Poly(3,4-dinitrothiophene)/SWCNT composite as a low overpotential hydrogen evolution metal-free catalyst†
Ke
Xie
,
Haiping
Wu
,
Yuena
Meng
,
Kun
Lu
,
Zhixiang
Wei
* and
Zhong
Zhang
*
National Center for Nanoscience and Technology, No.11 Beiyitiao, Zhongguancun, Beijing 100190, P. R. China. E-mail: weizx@nanoctr.cn; zhong.zhang@nanoctr.cn
First published on 30th October 2014
Abstract
A novel metal-free hydrogen evolution reaction catalyst made of poly(3,4-dinitrothiophene)/SWCNT was developed. This catalyst presents a good hydrogen evolution reaction activity with a lower overpotential than metallic catalysts. Its performance was optimized to an overpotential of ca. 0.040 V and a hydrogen generation rate of 44.2 μmol h−1 cm−2.
The global climate change and the rapidly diminishing reservoir of fossil fuels drive people to seek sustainable energy resources. As a consequence, solar and wind energies, coupled with the corresponding energy storage techniques, which are used to deal with the temporal and spatial variability of these types of energy, are attracting increasing world-wide interest.1 Electrochemically splitting water into hydrogen and oxygen to store chemical bond energy is a particularly promising way among various energy storage techniques, because the energy density stored in chemical bonds is high and the energy storing–releasing process is completely carbon-free.2 To integrate water splitting cells, the development of high performance (i.e. low overpotential and high exchange current) hydrogen and oxygen evolution catalysts is the most important scientific issue.3
Platinum and its composites have been commonly used in hydrogen evolution reactions (HERs) due to the superior electro-catalytic performance of Pt.4 However, the high price, low availability and carbon monoxide sensitivity of Pt preclude Pt-based water-splitting from being a true sustainable energy storage technique.1a,3b Therefore, non-precious metal alternatives, such as MoS(Se)x,5 Mo2C,2b,6a,b WS2,6c WC,2b,6d and Ni2P6e,f with overpotentials ranging from 0.110 to 0.150 V, have been intensively investigated as HER catalysts in acidic solution. These metallic catalysts usually suffer from corrosion and passivation in acidic electrolyte, which led to the very recent development of a non-transition metal catalyst (C60(OH)8 anion) and metal-free ones (C3N4@nitrogen-doped graphene and nitrogen–phosphorus dual-doped graphene) with onset overpotentials of ca. 0.110, 0.150 and 0.350 V, respectively.7 More metal-free catalysts need to be developed to provide possible candidates as alternatives to precious metal catalysts. Poly(3,4-ethylenedioxythiophene) (PEDOT) is a kind of polythiophene derivative with electron-donating groups. The HER activity of PEDOT has been achieved by successfully combining the electrocatalytic activity of PEDOT and the ability of polyethylene glycol (PEG) to coordinate protons.8 Strong electron-withdrawing groups in polythiophene derivatives are expected to further decrease the overpotential for HER.
In this work, we report the synthesis and characterization of a novel metal-free HER catalyst, poly(3,4-dinitrothiophene) (PDNT). The catalyst was loaded onto single-walled carbon nanotubes (SWCNT) for the purpose of increasing the effective surface area and overall conductivity. The hydrogen gas evolution rate was measured using gas chromatography (GC). The electron-withdrawing nitro groups on the thiophene rings improve their ability to take up electrons and eventually lead to the low HER overpotential of ca. 0.040 V. PDNT/SWCNT composites were prepared via the one-step in situ Yamamoto polymerization9 of 2,5-dibromo-3,4-dinitrothiophene with SWCNT in DMF, as displayed in Scheme 1.
By adding different amounts of SWCNT, the content of PDNT in the composites could be adjusted, as measured using thermogravimetric analysis (see Fig. S1 in ESI†). The products are referred to as PDNT0, PDNT1, PDNT2 or PDNT3, corresponding to the PDNT content in the products as 100%, 13.3%, 41.9% or 62.6%, respectively. Typical scanning electron microscope (SEM) images of these composites are shown in Fig. 1a–d. The pure PDNT was obtained as aggregated particle clusters with sizes of ca. 0.2–0.5 μm, as seen in Fig. 1a. Observed in Fig. 1b (PDNT1), Fig. 1c and S2a† (PDNT2), and Fig. 1d (PDNT3), the sizes of the PDNT clusters attached to SWCNT range from ca. 0.2 μm to ca. 0.4 μm, consistent with those of PDNT in Fig. 1a.
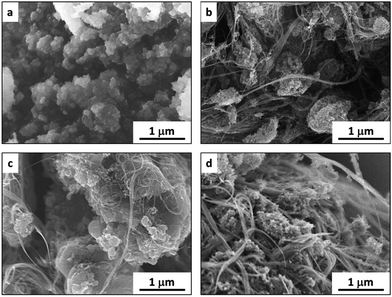 | ||
| Fig. 1 SEM images showing the morphologies of pure PDNT (a, PDNT0), and PDNT/SWCNT with a PDNT content of 13.3% (b, PNDT1), 41.9% (c, PDNT2) and 62.6% (d, PDNT3). | ||
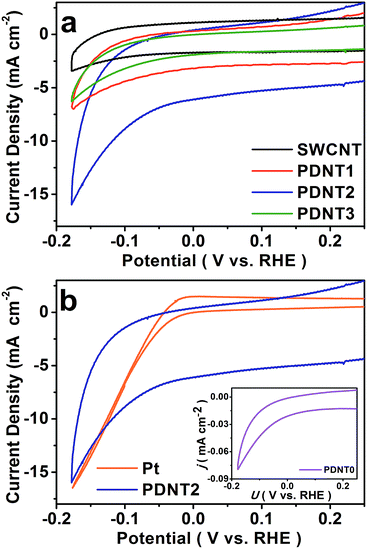
The HER performance of PDNT0 to PDNT3 and SWCNT deposited on glassy carbon electrodes was firstly investigated using cyclic voltammetry (CV) in 1 mol L−1 H2SO4 using a three-electrode setup. The HER performance of the Pt plate was also measured as the reference for the overpotential, since Pt possesses high HER activity with nearly zero overpotential.5a,e,8 The corresponding curves are illustrated in Fig. 2a and b, which reflect the 10th cycle of the samples with stable results. As seen in Fig. 2a, the CV curve of SWCNT shows the typical electrochemical double layer feature with negligible HER activity, while all the other samples exhibit a close HER onset potential at ca. −0.032 V vs. the reversible hydrogen electrode (RHE), ca. 0.040 V lower than that of Pt (ca. +0.008 V vs. RHE) measured under the same conditions as shown in Fig. 2b. Accordingly, the HER overpotential of PDNT is ca. 0.040 V, lower than those of non-precious metal catalysts in acidic electrolyte.5a,c,e,6c,e
As seen from the inset in Fig. 2b, PDNT0 presents HER activity despite the polarization current density being as low as 0.05 mA cm−2, implying insulation of the pure PDNT particles. In addition, as the PDNT content rises, the polarization current densities of the samples at −0.177 V vs. RHE firstly increase from 3.3 mA cm−2 (PDNT1) to 8.3 mA cm−2 (PDNT2), and then decrease to 3.1 mA cm−2 (PDNT3). A similar pattern is observed for the background current densities of those materials, which are influenced by both the electrochemical double layer capacitance from SWCNT and the pseudo-capacitance from the polythiophene derivative.11 The increase in polarization and background current from PDNT1 to PDNT2 is ascribed to the content increment of PDNT, which possesses HER activity and a capacitance higher than those of SWCNT. Further incremental increases in the PDNT content lead to a drop in both polarization and background current from PDNT2 to PDNT3 because the insulation of PDNT lowers the overall conductivity of the composite. The onset overpotential of ca. 0.040 V for PDNT2 is lower than those of the reported metal-free and transition metal based catalysts (e.g., sulfide and carbide of Mo and W) whose typical onset overpotentials range from 0.110 to 0.200 V (see ESI Table S1†). And the polarization current density of 8.3 mA cm−2 at a potential of −0.177 V vs. RHE (0.145 V lower than the onset potential of PDNT2) is also comparable to recent results of HER catalysts without a transition metal.7,8
The electrochemical kinetics of PDNT0 and PDNT2 on glassy carbon electrodes were studied using electrochemical impedance spectroscopy (EIS) in the range of 0.01–5 × 105 Hz. The corresponding Nyquist plots at typical potentials are displayed in Fig. 3a and b. The Nyquist plots of both samples show little difference in potentials that are higher than the HER onset potential of PDNT (0.223, 0.123 and 0.023 V vs. RHE), indicating similar electrode kinetics at those potentials. Further decrease of the potential from 0.023 to −0.077 V vs. RHE resulted in a dramatic reduction of the charge transfer resistance (Rct) for both PDNT0 and PDNT2 due to an evocation in HER, which is in agreement with the onset potential of ca. −0.032 V vs. RHE obtained using CV. Moreover, the HER Rct of PDNT2 is lower than that of PDNT0, as concluded from the Nyquist plots at −0.077 and −0.177 V vs. RHE.6c,d SWCNTs in the composite have basically two functions. Firstly, the SWCNT framework serves as the “electric cable framework” which transports electrons from an external power source to PDNT for HER catalysis. Furthermore, SWCNTs were used to anchor PDNT particles by π–π interaction and in this way disperse them within the framework, suppressing the aggregation of PDNT particles. The aggregation of nano-sized catalysts usually decreases their activity by reducing the accessibility of the catalyst’s surface. Therefore, in the case of PDNT2, the protons in the electrolyte are more likely to coordinate to the active site and hence it is easier for the electrons to move from PDNT to the protons, both of which result in lower Rct. The intercept of the Nyquist plot on the x-axis could be used to evaluate the system's equivalent series resistance (ESR), which is predominated by the conductivity of the electrode materials.12 From the Nyquist plots of PDNT0 and PDNT2 in the high-frequency region as illustrated in Fig. 3c and d, it is seen that the ESR of PDNT2 (8 ohm) is much lower than that of PDNT0 (350–400 ohm), demonstrating a much higher overall conductivity of PDNT2. This result is again consistent with that of the CV measurements. As observed, the SWCNTs in the composite significantly enhance the HER activity of PDNT by increasing the conductivity of the electrode and the effective surface area of the PDNT particles.
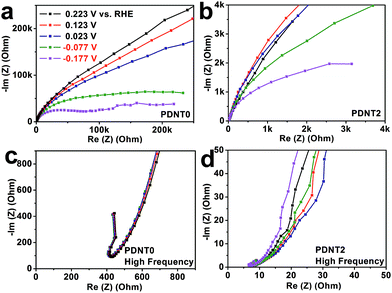 | ||
| Fig. 3 Nyquist plots from electrochemical impedance spectroscopy (EIS) of PDNT0 (a and c) and PDNT2 (b and d) at different potentials. | ||
To evaluate the performance of PDNT2 in a real system, the hydrogen gas evolution rates and electrochemical stability of PDNT2 were estimated using GC coupled with a static potential I–t test. As shown in Fig. 4, the current density (black line) firstly decayed from 7.50 to 1.08 mA cm−2 within ca. 1600 seconds and then remained nearly constant for the next ca. 5600 seconds at an operating potential of −0.177 V vs. RHE. The hydrogen gas evolution rate at this potential was 20.1 μmol h−1 cm−2, according to the fitted slope of the “normalized H2 amount–time plot” (red dots) in the stabilized part of this measurement (i.e. 1600–7200 s). After that, the potential applied to the electrode was changed to −0.277 V vs. RHE. The current density immediately leapt to 7.09 mA cm−2 and then progressively decayed to a stable value of 2.41 mA cm−2 during ca. 500 seconds. The hydrogen gas evolution rate at this potential achieved 44.2 mol h−1 cm−2. Typically the HER catalysts that contain organic groups (e.g., Ni and Co complexes) present Faraday efficiencies in the range of 20–95%,2a,3b which are lower than the nearly 100% Faraday efficiencies of inorganic HER catalysts,5g,10 probably due to some electrochemical side reactions (e.g., reduction of –NO2 groups). In this study, PDNT2 presented high Faraday efficiencies of 93.5% and 92.4% for −0.177 and −0.277 V vs. RHE, respectively, which are comparable with the highest value among catalysts based on organic compounds.2a,3b
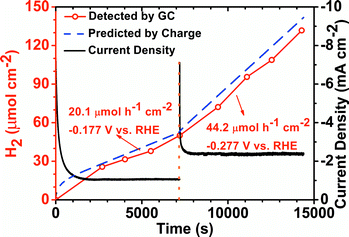 | ||
| Fig. 4 HER stability of PDNT2 measured using gas chromatography and static potential I–t at the potential indicated. | ||
The electron-withdrawing groups on the thiophene rings usually result in a decrease in the LUMO level of polythiophene, leading to an increase in the n-doping (reduction) potential, as widely reported.11b,13 In our case, there are two nitro groups, which are among the strongest inductive groups, on the thiophene ring. Therefore, the LUMO level is dramatically decreased, which improves the capability of PDNT to grasp electrons from an external power source at a relatively high potential, resulting in a negatively charged PDNT. The n-doping potential of PDNT2 was obtained from the CV measurement in organic electrolyte, as illustrated in Fig. S4 of the ESI.† As can be seen, the onset n-doping potential is −0.51 V vs. Ag+/Ag, which is, as we know, higher than that of any other polythiophene derivative under similar conditions.13 In other words, in the case of PDNT, the electrons transfer much easier from the external power source to PDNT and then are used to reduce the protons compared to other polythiophene derivatives. Moreover, negative charges on PDNT also improve the absorption of protons due to electrostatic forces, which is a critical step in the HER process.5,7a This is supported by the fact that the onset overpotential of pure PEDOT (∼0.5 V) is much higher than that of pure PDNT (∼0.04 V).8 On the other hand, this n-doping reaction is a potential initiation of PDNT chain–chain radical crosslinking, which leads to its deactivation in HER. Anchored on SWCNT, these radicals are stabilized due to the delocalization effect of the sp2 hybridized carbon atoms. So, the SWCNT can also enhance the stability of the catalyst. The pH of the electrolyte is another important factor for HER. In general, inorganic HER catalysts are used in acidic solutions because of their passivation in neutral or alkaline solutions which results from the surface oxidation of these materials.2b According to our study, the PDNT based catalysts are also incompatible with conventional neutral (KCl, NaCl) or alkaline (KOH, NaOH) electrolytes since their HER performance decays quickly during the electrochemical measurements in those electrolytes. This should be ascribed to the irreversible doping of alkali ions into the polythiophene matrix at a low potential.11b
Although the aggregation of PDNT particles was successfully suppressed to some degree (according to the improved performance from PDNT0 to PDNT1–3), it seems that the PDNT particles still have a tendency to aggregate into micron-sized clusters in the framework of SWCNT (see Fig. 1b and c and S2†). As a consequence, some of the PDNT might not be accessible for HER, and there is still potential to optimize the morphology and performance of PDNT based catalysts. In future, we can further improve the performance of PDNT based HER catalysts by optimizing the preparation process and using other carbonaceous supports such as graphene or surface functionalized CNTs.
In summary, PDNT/SWCNT composites are prepared via a one-step in situ Yamamoto polymerization of 2,5-dibromo-3,4-dinitrothiophene with SWCNT and applied as metal-free HER catalysts in 1 mol L−1 H2SO4. The polarization current could be tuned by regulating the ratio of SWCNT in the composites, which improves the overall performance by increasing the conductivity of the electrode and the effective surface area of PDNT. The HER performance of the PDNT/SWCNT composites has been optimized for PDNT2 with an overpotential of ca. 0.04 V and polarization current density of 8.3 mA cm−2 at the scan rate of 0.020 V s−1. The influence of electron-withdrawing groups demonstrated in this work could be used to develop and optimize other high efficiency metal-free HER catalysts.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 21125420), the Ministry of Science and Technology of China (no. 2012CB933001) and the Chinese Academy of Sciences.Notes and references
- (a) F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294 RSC; (b) Z. Li, W. Luo, M. Zhang, J. Feng and Z. Zou, Energy Environ. Sci., 2013, 6, 347 RSC.
- (a) V. Artero, M. Chavarot-Kerlidou and M. Fontecave, Angew. Chem., Int. Ed., 2011, 50, 7238 CrossRef CAS PubMed; (b) W. F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896 RSC.
- (a) A. B. Laursen, S. Kegnæs, S. Dahl and I. Chorkendorff, Energy Environ. Sci., 2012, 5, 5577 RSC; (b) V. S. Thoi, Y. Sun, J. R. Long and C. J. Chang, Chem. Soc. Rev., 2013, 42, 2388 RSC; (c) J. Yang and H. S. Shin, J. Mater. Chem. A, 2014, 2, 5979 RSC.
- (a) D. V Esposito, S. T. Hunt, A. L. Stottlemyer, K. D. Dobson, B. E. McCandless, R. W. Birkmire and J. G. Chen, Angew. Chem., Int. Ed., 2010, 49, 9859 CrossRef PubMed; (b) N. Danilovic, R. Subbaraman, D. Strmcnik, K. C. Chang, A. P. Paulikas, V. R. Stamenkovic and N. M. Markovic, Angew. Chem., Int. Ed., 2012, 51, 12495 CrossRef CAS PubMed; (c) D. V Esposito, S. T. Hunt, Y. C. Kimmel and J. G. Chen, J. Am. Chem. Soc., 2012, 134, 3025 CrossRef PubMed; (d) R. Subbaraman, D. Tripkovic, D. Strmcnik, K. C. Chang, M. Uchimura, A. P. Paulikas, V. Stamenkovic and N. M. Markovic, Science, 2011, 334, 1256 CrossRef CAS PubMed.
- (a) Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296 CrossRef CAS PubMed; (b) J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963 CrossRef CAS PubMed; (c) T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100 CrossRef CAS PubMed; (d) B. Hinnemann, P. G. Moses, J. Bonde, K. P. Jørgensen, J. H. Nielsen, S. Horch, I. Chorkendorff and J. K. Nørskov, J. Am. Chem. Soc., 2005, 127, 5308 CrossRef CAS PubMed; (e) M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. Li and S. Jin, J. Am. Chem. Soc., 2013, 135, 10274 CrossRef CAS PubMed; (f) H. Tang, K. Dou, C. C. Kaun, Q. Kuang and S. Yang, J. Mater. Chem. A, 2014, 2, 360 RSC; (g) Y. H. Chang, C. T. Lin, T. Y. Chen, C. L. Hsu, Y. H. Lee, W. Zhang, K. H. Wei and L. J. Li, Adv. Mater., 2013, 25, 756 CrossRef CAS PubMed; (h) J. Wang, Z. Guan, J. Huang, Q. Li and J. Yang, J. Mater. Chem. A, 2014, 2, 7960 RSC.
- (a) W. F. Chen, C. H. Wang, K. Sasaki, N. Marinkovic, W. Xu, J. T. Muckerman, Y. Zhu and R. R. Adzic, Energy Environ. Sci., 2013, 6, 943 RSC; (b) N. S. Alhajri, D. H. Anjum and K. Takanabe, J. Mater. Chem. A, 2014, 2, 10548 RSC; (c) D. Voiry, H. Yamaguchi, J. Li, R. Silva, D. C. B. Alves, T. Fujita, M. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nat. Mater., 2013, 12, 850 CrossRef CAS PubMed; (d) Y. Yan, B. Xia, X. Qi, H. Wang, R. Xu, J. Y. Wang, H. Zhang and X. Wang, Chem. Commun., 2013, 49, 4884 RSC; (e) E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267 CrossRef CAS PubMed; (f) P. Liu and J. A. Rodriguez, J. Am. Chem. Soc., 2005, 127, 14871 CrossRef CAS PubMed.
- (a) J. Zhuo, T. Wang, G. Zhang, L. Liu, L. Gan and M. Li, Angew. Chem., Int. Ed., 2013, 60, 10867 CrossRef PubMed; (b) Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 3783 Search PubMed; (c) Y. Zheng, Y. Jiao, L. H. Li, T. Xing, Y. Chen, M. Jaroniec and S. Z. Qiao, ACS Nano, 2014, 8, 5290 CrossRef CAS PubMed.
- B. Winther-Jensen, K. Fraser, C. Ong, M. Forsyth and D. R. MacFarlane, Adv. Mater., 2010, 22, 1727 CrossRef CAS PubMed.
- (a) R. D. McCullough, Adv. Mater., 1998, 10, 93 CrossRef CAS; (b) T. Yamamoto, Y. Miyazaki, T. Maruyama, H. Wakayama, Z. Zhou, Y. Nakamura, T. Kanbara, S. Sasaki and K. Kubota, Macromolecules, 1992, 25, 1214 CrossRef CAS; (c) J. Schmidt, M. Werner and A. Thomas, Macromolecules, 2009, 42, 4426 CrossRef CAS.
- P. D. Tran, M. Nguyen, S. S. Pramana, A. Bhattacharjee, S. Y. Chiam, J. Fize, M. J. Field, V. Artero, L. H. Wong, J. Loo and J. Barber, Energy Environ. Sci., 2012, 5, 8912 CAS.
- (a) B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481 CrossRef; (b) G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1 CrossRef CAS PubMed; (c) G. Lota, K. Fic and E. Frackowiak, Energy Environ. Sci., 2011, 4, 1592 RSC.
- Y. Qiu, X. Zhang and S. Yang, Phys. Chem. Chem. Phys., 2011, 13, 12554 RSC.
- (a) A. Rudge, I. Raistrick, S. Gottesfeld and J. P. Ferraris, Electrochim. Acta, 1994, 39, 273 CrossRef CAS; (b) A. Rudge, J. Davey, I. Raistrick, S. Gottesfeld and J. P. Ferraris, J. Power Sources, 1994, 47, 89 CrossRef CAS; (c) C. Arbizzani, M. Catellani, M. Mastrawstino and C. Migazzini, Electrochim. Acta, 1995, 40, 1871 CrossRef CAS; (d) P. Soudan, P. Lucas, H. A. Ho, D. Jobin, L. Breau and D. Bélanger, J. Mater. Chem., 2001, 11, 773 RSC; (e) F. Fusalba, H. A. Ho, L. Breau and D. Bélanger, Chem. Mater., 2000, 12, 2581 CrossRef CAS; (f) M. D. Levi and D. Aurbach, J. Power Sources, 2008, 180, 902 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, comparison of HER performance, thermogravimetric analysis data of catalysts, additional electron microscopy characterization, FT-IR spectrum of PDNT0 and CV measurement of PDNT2 in organic electrolyte. See DOI: 10.1039/c4ta04671d |
| This journal is © The Royal Society of Chemistry 2015 |