Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility†
Wang
Lin‡
abc,
Chen
Junjian‡
ab,
Cai
Chengzhi
c,
Shi
Lin
ab,
Liu
Sa
ab,
Ren
Li
*ab and
Wang
Yingjun
*ab
aNational Engineering Research Center for Tissue Restoration and Reconstruction, South China University of Technology, Guangzhou 510006, China. E-mail: psliren@scut.edu.cn
bSchool of Materials Science and Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: imwangyj@163.com
cDepartment of Chemistry, University of Houston, Houston, Texas 77204-5003, USA
First published on 28th October 2014
Abstract
In this paper, we developed a method for covalent multi-biofunctionalization of a titanium surface with mixtures of peptides at any desired ratios using click chemistry. We demonstrated that the optimization of the types and ratios of the peptides on a titanium surface resulted in an excellent antimicrobial activity as well as good biocompatibility.
Improving the biological property of titanium surfaces, such as biocompatibility or antimicrobial activity, is important for clinical application of titanium based implantable materials, as the defects of these properties would lead to the failure of the surgery, patient disability and morbidity, and even death.1 Peptides, which could exhibit excellent bioactivity with different amino sequences, are screened by researchers to solve this problem.2–6 However, to modify the titanium surface with a single peptide is often limited by undesirable effects. For example, to improve the antimicrobial activity of a titanium surface could lead to evident cytotoxicity.3,7 So how to multi-biofunctionalize titanium surfaces with different peptides to achieve proper biological property is of significant interest.
There have been several reports on the attachment of peptides onto titanium surfaces. Physical adsorption was a simple method to reach this goal.3,8 However, it is closely dependent on the morphology and composition of the surfaces,3 and the burst release, as well as the poor orientation of peptides on the surface would decrease the effect.3,8,9 Conventional covalent immobilization, such as amidation reaction between amino and carboxylic acid groups,10–12 would affect the orientation and flexibility of the immobilized peptides and decrease their activities9. Moreover, these methods do not allow for a control over the location and number of molecules binding to the substrate.9,13 Some other methods, such as integrating a peptide with an anchor, were also investigated.4,14,15 For example, Mas-Moruno et al. attempted to use this method to integrate PHSRN and RGD peptides onto a titanium surface at the same time. However, these two peptides, which they used have similar effects on improving the biocompatibility, and the ratios of the two peptides could not be changed to the desired ratios. To date, it is still interesting to find new methods, which could integrate several types of peptides with distinct functions at the same time at desired ratios.
In this paper, we developed a method to multi-biofunctionalize the titanium surface with peptides by immobilizing them with a silane coupling agent. Using a silane coupling agent is a useful method to integrate peptide onto implant surface.2 However, the conventional method is multistep, which should make it difficult to multi-biofunctionalize the titanium surface.2 In this research, we first select two types of peptides, HHC36 and RGD, as our model peptides. HHC36 (KRWWKWWRR, abbreviated as AMP) is a type of short antimicrobial peptide.16 Compared to other antimicrobial agents, antimicrobial peptides have several unique advantages, including a broad spectrum of antimicrobial activity, a low susceptibility to developing bacterial resistance and a short contact time to induce killing.9,17,18 In addition to these advantages, the HHC36 peptide also has improved antimicrobial activity.3,16 Moreover, as the amino acid sequences are short, they should have lower immunogenicity compared to traditional antimicrobial peptides and other cationic polymers.16,19 The RGD peptide, which could specifically combine with 11 species of integrin, is sometimes used to increase the biocompatibility of implants.2,20,21 As HHC36 and RGD have different bioactivities, we select these two peptides to multi-biofunctionalize the titanium surface with high antimicrobial activity, as well as good biocompatibility. We integrated azido-PEG12-acid or azido-PEG24-acid onto the peptide's “N” terminal by amide bond to introduce an azido group in the peptide, and prepared a type of silane coupling agent with an alkyl group (alkynyl-PEG-triethoxysilane, abbreviated as APTS). Then, we integrated the peptides with APTS by Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC). As a type of click chemistry, CuAAC could be processed in aqueous conditions at room temperature at a fast rate with high efficiency.22 In addition, as reported, it could control the orientation of the peptide integrated on the implant surface.9,13
The scheme of the experiment is shown in Fig. 1 and the detail of the experiment are shown in the ESI.† In brief, after integrating the APTS with AMP or RGD peptides at different ratios by CuAAC, we directly rotary evaporated the click solution and re-dissolved the system into 95% ethanol/5% water (pH = 4.6) to hydrolyze the APTS for 2 h. Then, we dripped the hydrolyzed solution onto the titanium surface to hydrolyze the APTS for another 2 h to form the film. For the control group, we used the peptide without azido group to replace the peptide–azide in the experimental groups, and the other processes were the same. All the experiments were performed at room temperature. The abbreviations of different surfaces are shown in Table 1S.†
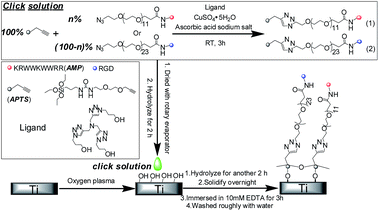 | ||
| Fig. 1 Scheme of the process to multi-biofunctionalize the titanium surface with a mixture of peptides. | ||
To demonstrate that this method could prepare bioactive molecules onto a titanium surface, we first used this method to prepare biotin with an azido group onto the substrate to replace the peptides. Then, the biotin in the film was stained with avidin–FITC, and observed with fluorescent microscopy. We used Ti and Ti-biotin-control (prepared in click solution without copper sulphate, and other processes were the same) as our control groups. The results shown in Fig. 2 show that only Ti-biotin exhibited evident fluorescence, which demonstrated that a probe molecule could be integrated with APTS and prepared onto the titanium surface by this method.
 | ||
| Fig. 2 Fluorescent images of the surfaces stain with avidin–FITC: (a) Ti; (b) Ti-biotin-control and (c) Ti-biotin. | ||
Then, we characterized the morphology and composition of Ti-100%AMP. The AFM results shown in Fig. 3(a) show that the Ti sample exhibited bulk morphology, which is supposed to be caused by the vapor deposition of titanium particles around 20 nm. After the APTS film is formed, the morphology of Ti-100%AMP evidently changed and the bulk morphology disappeared. We also used ellipsometry to estimate the thickness of molecule (1) (as shown in Fig. 1) on Ti-100%AMP, which was about 9.9 ± 0.2 nm. Note that, the film thickness derived from ellipsometry is only a rough estimate, as Fig. 3(a) showed that the titanium surface obtained by evaporating titanium particles onto Si (100) was not very smooth, which would impact the ellipsometry results. Then, we used the ellipsometry results and the formula (1)13,23 in the ESI† to estimate the density of molecule (1) on Ti-100%AMP, which was about 2.3 × 1014 cm−2.
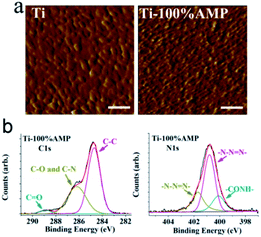 | ||
| Fig. 3 (a) AFM images of the indicated sample, the scale in the images denotes 100 nm, and the z scale for both images is 3 nm; (b) the C1s and N1s XPS spectrum of Ti-100%AMP. | ||
Furthermore, the C1s and N1s XPS spectrum of Ti-100%AMP shown in Fig. 3(b) demonstrate the formation of the film. The C1s signal could be deconvoluted into the peaks of C–C, C–O and C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the results, in which the peak of C–O from OEG on the peptide or APTS, and the peak of C–N from triazole were overlapped.24 The C1s spectrum of Ti-AMP-control shown in Fig. 1S† showed that there should be only a C–O peak from the OEG on APTS, which was considerably lower than that of Ti-100%AMP. The narrow N1s scans of Ti-100%AMP in Fig. 3(b) could be fitted into three peaks, which are assigned to –CONH– and –N–N
O in the results, in which the peak of C–O from OEG on the peptide or APTS, and the peak of C–N from triazole were overlapped.24 The C1s spectrum of Ti-AMP-control shown in Fig. 1S† showed that there should be only a C–O peak from the OEG on APTS, which was considerably lower than that of Ti-100%AMP. The narrow N1s scans of Ti-100%AMP in Fig. 3(b) could be fitted into three peaks, which are assigned to –CONH– and –N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–. The ratio of the two peak areas of –N–N
N–. The ratio of the two peak areas of –N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– was about 1
N– was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which was consistent with the structure of the triazole.
2, which was consistent with the structure of the triazole.
The XPS results shown in Fig. 2S† show that the Ti2p signals of Ti-100%AMP and Ti-AMP-control became weaker compared to that of Ti, which demonstrated that there was APTS film on the titanium surface to shield the Ti signal from the substrate. Fig. 3S† shows that the Si 2p signal of the Ti-100%AMP was lower compared to Ti-AMP-control, which is supposed to be caused by the peptide on the APTS shielding the Si signal.
The results above demonstrate that the bioactive molecule or peptide could be prepared onto a pristine titanium surface by this method. In order to multi-biofunctionalize the titanium surface, we then prepared different surfaces by controlling the ratio of AMP and RGD. The contact angle results of these surfaces are shown in Table 2S.† It shows that Ti had the lowest contact angle. As AMP has a hydrophobic amino acid, such as tryptophan, Ti-100%AMP has larger contact angle compared to Ti-100%RGD. It also showed that Ti-100%RGD, Ti-50%AMP–50%RGD, Ti-65%AMP–35%RGD and Ti-80%AMP–20%RGD had similar contact angles, which were arranged from 58.83° to 63.03°. When the concentration of AMP was up to 90%, the contact angle of Ti-90%AMP–10%RGD had a sudden rise to 73.43°. This might be caused by the difference in the OEG on the peptides. At the “N” terminal, the RGD had a longer OEG (EG24) compared to AMP (EG12), which should make it higher than the AMP. It might affect the hydrophilic–hydrophobic property of the surface even at low concentration.
The antimicrobial activity and the biocompatibility of the surfaces with different concentrations of AMP and RGD are shown in Fig. 4. The results shown in Fig. 4(a) show that without AMP, the Ti, Ti-AMP-control and Ti-100%RGD exhibited no antimicrobial activity. With the increase of AMP ratio on the surface, the antimicrobial activity of the sample increased. Ti-50%AMP–50%RGD could kill about 76.76% of S. aureus and 57.81% of E. coli on it. Ti-80%AMP–20%RGD could kill about 97.71% of S. aureus and 86.72% of E. coli on it. Ti-90%AMP–10%RGD and Ti-100%AMP could kill about 98.77% and 99.12% of S. aureus, as well as 92.03% and 94.24% of E. coli, respectively. In addition, the fluorescent images of the live/dead assay in Fig. 4S† also show that the bacteria on Ti-100%AMP were dead and those on Ti-AMP-control were alive. Interestingly, as shown in Fig. 5S,† when there was no RGD peptide, the antimicrobial activity of the surface with the same concentration of AMP could increase. For example, the Ti-50%AMP could kill about 98.34% of S. aureus and 80.00% of E. coli on it, which was considerably better than that of Ti-50%AMP–50%RGD.
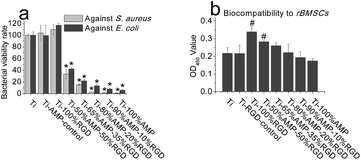 | ||
| Fig. 4 The antimicrobial activity (n = 3) and biocompatibility (n = 3) of the indicated samples (# denotes p < 0.05, and * denotes p < 0.001 compared to Ti sample). | ||
The biocompatibility assay shown in Fig. 4(b) shows that the Ti-100%AMP exhibited cytotoxicity evidently, and there was 80.03% of cells on it compared to those on Ti. With the decrease of AMP and the increase of RGD, the biocompatibility of the samples increased. The cells on Ti-90%AMP–10%RGD, Ti-80%AMP–20%RGD, Ti-65%AMP–35%RGD and Ti-50%AMP–50%RGD were about 0.89, 1.02, 1.20 and 1.30 times compared to those on Ti respectively. Ti-100%RGD had the best biocompatibility, which was about 1.56 times of Ti, and about 1.95 times of Ti-100%AMP. Among these groups, the ideal group should be Ti-80%AMP–20%RGD, which had similar biocompatibility to Ti and exhibited excellent antimicrobial activity. The Ti-RGD-control showed similar biocompatibility to Ti, which illustrated that there should not be RGD on the surface and the APTS film would not affect the biocompatibility.
Conclusions
In this paper, we prepared different types of peptides onto a titanium surface at desired ratios to multi-biofuntionalize the titanium surface. After modifying with the peptides HHC36 and RGD at certain ratios, the surface could exhibit excellent antimicrobial activity, as well as good biocompatibility.Acknowledgements
The authors gratefully acknowledge the financial support of National Natural Science Foundation of China (Grant 51232002), the Basic Research Project of China (2012CB619100), National Natural Science Foundation of China (Grant 51302088) and China Postdoctoral Science Foundation (Grant 2013M531844).Notes and references
- M. Geetha, A. K. Singh, R. Asokamani and A. K. Gogia, Prog. Mater. Sci., 2009, 54, 397–425 CrossRef CAS PubMed.
- S. J. Xiao, M. Textor, N. D. Spencer, M. Wieland, B. Keller and H. Sigrist, J. Mater. Sci.: Mater. Med., 1997, 8, 867–872 CrossRef CAS.
- M. Kazemzadeh-Narbat, J. Kindrachuk, K. Duan, H. Jenssen, R. E. W. Hancock and R. Wang, Biomaterials, 2010, 31, 9519–9526 CrossRef CAS PubMed.
- C. Mas-Moruno, R. Fraioli, F. Abericio, J. M. Manero and F. J. Gil, ACS Appl. Mater. Interfaces, 2014, 6, 6525–6536 CAS.
- A. W. G. Nijhuis, J. J. J. P. van den Beucken, O. C. Boerman, J. A. Jansen and S. C. G. Leeuwenburgh, Tissue Eng., Part C, 2013, 19, 610–619 CrossRef CAS PubMed.
- L. Wang, M. Zhao, S. Li, U. J. Erasquin, H. Wang, L. Ren, C. Chen, Y. Wang and C. Cai, ACS Appl. Mater. Interfaces, 2014, 6, 8401–8406 CAS.
- L. Wang, U. J. Erasquin, M. Zhao, L. Ren, M. Y. Zhang, G. J. Cheng, Y. Wang and C. Cai, ACS Appl. Mater. Interfaces, 2011, 3, 2885–2894 CAS.
- M. Ma, M. Kazemzadeh-Narbat, Y. Hui, S. Lu, C. Ding, D. D. Y. Chen, R. E. W. Hancock and R. Wang, J. Biomed. Mater. Res., Part A, 2012, 100A, 278–285 CrossRef CAS PubMed.
- F. Costa, I. F. Carvalho, R. C. Montelaro, P. Gomes and M. C. L. Martins, Acta Biomater., 2011, 7, 1431–1440 CrossRef CAS PubMed.
- M. D. Steven and J. H. Hotchkiss, J. Appl. Polym. Sci., 2008, 110, 2665–2670 CrossRef CAS.
- M. D. P. Willcox, E. B. H. Hume, Y. Aliwarga, N. Kumar and N. Cole, J. Appl. Microbiol., 2008, 105, 1817–1825 CrossRef CAS PubMed.
- M. S. Mannoor, S. Zhang, A. J. Link and M. C. McAlpine, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 19207–19212 CrossRef CAS PubMed.
- Y. Li, C. M. Santos, A. Kumar, M. Zhao, A. I. Lopez, G. Qin, A. M. McDermott and C. Cai, Chem.–Eur. J., 2011, 17, 2656–2665 CrossRef CAS PubMed.
- R. Beutner, J. Michael, B. Schwenzer and D. Scharnweber, J. R. Soc., Interface, 2010, 7, S93–S105 CrossRef CAS PubMed.
- J. Michael, L. Schoenzart, I. Israel, R. Beutner, D. Scharnweber, H. Worch, U. Hempel and B. Schwenzer, Bioconjugate Chem., 2009, 20, 710–718 CrossRef CAS PubMed.
- K. Hilpert, M. Elliott, H. Jenssen, J. Kindrachuk, C. D. Fjell, J. Koerner, D. F. H. Winkler, L. L. Weaver, P. Henklein, A. S. Ulrich, S. H. Y. Chiang, S. W. Farmer, N. Pante, R. Volkmer and R. E. W. Hancock, Chem. Biol., 2009, 16, 58–69 CrossRef CAS PubMed.
- M. Chromek, Z. Slamova, P. Bergman, L. Kovacs, L. Podracka, I. Ehren, T. Hokfelt, G. H. Gudmundsson, R. L. Gallo, B. Agerberth and A. Brauner, Nat. Med., 2006, 12, 636–641 CrossRef CAS PubMed.
- F. H. Login, S. Balmand, A. Vallier, C. Vincent-Monegat, A. Vigneron, M. Weiss-Gayet, D. Rochat and A. Heddi, Science, 2011, 334, 362–365 CrossRef CAS PubMed.
- M. Malmsten, Soft Matter, 2011, 7, 8725–8736 RSC.
- G. Vidal, T. Blanchi, A. J. Mieszawska, R. Calabrese, C. Rossi, P. Vigneron, J.-L. Duval, D. L. Kaplan and C. Egles, Acta Biomater., 2013, 9, 4935–4943 CrossRef CAS PubMed.
- U. Hersel, C. Dahmen and H. Kessler, Biomaterials, 2003, 24, 4385–4415 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- S. J. Sofia, V. Premnath and E. W. Merrill, Macromolecules, 1998, 31, 5059–5070 CrossRef CAS PubMed.
- Y. Li, M. Zhao, J. Wang, K. Liu and C. Cai, Langmuir, 2011, 27, 4848–4856 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental methods, abbreviation of the samples, XPS results, contact angle results and antimicrobial activity assay. See DOI: 10.1039/c4tb01318b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |
