 CrossRef CAS PubMed.
- V. Girijavallabhan, C. Alvarez and F. G. Njoroge, J. Org. Chem., 2011, 76, 6442–6446 CrossRef CAS PubMed.
- E. K. Leggans, T. J. Barker, K. K. Duncan and D. L. Boger, Org. Lett., 2012, 14, 1428–1431 CrossRef CAS PubMed.
- T. J. Barker and D. L. Boger, J. Am. Chem. Soc., 2012, 134, 13588–13591 CrossRef CAS PubMed.
- H. Shigehisa, T. Aoki, S. Yamaguchi, N. Shimizu and K. Hiroya, J. Am. Chem. Soc., 2013, 135, 10306–10309 CrossRef CAS PubMed.
- H. Shigehisa, E. Nishi, M. Fujisawa and K. Hiroya, Org. Lett., 2013, 15, 5158–5161 CrossRef CAS PubMed.
- J. C. Lo, Y. Yabe and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 1304–1307 CrossRef CAS PubMed.
- J. C. Lo, J. Gui, Y. Yabe, C.-M. Pan and P. S. Baran, Nature, 2014, 516, 343–348 CrossRef CAS PubMed.
- J. Gui, C.-M. Pan, Y. Jin, T. Qin, J. C. Lo, B. J. Lee, S. H. Spergel, M. E. Mertzman, W. J. Pitts, T. E. la Cruz, M. A. Schmidt, N. Darvatkar, S. R. Natarajan and P. S. Baran, Science, 2015, 348, 886–891 CrossRef CAS PubMed.
- S. M. King, X. Ma and S. B. Herzon, J. Am. Chem. Soc., 2014, 136, 6884–6887 CrossRef CAS PubMed.
- K. Iwasaki, K. K. Wan, A. Oppedisano, S. W. M. Crossley and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 1300–1303 CrossRef CAS PubMed.
- S.-K. Chung, J. Org. Chem., 1979, 44, 1014–1016 CrossRef CAS.
- P. Magnus, M. J. Waring and D. A. Scott, Tetrahedron Lett., 2000, 41, 9731–9733 CrossRef CAS.
- A. A. Peterson, K. A. Thoreson and K. McNeill, Organometallics, 2009, 28, 5982–5991 CrossRef CAS.
- S. W. M. Crossley, F. Barabé and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 16788–16791 CrossRef CAS PubMed.
- L. Tang, E. T. Papish, G. P. Abramo, J. R. Norton, M.-H. Baik, R. A. Friesner and A. Rappé, J. Am. Chem. Soc., 2003, 125, 10093–10102 CrossRef CAS PubMed.
- J. Choi, L. Tang and J. R. Norton, J. Am. Chem. Soc., 2007, 129, 234–240 CrossRef CAS PubMed.
- R. L. Augustine, Heterogeneous Catalysis for the Synthetic Chemist, Taylor & Francis, 1995 Search PubMed.
- A. H. Hoveyda, D. A. Evans and G. C. Fu, Chem. Rev., 1993, 93, 1307–1370 CrossRef CAS.
- For example, the bulky complex manganese tris(dipivaloylmethane) mediates the reduction of α-olefins faster than 2,2-disubstituted or trisubstituted alkenes, despite the lower stability of the secondary alkylradical intermediate, see ref. 44.
- Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, Boca Raton, 2007 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterization data for all new compounds. See DOI: 10.1039/c5sc02476e |
| This journal is © The Royal Society of Chemistry 2015 |